Abstract
Background and purpose
Despite the increasing number of reports on the spectrum of neurological manifestations of COVID‐19 (neuro‐COVID), few studies have assessed short‐ and long‐term outcome of the disease.
Methods
This is a cohort study enrolling adult patients with neuro‐COVID seen in neurological consultation. Data were collected prospectively or retrospectively in the European Academy of Neurology NEuro‐covid ReGistrY ((ENERGY). The outcome at discharge was measured using the modified Rankin Scale and defined as ‘stable/improved’ if the modified Rankin Scale score was equal to or lower than the pre‐morbid score, ‘worse’ if the score was higher than the pre‐morbid score. Status at 6 months was also recorded. Demographic and clinical variables were assessed as predictors of outcome at discharge and 6 months.
Results
From July 2020 to March 2021, 971 patients from 19 countries were included. 810 (83.4%) were hospitalized. 432 (53.3%) were discharged with worse functional status. Older age, stupor/coma, stroke and intensive care unit (ICU) admission were predictors of worse outcome at discharge. 132 (16.3%) died in hospital. Older age, cancer, cardiovascular complications, refractory shock, stupor/coma and ICU admission were associated with death. 262 were followed for 6 months. Acute stroke or ataxia, ICU admission and degree of functional impairment at discharge were predictors of worse outcome. 65/221 hospitalized patients (29.4%) and 10/32 non‐hospitalized patients (24.4%) experienced persisting neurological symptoms/signs. 10/262 patients (3.8%) developed new neurological complaints during the 6 months of follow‐up.
Conclusions
Neuro‐COVID is a severe disease associated with worse functional status at discharge, particularly in older subjects and those with comorbidities and acute complications of infection.
Keywords: COVID‐19, neurological disorders, outcome, predictors, SARS‐CoV‐2
From July 2020 to March 2021, 971 patients from 19 countries were included. 810 (83.4%) were hospitalized during the acute phase of the COVID‐19 infection, of whom 432 (53.3%) were discharged with worse functional status and 132 (16.3%) died in hospital. 262 were followed for 6 months: 75/262 patients (28.6%) experienced persisting neurological symptoms/signs, whilst 10/262 patients (3.8%) developed new neurological complaints during the 6 months of follow‐up.
INTRODUCTION
The spectrum of coronavirus disease 2019 (COVID‐19) includes several neurological manifestations that, when present, are associated with higher severity and worse outcome [1, 2, 3, 4]. However, neurological symptoms, signs and diagnoses in patients with COVID‐19 (neuro‐COVID) vary according to the target populations, setting (inpatients vs. outpatients), diagnostic criteria and the background of those in charge of data collection [5]. At present, there are only few publications with follow‐up, mainly from single centre studies [6] or non‐hospitalized patients [7], or based on self‐reports [8], electronic databases [9, 10], small samples [11], or with short follow‐up [12, 13] or high attrition rates [14]. Thus, available evidence is insufficient to define the full spectrum of neuro‐COVID and verify how patients’ profile (demographics, baseline clinical features) and acute manifestations of infection predict the outcome of the disease.
On this background, an international registry of patients with COVID‐19 and neurological symptoms, signs or diagnoses was established for a better understanding of the disease spectrum, along with risk factors, comorbidities and outcome [15]. The advantage of such a registry is the investigation of a large sample of patients from various countries, from which data on neuro‐COVID are collected using uniform diagnostic criteria and standardized methods.
The aims of this study were (1) to compare the outcome of neuro‐COVID at hospital discharge and at 6 months with patients’ profile (comorbidities, general and neurological findings during the acute phase) and find outcome predictors; (2) to illustrate the demographic and clinical features of inpatients and outpatients with neuro‐COVID from different countries; (3) to define incidence and types of new neurological manifestations after the acute phase.
PATIENTS AND METHODS
A multinational registry of patients with neuro‐COVID was activated in May 2020 by the European Academy of Neurology (EAN) to provide epidemiological data on neurological signs and symptoms in patients with COVID‐19 infection reported by neurologists in outpatient services, emergency rooms and hospital departments (the EAN NEuro‐covid ReGistrY, ENERGY). Details on the ENERGY structure and organization have been published [15]. Briefly, all neurologists participating in the registry were asked to record neurological symptoms, signs and diagnoses in clinically or laboratory‐confirmed COVID‐19 patients in an electronic case record form (e‐CRF) (Appendix S1). Data were collected prospectively or retrospectively and included patients’ demographics and lifestyle habits, comorbidities, date of first symptoms of infection, hospital and intensive care unit (ICU) admission, incident general and neurological manifestations during the acute phase, diagnostic tests and outcome. Each variable was reported as ‘Yes’, ‘No’ or ‘Unknown’. In addition, for each documented neurological manifestation, the local investigator was asked to indicate whether or not it was associated with COVID‐19.
All adult patients with symptoms and/or signs and/or diseases requiring neurological consultation were eligible for inclusion. A guide is included in the e‐CRF (Appendix S2) to define each variable and facilitate data collection in the e‐CRF at study entry and during follow‐up. Registration and follow‐up of eligible patients is ongoing.
All registered patients were followed through telephone contacts at 6 and 12 months. At each contact, the modified Rankin Scale (mRS) score was assigned and new neurological manifestations were noted; for patients who died, date of death and, if performed, autopsy were noted. As the mRS is reliable even when applied by telephone [16], in addition to follow‐up, functional disability at baseline was measured enquiring of patients or caregivers their pre‐morbid functional status.
Descriptive statistics were performed on all variables collected during the acute phase in the entire sample and comparing hospitalized and non‐hospitalized patients and prospective and retrospective observations. The outcome of the infection, in terms of functional impairment, was defined as ‘stable/improved’ if mRS at discharge was equal to or lower than the baseline score; ‘worse’ if mRS score at discharge was higher than the baseline score. Stable/improved and worse outcome were also assessed in patients who died during hospital stay compared to those discharged alive.
Neurological symptoms, signs and diagnoses persisting at 6 months were listed. The demographic and clinical profile of patients with new neurological manifestations occurring during follow‐up was illustrated. The same methods were used to assess the effect of variables collected during the acute phase or at discharge on the 6‐month outcome.
The association of all variables included in the registry with outcome (worse vs. stable/improved) and status (dead vs. alive) at hospital discharge was evaluated using univariable logistic regression models. Variables identified as statistically significant in univariable models were included in multivariable models, and a stepwise selection (with p < 0.05 as the criterion for entering and removing effects) was applied to identify variables most strongly associated with the outcome and status at discharge. Results of univariable and multivariable logistic regression models are presented as odds ratios (ORs) and adjusted odds ratios with 95% confidence intervals (CIs). Significance was set at the 5% level (0.05).
For demographic and lifestyle variables, mRS and outcome, the number of missing data was reported in the tables and indicated as unknown or missing. For all other variables, ‘unknown’ values were grouped with ‘No’. For neurological findings, the categories ‘present, not COVID associated’ and ‘present, likely COVID associated’ were combined. Data presented as numbers with percentages or as means with standard deviations or medians and ranges were calculated only in subjects with the corresponding values.
The study was approved by the ethics committees of all participating sites and informed consent was obtained from all eligible patients in line with each participating country's legal requirements.
RESULTS
As of 31 March 2021, 1004 patients were enrolled. COVID‐19 infection was not laboratory‐confirmed in 33 cases, which were excluded from further analyses. The final sample included 971 patients from 19 countries (Europe 14; Asia 2; Africa 2; South America 1) (Table 1). A flowchart of the study is illustrated in Figure 1.
TABLE 1.
Confirmed COVID‐19 cases, hospitalized and not hospitalized cases
| All COVID‐19 confirmed (n = 971) a | Hospitalized (n = 810) | Not hospitalized (n = 154) | p value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Country | |||||||
| Austria | 66 | 6.80 | 64 | 7.90 | 2 | 1.30 | <0.0001 |
| Brazil | 3 | 0.31 | 1 | 0.12 | 2 | 1.30 | |
| Egypt | 6 | 0.62 | 5 | 0.62 | 1 | 0.65 | |
| Estonia | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| France | 22 | 2.27 | 19 | 2.35 | 3 | 1.95 | |
| Hungary | 101 | 10.40 | 85 | 10.49 | 16 | 10.39 | |
| Israel | 30 | 3.09 | 30 | 3.70 | 0 | 0.00 | |
| Italy | 165 | 16.99 | 96 | 11.85 | 69 | 44.81 | |
| Macedonia | 1 | 0.10 | 1 | 0.12 | 0 | 0.00 | |
| Moldova | 118 | 12.15 | 116 | 14.32 | 2 | 1.30 | |
| Norway | 50 | 5.15 | 39 | 4.81 | 11 | 7.14 | |
| Poland | 26 | 2.68 | 9 | 1.11 | 17 | 11.04 | |
| Portugal | 56 | 5.77 | 53 | 6.54 | 0 | 0.00 | |
| Romania | 84 | 8.65 | 84 | 10.37 | 0 | 0.00 | |
| Russia | 13 | 1.34 | 7 | 0.86 | 6 | 3.90 | |
| Switzerland | 42 | 4.33 | 22 | 2.72 | 19 | 12.34 | |
| Tunisia | 19 | 1.96 | 14 | 1.73 | 5 | 3.25 | |
| Turkey | 145 | 14.93 | 141 | 17.41 | 1 | 0.65 | |
| Ukraine | 24 | 2.47 | 24 | 2.96 | 0 | 0.00 | |
| Sex | |||||||
| Male | 497 | 51.18 | 434 | 53.58 | 59 | 38.31 | 0.0033 |
| Female | 466 | 47.99 | 368 | 45.43 | 95 | 61.69 | |
| Intersex | 2 | 0.21 | 2 | 0.25 | 0 | 0.00 | |
| Unknown | 6 | 0.62 | 6 | 0.74 | 0 | 0.00 | |
| Smoking | |||||||
| Yes | 122 | 12.56 | 109 | 13.46 | 13 | 8.44 | 0.0949 |
| No | 729 | 75.08 | 606 | 74.81 | 120 | 77.92 | |
| Unknown | 120 | 12.36 | 95 | 11.73 | 21 | 13.64 | |
| Source of COVID‐19 contact | |||||||
| Occupation | 75 | 7.72 | 42 | 5.19 | 33 | 21.43 | <0.0001 |
| Family member | 168 | 17.30 | 119 | 14.69 | 46 | 29.87 | |
| Social | 86 | 8.86 | 66 | 8.15 | 18 | 11.69 | |
| Travel | 17 | 1.75 | 16 | 1.98 | 1 | 0.65 | |
| Other | 61 | 6.28 | 61 | 7.53 | 0 | 0.00 | |
| Unknown | 564 | 58.08 | 506 | 62.47 | 56 | 36.36 | |
| Median (n) | IQR | Median (n) | IQR | Median (n) | IQR | ||
|---|---|---|---|---|---|---|---|
| Age at COVID onset | 63 (909) | 48–74 | 66 (751) | 52–76 | 48 (151) | 34–61 | <0.0001 |
| BMI | 25 (840) | 23–28 | 26 (706) | 23–29 | 24 (130) | 22–28 | 0.0455 |
| n | % | n | % | n | % | ||
|---|---|---|---|---|---|---|---|
| Any comorbidity | 619 | 63.75 | 565 | 69.75 | 49 | 31.82 | <0.0001 |
| Hypertension | 505 | 52.01 | 464 | 57.28 | 36 | 23.38 | <0.0001 |
| Diabetes type 1 | 8 | 0.82 | 8 | 0.99 | 0 | 0.00 | 0.2054 |
| Diabetes type 2 | 206 | 21.22 | 191 | 23.58 | 14 | 9.09 | <0.0001 |
| Cardiovascular disease | 289 | 29.76 | 269 | 33.21 | 17 | 11.04 | <0.0001 |
| Chronic kidney disease | 88 | 9.06 | 87 | 10.74 | 1 | 0.65 | <0.0001 |
| Chronic liver disease | 37 | 3.81 | 37 | 4.57 | 0 | 0.00 | 0.0057 |
| Chronic pulmonary disease | 93 | 9.58 | 86 | 10.62 | 6 | 3.90 | 0.0136 |
| Anaemia | 50 | 5.15 | 47 | 5.80 | 3 | 1.95 | 0.0389 |
| Cancer | 85 | 8.75 | 80 | 9.88 | 4 | 2.60 | 0.0055 |
| Immunosuppressed state | 50 | 5.15 | 44 | 5.43 | 6 | 3.90 | 0.3712 |
| Other non‐neurological comorbidity | 232 | 23.89 | 216 | 26.67 | 15 | 9.74 | <0.0001 |
| Dementia | 86 | 8.86 | 79 | 9.75 | 6 | 3.90 | 0.0275 |
| Parkinson's disease | 35 | 3.60 | 21 | 2.59 | 14 | 9.09 | 0.0001 |
| Stroke: ICH, ischaemic stroke, TIA | 154 | 15.86 | 147 | 18.15 | 6 | 3.90 | <0.0001 |
| Multiple sclerosis | 47 | 4.84 | 19 | 2.35 | 28 | 18.18 | <0.0001 |
| Motor neuron disease | 4 | 0.41 | 3 | 0.37 | 1 | 0.65 | 0.6501 |
| Neuromuscular disorder | 12 | 1.24 | 11 | 1.36 | 1 | 0.65 | 0.4395 |
| Neuropathy | 34 | 3.50 | 31 | 3.83 | 3 | 1.95 | 0.2157 |
| Other neurological disease | 99 | 10.20 | 82 | 10.12 | 16 | 10.39 | 0.8675 |
| COVID systemic complications | 501 | 51.60 | 480 | 59.26 | 17 | 11.04 | <0.0001 |
| Dyspnoea | 503 | 51.80 | 453 | 55.93 | 46 | 29.87 | <0.0001 |
| Pneumonia | 528 | 54.38 | 501 | 61.85 | 22 | 14.29 | <0.0001 |
| Cardiovascular | 121 | 12.46 | 117 | 14.44 | 1 | 0.65 | <0.0001 |
| Renal insufficiency/dialysis | 65 | 6.69 | 65 | 8.02 | 0 | 0.00 | 0.0002 |
| Coagulation disorder/disseminated intravascular coagulation | 45 | 4.63 | 44 | 5.43 | 1 | 0.65 | 0.0080 |
| Refractory shock | 38 | 3.91 | 37 | 4.57 | 0 | 0.00 | 0.0183 |
| Extra‐corporeal membrane oxygenation (ECMO) | 5 | 0.51 | 5 | 0.62 | 0 | 0.00 | 0.3176 |
| Mechanical ventilation | 121 | 12.46 | 116 | 14.32 | 0 | 0.00 | <0.0001 |
| Neurological findings | 747 | 76.93 | 633 | 78.15 | 107 | 69.48 | 0.0434 |
| Headache | 394 | 40.58 | 310 | 38.27 | 81 | 52.60 | 0.0010 |
| Hyposmia/hypogeusia | 291 | 29.97 | 199 | 24.57 | 90 | 58.44 | <0.0001 |
| Dysautonomia | 139 | 14.32 | 107 | 13.21 | 31 | 20.13 | 0.0274 |
| Vertigo | 194 | 19.98 | 159 | 19.63 | 32 | 20.78 | 0.5409 |
| Myalgia | 284 | 29.25 | 202 | 24.94 | 80 | 51.95 | <0.0001 |
| Sleep disorders | 161 | 16.58 | 120 | 14.81 | 39 | 25.39 | 0.0009 |
| Cognitive impairment (including dysexecutive syndrome) | 288 | 29.66 | 256 | 31.60 | 32 | 20.78 | 0.0029 |
| Hyperactive delirium | 122 | 12.56 | 111 | 13.70 | 10 | 6.49 | 0.0163 |
| Hypoactive delirium/acute encephalopathy | 112 | 11.53 | 106 | 13.09 | 6 | 3.90 | 0.0007 |
| Stupor/coma | 124 | 12.77 | 119 | 14.69 | 4 | 2.60 | <0.0001 |
| Syncope | 51 | 5.25 | 46 | 5.68 | 5 | 3.25 | 0.1813 |
| Seizures/status epilepticus | 81 | 8.34 | 76 | 9.38 | 5 | 3.25 | 0.0085 |
| Meningitis/encephalitis | 42 | 4.33 | 38 | 4.69 | 4 | 2.60 | 0.2087 |
| Stroke | 253 | 26.06 | 244 | 30.12 | 6 | 3.90 | <0.0001 |
| Tremor | 68 | 7.00 | 60 | 7.41 | 8 | 5.19 | 0.2681 |
| Chorea | 1 | 0.10 | 1 | 0.12 | 0 | 0.00 | 0.6556 |
| Dystonia | 18 | 1.85 | 18 | 2.22 | 0 | 0.00 | 0.0562 |
| Myoclonus | 15 | 1.54 | 15 | 1.85 | 0 | 0.00 | 0.0818 |
| Dyskinesia | 12 | 1.24 | 9 | 1.11 | 3 | 1.95 | 0.4301 |
| Parkinsonism | 26 | 2.68 | 21 | 2.59 | 5 | 3.25 | 0.7217 |
| Ataxia | 86 | 8.86 | 78 | 9.63 | 8 | 5.19 | 0.0573 |
| Spinal cord disorder | 38 | 3.91 | 36 | 4.44 | 2 | 1.30 | 0.0557 |
| Peripheral neuropathy | 92 | 9.47 | 85 | 10.49 | 7 | 4.55 | 0.0150 |
| Other neurological findings | 121 | 12.46 | 106 | 13.09 | 15 | 9.74 | 0.1859 |
| Hospital admission | 810 | 83.42 | 810 | 100.00 | 0 | 0.00 | <0.0001 |
| ICU admission | 227 | 23.38 | 224 | 27.65 | 0 | 0.00 | <0.0001 |
| Pre‐morbid mRS | |||||||
| 0 | 488 | 52.87 | 381 | 48.97 | 104 | 75.36 | <0.0001 |
| 1 | 153 | 16.58 | 132 | 16.97 | 20 | 14.49 | |
| 2 | 95 | 10.29 | 84 | 10.80 | 10 | 7.25 | |
| 3 | 96 | 10.40 | 90 | 11.57 | 4 | 2.90 | |
| 4 | 64 | 6.93 | 64 | 8.23 | 0 | 0.00 | |
| 5 | 27 | 2.93 | 27 | 3.47 | 0 | 0.00 | |
| Missing | 48 | 32 | 16 | ||||
| Discharge mRS | |||||||
| 0 | 264 | 28.12 | 170 | 21.49 | 94 | 64.83 | <0.0001 |
| 1 | 158 | 16.83 | 128 | 16.18 | 30 | 20.69 | |
| 2 | 116 | 12.35 | 101 | 12.77 | 15 | 10.34 | |
| 3 | 130 | 13.84 | 123 | 15.55 | 6 | 4.14 | |
| 4 | 88 | 9.37 | 87 | 11.00 | 0 | 0.00 | |
| 5 | 51 | 5.43 | 50 | 6.32 | 0 | 0.00 | |
| 6 | 132 | 14.06 | 132 | 16.69 | 0 | 0.00 | |
| Missing | 32 | 19 | 9 | ||||
| Outcome | |||||||
| Worse b | 448 | 49.12 | 432 | 56.10 | 14 | 10.07 | <0.0001 |
| Stable/improved b | 464 | 50.88 | 338 | 43.90 | 125 | 89.93 | |
| Not available | 59 | 40 | 19 | ||||
Abbreviations: BMI, body mass index; ICH, intracerebral haemorrhage; ICU, intensive care unit; IQR, interquartile range; mRS, modified Rankin Scale; TIA, transient ischaemic attack.
Setting was unknown in seven cases: Portugal three, Switzerland one, Turkey three.
Worse outcome, mRS score at discharge higher than pre‐morbid mRS score; stable/improved outcome, mRS score at discharge equal to or lower than pre‐morbid mRS score.
FIGURE 1.
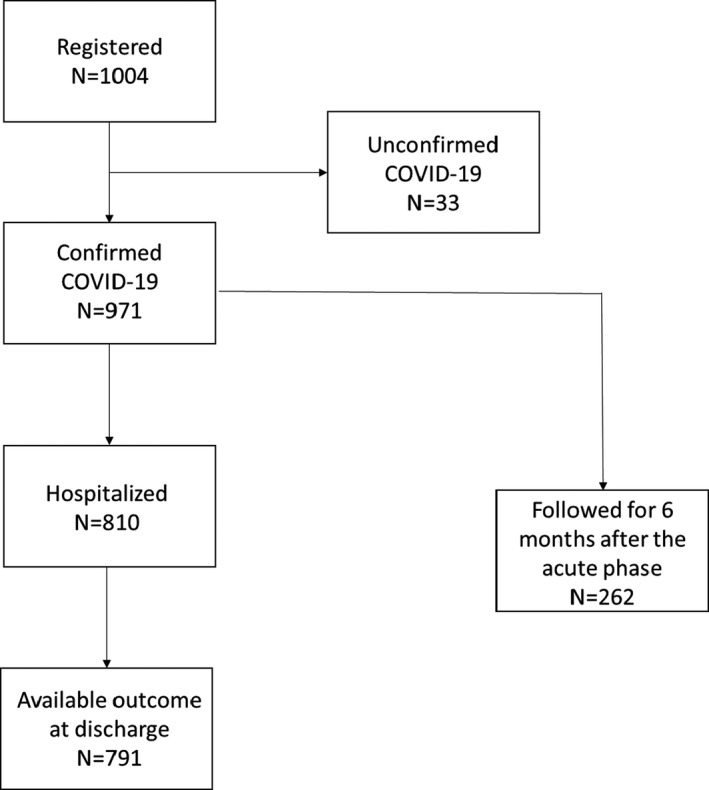
Study flowchart
With few exceptions, there were no major differences in the general characteristics of prospective compared to retrospective cases (Table S1). There were 497 men and 466 women (plus eight intersex or unknown) aged 16–101 years (median 63; interquartile range [IQR] 48–74). One or more comorbidities were present in 619 cases (63.75%). The most frequent was hypertension (52.0%), followed by cardiovascular disease (29.8%) and diabetes (22.0%). A history of transient ischaemic attacks or stroke (154 cases, 15.9%), dementia (86 cases, 8.9%) and Parkinson's disease (35 cases, 3.6%) were the commonest neurological comorbidities.
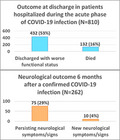
In all, 810 patients (83.4%) were hospitalized. Compared to non‐hospitalized patients, hospitalized patients were older, more often men, with one or more baseline comorbidities, and with functional impairment at baseline (Table 1). Functional disability at admission (mRS 2+) was present in 34.1% of hospitalized patients and 10.6% of non‐hospitalized cases. Hospitalized patients experienced more systemic COVID‐19 complications and had more neurological manifestations during the acute phase. The most common neurological complaints/manifestations during the acute phase included headache (38.2% in hospitalized and 52.6.9% in non‐hospitalized patients), cognitive impairment (31.6% vs. 20.8%), stroke (30.1% vs. 3.9%), delirium (26.7% vs. 10.4%), hyposmia/hypogeusia (24.6% vs. 58.4%), sleep disorders (14.8% vs. 25.4%), myalgias (24.9% vs. 51.9%) and stupor/coma (14.7% vs. 2.6%). 224 patients (27.6%) were admitted to the ICU.
At discharge, the proportion of hospitalized patients with functional impairment (mRS 2+) increased to 62.3% (vs. 14.5% of non‐hospitalized subjects). 432 hospitalized patients (53.3%) were discharged with a worse functional status compared to admission (Table 2, columns A). Compared to patients who improved or were stable, patients with worse outcome were older, had more non‐neurological (hypertension, cardiovascular and renal diseases) and neurological comorbidities (history of transient ischaemic attacks or stroke, dementia) and presented more systemic complications during the acute phase. Stroke was the most common neurological manifestation in patients with worse outcome (40.7%) followed by cognitive impairment (34.0%), headache (31.0%), stupor/coma (23.6%) and myalgia (20.8%). In contrast, in patients with stable/improved outcome the commonest manifestations were, in decreasing order, headache (48.2%), hyposmia/hypogeusia (32.3%), myalgias (30.5%), vertigo (23.4%) and cognitive impairment (20.7%).
TABLE 2.
Outcome at discharge (only confirmed and hospitalized COVID‐19 cases)
| A | p value | B | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Worse outcome a (n = 432) | Stable/improved outcome a (n = 338) | Alive (n = 659) | Dead (n = 132) | |||||||
| n | % | n | % | n | % | n | % | |||
| Country | ||||||||||
| Austria | 35 | 8.10 | 22 | 6.51 | <0.0001 | 52 | 7.89 | 10 | 7.58 | <0.0001 |
| Brazil | 0 | 0.00 | 1 | 0.30 | 1 | 0.15 | 0 | 0.00 | ||
| Egypt | 0 | 0.00 | 1 | 0.30 | 5 | 0.76 | 0 | 0.00 | ||
| Estonia | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | ||
| France | 15 | 3.47 | 3 | 0.89 | 14 | 2.12 | 4 | 3.03 | ||
| Hungary | 28 | 6.48 | 56 | 16.57 | 75 | 11.38 | 10 | 7.58 | ||
| Israel | 8 | 1.85 | 22 | 6.51 | 24 | 3.64 | 6 | 4.55 | ||
| Italy | 70 | 16.20 | 23 | 6.80 | 69 | 10.47 | 26 | 19.70 | ||
| Macedonia | 0 | 0.00 | 1 | 0.30 | 1 | 0.15 | 0 | 0.00 | ||
| Moldova | 97 | 22.45 | 19 | 5.62 | 89 | 13.51 | 27 | 20.45 | ||
| Norway | 23 | 5.32 | 7 | 2.07 | 31 | 4.70 | 0 | 0.00 | ||
| Poland | 4 | 0.93 | 3 | 0.89 | 7 | 1.06 | 0 | 0.00 | ||
| Portugal | 35 | 8.10 | 18 | 5.33 | 44 | 6.68 | 9 | 6.82 | ||
| Romania | 51 | 11.81 | 33 | 9.76 | 56 | 8.50 | 28 | 21.21 | ||
| Russia | 2 | 0.46 | 5 | 1.48 | 7 | 1.06 | 0 | 0.00 | ||
| Switzerland | 6 | 1.39 | 5 | 1.48 | 17 | 2.58 | 1 | 0.76 | ||
| Tunisia | 10 | 2.31 | 4 | 1.18 | 11 | 1.67 | 3 | 2.27 | ||
| Turkey | 48 | 11.11 | 92 | 27.22 | 132 | 20.03 | 8 | 6.06 | ||
| Ukraine | 0 | 0.00 | 23 | 6.80 | 24 | 3.64 | 0 | 0.00 | ||
| Sex | ||||||||||
| Male | 237 | 54.86 | 170 | 50.30 | 0.1716 | 349 | 52.96 | 69 | 52.27 | 0.6373 |
| Female | 193 | 44.68 | 162 | 47.93 | 302 | 45.83 | 63 | 47.73 | ||
| Intersex | 1 | 0.23 | 1 | 0.30 | 2 | 0.30 | 0 | 0.00 | ||
| Unknown | 1 | 0.23 | 5 | 1.48 | 6 | 0.91 | 0 | 0.00 | ||
| Smoking | ||||||||||
| Yes | 40 | 9.26 | 67 | 19.82 | <0.0001 | 100 | 15.17 | 8 | 6.06 | 0.0027 |
| No | 336 | 77.78 | 240 | 71.01 | 492 | 74.66 | 101 | 76.52 | ||
| Unknown | 56 | 12.96 | 31 | 9.17 | 67 | 10.17 | 23 | 17.42 | ||
| Source of COVID‐19 contact | ||||||||||
| Occupation | 13 | 3.01 | 25 | 7.40 | 0.0002 | 39 | 5.92 | 1 | 0.76 | <0.0001 |
| Family member | 50 | 11.57 | 61 | 18.05 | 103 | 15.63 | 12 | 9.09 | ||
| Social | 31 | 7.18 | 34 | 10.06 | 51 | 7.74 | 14 | 10.61 | ||
| Travel | 9 | 2.08 | 6 | 1.78 | 15 | 2.28 | 0 | 0.00 | ||
| Other | 44 | 10.19 | 16 | 4.73 | 38 | 5.77 | 22 | 16.67 | ||
| Unknown | 285 | 65.97 | 196 | 57.99 | 413 | 62.67 | 83 | 62.88 | ||
| Median (n) | IQR | Median (n) | IQR | Median (n) | IQR | Median (n) | IQR | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Age at COVID onset | 70 (405) | 60–79 | 58 (308) | 43–71 | <0.0001 | 63 (606) | 49–73 | 76 (127) | 67–85 | <0.0001 |
| BMI | 26 (379) | 23–29 | 25 (302) | 23–28 | 0.0135 | 26 (573) | 23–28 | 25 (117) | 22–29 | 0.6456 |
| n | % | n | % | n | % | n | % | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Any comorbidity | 335 | 77.55 | 202 | 59.76 | <0.0001 | 434 | 65.86 | 118 | 89.39 | <0.0001 |
| Hypertension | 289 | 66.90 | 158 | 46.75 | <0.0001 | 348 | 52.81 | 107 | 81.06 | <0.0001 |
| Diabetes type 1 | 3 | 0.69 | 5 | 1.48 | 0.2865 | 8 | 1.21 | 0 | 0.00 | 0.2033 |
| Diabetes type 2 | 112 | 25.93 | 71 | 21.01 | 0.1114 | 149 | 22.61 | 40 | 30.30 | 0.0585 |
| Cardiovascular disease | 165 | 38.19 | 92 | 27.22 | 0.0014 | 195 | 29.59 | 68 | 51.52 | <0.0001 |
| Chronic kidney disease | 57 | 13.19 | 28 | 8.28 | 0.0309 | 54 | 8.19 | 32 | 24.24 | <0.0001 |
| Chronic liver disease | 14 | 3.24 | 20 | 5.92 | 0.0728 | 30 | 4.55 | 7 | 5.30 | 0.7093 |
| Chronic pulmonary disease | 47 | 10.88 | 35 | 10.36 | 0.8148 | 68 | 10.32 | 15 | 11.36 | 0.7207 |
| Anaemia | 22 | 5.09 | 20 | 5.92 | 0.6171 | 34 | 5.16 | 13 | 9.85 | 0.0375 |
| Cancer | 46 | 10.65 | 29 | 8.58 | 0.3368 | 53 | 8.04 | 24 | 18.18 | 0.0003 |
| Immunosuppressed state | 20 | 4.63 | 19 | 5.62 | 0.5334 | 33 | 5.01 | 8 | 6.06 | 0.6184 |
| Other non‐neurological comorbidity | 145 | 33.56 | 63 | 18.64 | <0.0001 | 153 | 23.22 | 60 | 45.45 | <0.0001 |
| Dementia | 49 | 11.34 | 27 | 7.99 | 0.1214 | 53 | 8.04 | 26 | 19.70 | <0.0001 |
| Parkinson's disease | 14 | 3.24 | 7 | 2.07 | 0.3227 | 14 | 2.12 | 7 | 5.30 | 0.0381 |
| Stroke: ICH, ischaemic stroke, TIA | 98 | 22.69 | 44 | 13.02 | 0.0006 | 102 | 15.48 | 43 | 32.58 | <0.0001 |
| Multiple sclerosis | 3 | 0.69 | 13 | 3.85 | 0.0023 | 18 | 2.73 | 1 | 0.76 | 0.1764 |
| Motor neuron disease | 0 | 0.00 | 2 | 0.59 | 0.1094 | 3 | 0.46 | 0 | 0.00 | 0.4374 |
| Neuromuscular disorder | 5 | 1.16 | 4 | 1.18 | 0.9734 | 9 | 1.37 | 1 | 0.76 | 0.5681 |
| Neuropathy | 15 | 3.47 | 13 | 3.85 | 0.7833 | 26 | 3.95 | 5 | 3.79 | 0.9322 |
| Other neurological disease | 38 | 8.80 | 37 | 10.95 | 0.3179 | 60 | 9.10 | 17 | 12.88 | 0.1818 |
| COVID systemic complications | 312 | 72.22 | 151 | 44.67 | <0.0001 | 350 | 53.11 | 122 | 92.42 | <0.0001 |
| Dyspnoea | 270 | 62.50 | 163 | 48.22 | <0.0001 | 335 | 50.83 | 105 | 79.55 | <0.0001 |
| Pneumonia | 306 | 70.83 | 172 | 50.89 | <0.0001 | 374 | 56.75 | 112 | 84.85 | <0.0001 |
| Cardiovascular | 76 | 17.59 | 39 | 11.54 | 0.0193 | 79 | 11.99 | 38 | 28.79 | <0.0001 |
| Renal insufficiency/dialysis | 46 | 10.65 | 17 | 5.03 | 0.0048 | 42 | 6.37 | 22 | 16.67 | <0.0001 |
| Coagulation disorder/disseminated intravascular coagulation | 28 | 6.48 | 13 | 3.85 | 0.106 | 33 | 5.01 | 9 | 6.82 | 0.3971 |
| Refractory shock | 36 | 8.33 | 1 | 0.30 | <0.0001 | 1 | 0.15 | 36 | 27.27 | <0.0001 |
| Extra‐corporeal membrane oxygenation (ECMO) | 4 | 0.93 | 0 | 0.00 | 0.0761 | 4 | 0.61 | 0 | 0.00 | 0.3695 |
| Mechanical ventilation | 99 | 22.92 | 12 | 3.55 | <0.0001 | 66 | 10.02 | 48 | 36.36 | <0.0001 |
| Neurological findings | 377 | 87.27 | 230 | 68.05 | <0.0001 | 498 | 75.57 | 125 | 94.70 | <0.0001 |
| Headache | 134 | 31.02 | 163 | 48.22 | <0.0001 | 279 | 42.34 | 23 | 17.42 | <0.0001 |
| Hyposmia/hypogeusia | 79 | 18.29 | 109 | 32.25 | <0.0001 | 184 | 27.92 | 7 | 5.30 | <0.0001 |
| Dysautonomia | 50 | 11.57 | 54 | 15.98 | 0.0761 | 93 | 14.11 | 13 | 9.85 | 0.1893 |
| Vertigo | 72 | 16.67 | 79 | 23.37 | 0.02 | 140 | 21.24 | 14 | 10.61 | 0.0048 |
| Myalgia | 90 | 20.83 | 103 | 30.47 | 0.0022 | 184 | 27.92 | 12 | 9.09 | <0.0001 |
| Sleep disorders | 63 | 14.58 | 51 | 15.09 | 0.8446 | 105 | 15.93 | 14 | 10.61 | 0.1181 |
| Cognitive impairment (including dysexecutive syndrome) | 157 | 36.34 | 86 | 25.44 | 0.0012 | 183 | 27.77 | 67 | 50.76 | <0.0001 |
| Hyperactive delirium | 76 | 17.59 | 32 | 9.47 | 0.0013 | 86 | 13.05 | 23 | 17.42 | 0.1833 |
| Hypoactive delirium/acute encephalopathy | 76 | 17.59 | 29 | 8.58 | 0.0003 | 74 | 11.23 | 32 | 24.24 | <0.0001 |
| Stupor/coma | 102 | 23.61 | 16 | 4.73 | <0.0001 | 41 | 6.22 | 78 | 59.09 | <0.0001 |
| Syncope | 14 | 3.24 | 30 | 8.88 | 0.0008 | 41 | 6.22 | 4 | 3.03 | 0.1485 |
| Seizures/status epilepticus | 37 | 8.56 | 38 | 11.24 | 0.2136 | 62 | 9.41 | 14 | 10.61 | 0.6699 |
| Meningitis/encephalitis | 19 | 4.40 | 19 | 5.62 | 0.4368 | 33 | 5.01 | 5 | 3.79 | 0.5498 |
| Stroke | 176 | 40.74 | 62 | 18.34 | <0.0001 | 180 | 27.31 | 63 | 47.73 | <0.0001 |
| Tremor | 24 | 5.56 | 32 | 9.47 | 0.038 | 54 | 8.19 | 5 | 3.79 | 0.0786 |
| Chorea | 0 | 0.00 | 1 | 0.30 | 0.2579 | 1 | 0.15 | 0 | 0.00 | 0.6543 |
| Dystonia | 2 | 0.46 | 15 | 4.44 | 0.0002 | 17 | 2.58 | 1 | 0.76 | 0.2001 |
| Myoclonus | 8 | 1.85 | 7 | 2.07 | 0.8271 | 12 | 1.82 | 3 | 2.27 | 0.7283 |
| Dyskinesia | 2 | 0.46 | 7 | 2.07 | 0.0394 | 7 | 1.06 | 2 | 1.52 | 0.6543 |
| Parkinsonism | 9 | 2.08 | 12 | 3.55 | 0.2149 | 20 | 3.03 | 1 | 0.76 | 0.1374 |
| Ataxia | 34 | 7.87 | 43 | 12.72 | 0.026 | 72 | 10.93 | 6 | 4.55 | 0.0248 |
| Spinal cord disorder | 19 | 4.40 | 16 | 4.73 | 0.8244 | 34 | 5.16 | 2 | 1.52 | 0.0667 |
| Peripheral neuropathy | 49 | 11.34 | 26 | 7.69 | 0.09 | 73 | 11.08 | 7 | 5.30 | 0.0466 |
| Other neurological findings | 62 | 14.35 | 37 | 10.95 | 0.1613 | 88 | 13.35 | 16 | 12.12 | 0.7021 |
| ICU admission | 180 | 41.67 | 37 | 10.95 | <0.0001 | 157 | 23.82 | 64 | 48.48 | <0.0001 |
| Pre‐morbid mRS | ||||||||||
| 0 | 206 | 48.02 | 163 | 48.95 | 0.6792 | 331 | 52.29 | 38 | 29.46 | <0.0001 |
| 1 | 78 | 18.18 | 53 | 15.92 | 118 | 18.64 | 13 | 10.08 | ||
| 2 | 50 | 11.66 | 32 | 9.61 | 64 | 10.11 | 18 | 13.95 | ||
| 3 | 48 | 11.19 | 41 | 12.31 | 66 | 10.43 | 23 | 17.83 | ||
| 4 | 35 | 8.16 | 29 | 8.71 | 39 | 6.16 | 25 | 19.38 | ||
| 5 | 12 | 2.80 | 15 | 4.50 | 15 | 2.37 | 12 | 9.30 | ||
| Missing | 3 | 5 | 26 | 3 | ||||||
| Discharge mRS | ||||||||||
| 0 | 0 | 0.00 | 170 | 50.30 | <0.0001 | 170 | 25.80 | 0 | 0.00 | <0.0001 |
| 1 | 58 | 13.43 | 62 | 18.34 | 128 | 19.42 | 0 | 0.00 | ||
| 2 | 57 | 13.19 | 38 | 11.24 | 101 | 15.33 | 0 | 0.00 | ||
| 3 | 88 | 20.37 | 35 | 10.36 | 123 | 18.66 | 0 | 0.00 | ||
| 4 | 61 | 14.12 | 22 | 6.51 | 87 | 13.20 | 0 | 0.00 | ||
| 5 | 36 | 8.33 | 11 | 3.25 | 50 | 7.59 | 0 | 0.00 | ||
| 6 | 132 | 30.56 | 0 | 0.00 | 0 | 0.00 | 132 | 100.00 | ||
Abbreviations: BMI, body mass index; ICH, intracerebral haemorrhage; ICU, intensive care unit; IQR, interquartile range; mRS, modified Rankin Score; TIA, transient ischaemic attack.
Worse outcome, mRS score at discharge higher than pre‐morbid mRS score; stable/improved outcome, mRS score at discharge equal to or lower than pre‐morbid mRS score.
In all, 132 patients died in hospital (Table 2, columns B). Compared to those discharged alive, patients who died were older (median age at COVID‐19 onset 76 years, IQR 67–85), with more comorbidities (hypertension, cardiovascular and renal diseases, cancer and, amongst neurological diseases, stroke, dementia and Parkinson's disease). Stupor/coma, stroke and cognitive disturbances were the commonest neurological manifestations/complaints in patients who died in hospital (59.1%, 47.7% and 49.2%, respectively) along with dysexecutive syndrome (23.5%) and hypoactive delirium (24.2%). Almost all deceased patients presented systemic COVID‐19 complications (predominantly pneumonia, 84.9% of cases), 48.5% were admitted in ICU and 36.4% required mechanical ventilation. Refractory shock occurred in 27.3% of in‐hospital deaths.
The variable most highly associated with worse outcome was refractory shock (OR 30.6; 95% CI 4.2–224.5) (Table 3, columns A). Increasing age also predicted worse outcome (OR 1.04 for each additional year; 95% CI 1.03–1.05). Amongst neurological manifestations, stupor/coma (OR 6.2; 95% CI 3.6–10.8) and stroke (OR 3.1; 95% CI 2.2–4.3) showed the highest risk for worse outcome. The need for mechanical ventilation (OR 8.1; 95% CI 4.35–15.0) and ICU admission (OR 5.8; 95% CI 3.9–8.6) indicated worse outcome. Older age, stupor/coma, stroke and ICU admission were confirmed as predictors of worse outcome at discharge in a multivariable model, whilst syncope and dystonia were predictors of stable/improved outcome. In univariable models, stupor/coma carried the highest death risk (OR 21.8; 95% CI 13.6–34.8), followed by cognitive impairment (3.05; 95% CI 2.1–4.5), hypoactive delirium (OR 2.5; 95% CI 1.6–4.0) and stroke (OR 2.4; 95% CI 1.7–3.56). Use of mechanical ventilation (OR 5.1; 95% CI 3.3–7.9), pneumonia (OR 4.3; 95% CI 2.6–7.0), ICU admission (OR 3.0; 95% CI 2.05–4.4), cardiovascular complications (OR 3.0; 95% CI 1.9–4.6) and renal insufficiency (OR 2.9; 95% CI 1.7–5.1) also predicted in‐hospital mortality. Amongst pre‐existing comorbidities, hypertension (OR 3.8; 95% CI 2.4–6.1), chronic kidney diseases (OR 3.6; 95% CI 2.2–5.8), cardiovascular diseases (OR 2.5; 95% 1.7–3.7) and cancer (OR 2.5; 95% CI 1.5–4.3) were the variables most highly associated with in‐hospital death. Amongst pre‐existing neurological comorbidities, stroke (OR 2.6; 95% CI 1.7–4.0) and Parkinson's disease (OR 2.6; 95% CI 1.02–6.5) carried the highest risk. Pre‐morbid mRS score was significantly associated with in‐hospital mortality, showing an increasing risk with increase of the disability score (Table 3, columns B). Older age, cancer, cardiovascular complications, refractory shock, stupor/coma and ICU admission were predictors of death in the multivariable model, whilst hyposmia/hypogeusia predicted a lower risk of death.
TABLE 3.
Predictors of outcome at discharge (N = 971)
|
A Worse outcome vs. stable/improved outcome a |
B Dead vs. alive at discharge |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable model | Multivariable model | Univariable model | Multivariable model | |||||||||
| OR | 95% CI | p value | Adj. OR | 95% CI | p value | OR | 95% CI | p value | Adj. OR | 95% CI | p value | |
| Sex | ||||||||||||
| Male | 1 (ref.) | 0.1432 | 1 (ref.) | 0.9611 | ||||||||
| Female | 0.86 | 0.64–1.14 | 1.05 | 0.73–1.54 | ||||||||
| Intersex/unknown | 0.24 | 0.05–1.20 | ne | ne | ||||||||
| Smoking | ||||||||||||
| Yes | 0.43 | 0.28–0.65 | 0.0001 | 0.39 | 0.18–0.83 | 0.0039 | ||||||
| No | 1 (ref.) | – | 1 (ref.) | – | ||||||||
| Unknown | 1.29 | 0.81–2.06 | 1.67 | 0.99–2.81 | ||||||||
| Source of COVID‐19 contact | ||||||||||||
| Occupation | 0.53 | 0.35–0.80 | 0.0007 | 0.50 | 0.27–0.94 | 0.0471 | ||||||
| Family member | 0.33 | 0.16–0.67 | 0.11 | 0.02–0.81 | ||||||||
| Social | 0.59 | 0.35–0.99 | 1.18 | 0.63–2.21 | ||||||||
| Travel | 0.97 | 0.34–2.76 | ne | ne | ||||||||
| Other/unknown | 1 (ref.) | – | 1 (ref.) | – | ||||||||
| Age at admission (1‐year increase) | 1.04 | 1.03–1.05 | <0.0001 | 1.03 | 1.02–1.04 | <0.0001 | 1.06 | 1.05–1.08 | <0.0001 | 1.05 | 1.03–1.07 | <0.0001 |
| BMI (1‐unit increase) | 1.01 | 0.99–1.03 | 0.2869 | 0.99 | 0.97–1.02 | 0.5206 | ||||||
| Non‐neurological comorbidities | ||||||||||||
| Hypertension | 2.30 | 1.72–3.09 | <0.0001 | 3.82 | 2.41–6.07 | <0.0001 | ||||||
| Diabetes type 1/type 2 | 1.25 | 0.90–1.74 | 0.1878 | 1.39 | 0.92–2.10 | 0.1171 | ||||||
| Cardiovascular disease | 1.65 | 1.21–2.25 | 0.0014 | 2.53 | 1.73–3.70 | <0.0001 | ||||||
| Chronic kidney disease | 1.68 | 1.05–2.71 | 0.0324 | 3.59 | 2.21–5.83 | <0.0001 | ||||||
| Chronic liver disease | 0.53 | 0.27–1.07 | 0.0770 | 1.17 | 0.50–2.73 | 0.7095 | ||||||
| Chronic pulmonary disease | 1.06 | 0.67–1.68 | 0.8154 | 1.11 | 0.61–2.02 | 0.7208 | ||||||
| Anaemia | 0.85 | 0.46–1.59 | 0.6174 | 2.01 | 1.03–3.92 | 0.0409 | ||||||
| Cancer | 1.27 | 0.78–2.07 | 0.3345 | 2.54 | 1.51–4.29 | 0.0005 | 2.57 | 1.23–5.34 | 0.0117 | |||
| Immunosuppressed state | 0.82 | 0.43–1.55 | 0.5341 | 1.22 | 0.55–2.71 | 0.6189 | ||||||
| Neurological comorbidities | ||||||||||||
| Dementia | 1.47 | 0.90–2.41 | 0.1232 | 2.81 | 1.68–4.68 | <0.0001 | ||||||
| Parkinson's disease | 1.58 | 0.63–3.97 | 0.3267 | 2.58 | 1.02–6.52 | 0.0451 | ||||||
| Stroke: ICH, ischaemic stroke, TIA | 1.96 | 1.33–2.89 | 0.0007 | 2.64 | 1.73–4.02 | <0.0001 | ||||||
| Multiple sclerosis | 0.18 | 0.05–0.62 | 0.0068 | 0.27 | 0.04–2.05 | 0.2068 | ||||||
| Motor neuron disease | ne | ne | 0.9796 | ne | ne | 0.9837 | ||||||
| Neuromuscular disorder | 0.97 | 0.26–3.67 | 0.9734 | 0.55 | 0.07–4.39 | 0.5737 | ||||||
| Neuropathy | 0.90 | 0.42–1.92 | 0.7822 | 0.96 | 0.36–2.54 | 0.9330 | ||||||
| COVID systemic complications | ||||||||||||
| Dyspnoea | 1.79 | 1.34–2.39 | <0.0001 | 3.76 | 2.40–5.90 | <0.0001 | ||||||
| Pneumonia | 2.34 | 1.74–3.16 | <0.0001 | 4.27 | 2.59–7.04 | <0.0001 | ||||||
| Cardiovascular | 1.64 | 1.08–2.48 | 0.0201 | 2.97 | 1.90–4.63 | <0.0001 | 2.08 | 1.07–4.06 | 0.0311 | |||
| Renal insufficiency/dialysis | 2.25 | 1.27–4.00 | 0.0058 | 2.94 | 1.69–5.11 | 0.0001 | ||||||
| Coagulation disorder/disseminated intravascular coagulation | 1.73 | 0.88–3.40 | 0.1099 | 1.39 | 0.65–2.98 | 0.3986 | ||||||
| Refractory shock | 30.63 | 4.18–224.56 | 0.0008 | ne | ne | <0.0001 | 44.72 | 5.68–352.5 | 0.0003 | |||
| Extra‐corporeal membrane oxygenation (ECMO) | ne | ne | 0.9818 | ne | ne | 0.9877 | ||||||
| Mechanical ventilation | 8.08 | 4.35–14.99 | <0.0001 | 5.13 | 3.31–7.94 | <0.0001 | ||||||
| Neurological findings | ||||||||||||
| Headache | 0.48 | 0.36–0.65 | <0.0001 | 0.29 | 0.18–0.46 | <0.0001 | ||||||
| Hyposmia/hypogeusia | 0.47 | 0.34–0.66 | <0.0001 | 0.15 | 0.07–0.32 | <0.0001 | 0.12 | 0.04–0.40 | 0.0006 | |||
| Dysautonomia | 0.69 | 0.46–1.04 | 0.0772 | 0.67 | 0.36–1.23 | 0.1919 | ||||||
| Vertigo | 0.66 | 0.46–0.94 | 0.0205 | 0.44 | 0.25–0.79 | 0.0059 | ||||||
| Myalgia | 0.60 | 0.43–0.83 | 0.0023 | 0.26 | 0.14–0.48 | <0.0001 | ||||||
| Sleep disorders | 0.96 | 0.64–1.43 | 0.8444 | 0.63 | 0.35–1.13 | 0.1209 | ||||||
| Cognitive impairment (including dysexecutive syndrome) | 1.67 | 1.22–2.29 | 0.0013 | 2.68 | 1.83–3.93 | <0.0001 | ||||||
| Hyperactive delirium | 2.04 | 1.31–3.17 | 0.0015 | 1.40 | 0.85–2.33 | 0.1846 | ||||||
| Hypoactive delirium/acute encephalopathy | 2.28 | 1.44–3.58 | 0.0004 | 2.53 | 1.59–4.03 | <0.0001 | ||||||
| Stupor/coma | 6.22 | 3.59–10.77 | <0.0001 | 12.01 | 4.35–33.11 | <0.0001 | 21.77 | 13.62–34.81 | <0.0001 | 22.77 | 12.1–42.86 | <0.0001 |
| Syncope | 0.34 | 0.18–0.66 | 0.0013 | 0.10 | 0.03–0.31 | <0.0001 | 0.47 | 0.17–1.34 | 0.1576 | |||
| Seizures/status epilepticus | 0.74 | 0.46–1.19 | 0.2149 | 1.14 | 0.62–2.11 | 0.6701 | ||||||
| Meningitis/encephalitis | 0.77 | 0.40–1.48 | 0.4379 | 0.75 | 0.29–1.95 | 0.5514 | ||||||
| Stroke | 3.06 | 2.19–4.28 | <0.0001 | 2.89 | 1.88–4.44 | <0.0001 | 2.43 | 1.66–3.56 | <0.0001 | |||
| Tremor | 0.56 | 0.33–0.98 | 0.0402 | 0.44 | 0.17–1.13 | 0.0865 | ||||||
| Chorea | ne | ne | 0.9780 | ne | ne | 0.9858 | ||||||
| Dystonia | 0.10 | 0.02–0.44 | 0.0024 | 0.02 | 0.00–0.14 | <0.0001 | 0.29 | 0.04–2.19 | 0.2288 | |||
| Myoclonus | 0.89 | 0.32–2.48 | 0.8263 | 1.25 | 0.35–4.51 | 0.7288 | ||||||
| Dyskinesia | 0.22 | 0.05–1.07 | 0.0600 | 1.43 | 0.30–6.98 | 0.6555 | ||||||
| Parkinsonism | 0.58 | 0.24–1.39 | 0.2202 | 0.24 | 0.03–1.83 | 0.1704 | ||||||
| Ataxia | 0.59 | 0.37–0.94 | 0.0273 | 0.39 | 0.17–0.91 | 0.0301 | ||||||
| Spinal cord disorder | 0.93 | 0.47–1.83 | 0.8238 | 0.28 | 0.07–1.19 | 0.0853 | ||||||
| Peripheral neuropathy | 1.54 | 0.93–2.53 | 0.0919 | 0.45 | 0.20–1.00 | 0.0499 | ||||||
| ICU admission | 5.81 | 3.93–8.59 | <0.0001 | 5.62 | 3.54–8.95 | <0.0001 | 3.01 | 2.05–4.43 | <0.0001 | 2.17 | 1.18–4.00 | 0.0130 |
| Pre‐morbid mRS | ||||||||||||
| 0 | 1 (ref.) | – | 0.6842 | 1 (ref.) | – | <0.0001 | ||||||
| 1 | 1.16 | 0.77–1.75 | 0.96 | 0.49–1.86 | ||||||||
| 2 | 1.24 | 0.76–2.02 | 2.45 | 1.32–4.56 | ||||||||
| 3 | 0.93 | 0.58–1.47 | 3.04 | 1.70–5.43 | ||||||||
| 4 | 0.96 | 0.56–1.63 | 5.58 | 3.06–10.22 | ||||||||
| 5 | 0.63 | 0.29–1.39 | 6.97 | 3.04–15.98 | ||||||||
Abbreviations: Adj. OR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; ICH, intracerebral haemorrhage; ICU, intensive care unit; mRS, modified Rankin Scale; OR, odds ratio; ne, not estimable; TIA, transient ischaemic attack.
Worse outcome, mRS score at discharge higher than pre‐morbid mRS score; stable/improved outcome, mRS score at discharge equal to or lower than pre‐morbid mRS score.
At the time of data collection, a total of 269 patients (224 hospitalized and 45 non‐hospitalized) had been followed for 6 months. Of them, 262 had laboratory‐confirmed COVID‐19 infection. This sample included 131 men and 130 women (unknown sex in 1) aged 19 through 91 years (IQR 47–71) (Table S2). 199 patients (76.0%) had neurological manifestations during the acute phase of the COVID‐19 infection, predominantly headache (40.8%), hyposmia/hypogeusia (34.7%), myalgia (29.1%), delirium (25.2%), cognitive impairment (23.3%), stroke sequelae (21.0%) and sleep disorders (17.1%). A mild‐to‐severe functional impairment (mRS 2+) was present in 48 patients (18.9%) before the onset of symptoms, in 133 patients (53.8%) at the end of the acute phase of the infection, and in 118 patients (46.1%) at the 6‐month follow‐up. Almost all the variables associated with worse outcome at discharge were negative prognostic predictors for outcome at 6 months (Table 4). Experiencing stroke or ataxia during the acute phase (OR 8.5, 95% CI 2.8–26.1; and, respectively, OR 6.9, 95% CI 1.2–40.7) and ICU admission (OR 3.6; 95% CI 1.5–8.7) were confirmed as predictors of worse outcome at 6 months, along with functional impairment at discharge. In contrast, history of stroke was associated with stable/improved outcome (OR 0.3; 95% CI 0.1–0.9).
TABLE 4.
Predictors of outcome at 6 months (N = 262)
| Worse outcome vs. stable/improved outcome a | ||||||
|---|---|---|---|---|---|---|
| Univariable model | Multivariable model | |||||
| OR | 95% CI | p value | Adj. OR | 95% CI | p value | |
| Sex | ||||||
| Male | 1 (ref.) | 0.9979 | ||||
| Female | 1.02 | 0.62–1.67 | ||||
| Intersex/unknown | ne | ne | ||||
| Smoking | ||||||
| Yes | 0.80 | 0.35–1.84 | 0.5947 | |||
| No | 1 (ref.) | – | ||||
| Unknown | 1.63 | 0.52–5.14 | ||||
| Source of COVID‐19 contact | ||||||
| Occupation | 0.23 | 0.10–0.50 | 0.0008 | |||
| Family member | 0.36 | 0.17–0.78 | ||||
| Social | 0.36 | 0.13–1.02 | ||||
| Travel | 0.79 | 0.23–2.71 | ||||
| Other/unknown | 1 (ref.) | – | ||||
| Age at admission (1‐year increase) | 1.03 | 1.02–1.05 | 0.0001 | |||
| BMI (1‐unit increase) | 0.99 | 0.99–1.00 | 0.6458 | |||
| Non‐neurological comorbidities | ||||||
| Hypertension | 1.96 | 1.19–3.23 | 0.0085 | |||
| Diabetes type 1/type 2 | 1.26 | 0.66–2.40 | 0.4771 | |||
| Cardiovascular disease | 2.25 | 1.22–4.15 | 0.0095 | |||
| Chronic kidney disease | 7.88 | 1.75–35.45 | 0.0071 | |||
| Chronic liver disease | 0.76 | 0.17–3–45 | 0.7181 | |||
| Chronic pulmonary disease | 0.95 | 0.45–2.01 | 0.8851 | |||
| Anaemia | 2.08 | 0.51–8.52 | 0.3070 | |||
| Cancer | 0.47 | 0.19–1.15 | 0.0996 | |||
| Immunosuppressed state | 1.02 | 0.29–3.60 | 0.9796 | |||
| Neurological comorbidities | ||||||
| Dementia | 0.32 | 0.08–1.22 | 0.0955 | |||
| Parkinson's disease | 0.67 | 0.11–4–09 | 0.6664 | |||
| Stroke: ICH, ischaemic stroke, TIA | 2.08 | 1.01–4.31 | 0.0475 | 0.27 | 0.08–0.91 | 0.0302 |
| Multiple sclerosis | 0.25 | 0.03–2.25 | 0.2153 | |||
| Motor neuron disease | ne | ne | – | |||
| Neuromuscular disorder | 1.54 | 0.25–9.36 | 0.6410 | |||
| Neuropathy | 0.33 | 0.03–3.25 | 0.3443 | |||
| COVID‐19 systemic complications | ||||||
| Dyspnoea | 1.13 | 0.69–1.86 | 0.6225 | |||
| Pneumonia | 1.34 | 0.81–2.20 | 0.2501 | |||
| Cardiovascular | 3.84 | 1.37–10.76 | 0.0105 | |||
| Renal insufficiency/dialysis | 4.19 | 1.35–13.01 | 0.0131 | |||
| Coagulation disorder/disseminated intravascular coagulation | 2.83 | 0.73–10.91 | 0.1317 | |||
| Refractory shock | ne | ne | 0.9907 | |||
| Extra‐corporeal membrane oxygenation (ECMO) | 1.02 | 0.14–7.33 | 0.9872 | |||
| Mechanical ventilation | 2.77 | 1.30–5.88 | 0.0081 | |||
| Neurological findings | ||||||
| Headache | 0.71 | 0.43–1.18 | 0.1925 | |||
| Hyposmia/hypogeusia | 0.52 | 0.30–0.88 | 0.0152 | |||
| Dysautonomia | 1.22 | 0.53–2.84 | 0.6393 | |||
| Vertigo | 1.27 | 0.66–2.44 | 0.4711 | |||
| Myalgia | 0.95 | 0.55–1.63 | 0.8451 | |||
| Sleep disorders | 1.14 | 0.59–2.18 | 0.1209 | |||
| Cognitive impairment (including dysexecutive syndrome) | 2.09 | 1.17–3.74 | 0.0125 | |||
| Hyperactive delirium | 1.65 | 0.83–3.28 | 0.1542 | |||
| Hypoactive delirium/acute encephalopathy | 3.07 | 1.24–7.60 | 0.0151 | |||
| Stupor/coma | 5.43 | 1.17–25.32 | 0.0311 | |||
| Syncope | 0.67 | 0.11–4.09 | 0.6664 | |||
| Seizures/status epilepticus | 0.74 | 0.30–1.83 | 0.5196 | |||
| Meningitis/encephalitis | 1.37 | 0.30–6.23 | 0.6869 | |||
| Stroke | 6.35 | 3.02–13.36 | <0.0001 | 8.51 | 2.77–26.13 | 0.0007 |
| Tremor | 1.82 | 0.52–6.39 | 0.3475 | |||
| Chorea | ne | ne | 0.8997 | |||
| Dystonia | ne | ne | 0.9906 | |||
| Myoclonus | 1.02 | 0.06–16.43 | 0.9910 | |||
| Dyskinesia | ne | ne | 0.9907 | |||
| Parkinsonism | ne | ne | 0.9790 | |||
| Ataxia | 6.03 | 1.31–27.78 | 0.0212 | 6.94 | 1.18–40.68 | 0.0180 |
| Spinal cord disorder | 1.72 | 0.40–7.36 | 0.4635 | |||
| Peripheral neuropathy | 1.84 | 0.81–4.20 | 0.1462 | |||
| ICU admission | 6.39 | 3.19–12.79 | <0.0001 | 3.59 | 1.49–8.66 | 0.0017 |
| Pre‐morbid mRS | ||||||
| 0 | 1 (ref.) | – | 0.8153 | |||
| 1 | 1.01 | 0.53–1.92 | ||||
| 2 | 0.65 | 0.23–1–79 | ||||
| 3 | 0.82 | 0.30–2.24 | ||||
| 4 | 0.37 | 0.07–1.97 | ||||
| 5 | 0.69 | 0.15–3.20 | ||||
| Discharge mRS | ||||||
| 0 | 1 (ref.) | – | <0.0001 | 1 (ref) | – | <0.0001 |
| 1 | 11.60 | 3.96–33.97 | 6.71 | 2.03–22.15 | ||
| 2 | 19.33 | 6.34–58.98 | 12.96 | 3.60–46.66 | ||
| 3 | 39.44 | 12.44–125.0 | 21.46 | 5.92–77.73 | ||
| 4 | 28.35 | 8.55–93.98 | 19.38 | 4.87–77.14 | ||
| 5 | 50.26 | 10.64–237.4 | 23.64 | 4.32–129.3 | ||
Abbreviations: Adj. OR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; ICH, intracerebral haemorrhage; ICU, intensive care unit; mRS, modified Rankin Scale; OR, odds ratio; TIA, transient ischaemic attack.
Worse outcome, mRS score at discharge higher than pre‐morbid mRS score; stable/improved outcome, mRS score at discharge equal to or lower than pre‐morbid mRS score.
At 6 months, 65/221 hospitalized patients (29.4%) and 10/41 non‐hospitalized patients (24.4%) experienced persisting neurological symptoms/signs, the commonest being hemiparesis/plegia (11 patients), cognitive impairment (10 cases), anosmia/ageusia (10 cases), para/tetraparesis (six cases) and fatigue (five cases) (Figure 2).
FIGURE 2.
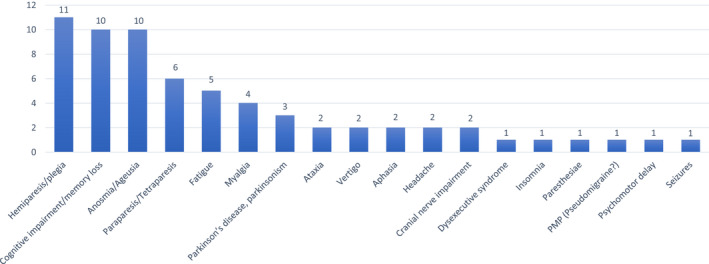
List of neurological symptoms, signs and diseases persisting at 6 months [Colour figure can be viewed at wileyonlinelibrary.com]
Ten patients (3.8%) developed new neurological complications during follow‐up. Two were not hospitalized during the acute phase. The general characteristics of these patients are illustrated in Table S3. Incident neurological manifestations varied in type and severity. The majority of patients had one or more comorbidities and complications of COVID‐19 (mostly pneumonia). Three patients had severe functional impairment at 6 months. These patients developed vertical diplopia and, respectively, status epilepticus and recurrent stroke during follow‐up. New neurological complaints were more severe in patients with sequelae at hospital discharge.
DISCUSSION
This is the largest international cohort study including 6‐month follow‐up in adult patients with neuro‐COVID seen by neurologists. It was found that neurological complications are highly prevalent and have a dramatic impact on the outcome of hospitalized patients. Further strengthening the relevance of neurological involvement, a 76% persistence of neurological involvement was found with mild‐to‐severe functional impact in 68%.
At admission, one or more comorbidities were present in 63.7% of cases and functional disability was documented in 34.1%; 51.6% of patients experienced systemic complications of SARS‐CoV‐2 infection, 83.4% were hospitalized, 23.4% were admitted to the ICU, 56.1% had worsening of their functional abilities at discharge, and 16.7% died whilst in hospital. Stupor or coma, ICU admission and stroke carried a worse outcome at discharge whereas history of cancer, development of cardiovascular complications and refractory shock were associated with increased mortality. Older age and coma were negative prognostic predictors (increased functional disability and death) but did not predict worse outcome at 6 months amongst survivors at discharge. ICU admission was a negative prognostic factor both at discharge and at 6 months.
At 6 months, 28.6% of patients still presented persistent neurological sequelae of the acute phase, the commonest being focal or generalized motor weakness and cognitive impairment. The development of stroke or ataxia, ICU admission and functional impairment at discharge were predictive of worse 6‐month outcome. These findings support the dispute that only the severity of the acute COVID‐19 spectrum and some neurological complications, rather than older age, the presence of comorbidities and the baseline functional impairment, are significant long‐term prognostic predictors.
A number of neurological symptoms or signs (hyposmia/hypogeusia, syncope, dystonia, history of stroke) were associated with stable/improved outcome. However, for some of them (hyposmia/hypogeusia, syncope, dystonia) interview bias is a possible explanation (as more severe cases were perhaps unable to report those symptoms). The protective role of history of stroke cannot be easily interpreted. Although the mechanisms of previous strokes might have been different from COVID‐19's mechanism of action, a coincidental finding cannot be excluded.
Another study assessed incident neurological symptoms, signs and diagnoses in 4491 hospitalized patients seen in neurological consultation [17]. In that study, 88% of patients had new neurological manifestations. The most common were toxic/metabolic encephalopathy (51%), stroke (14%), seizures (12%) and hypoxic/ischaemic brain injury (11%). In line with our study, those patients were older, more severely ill and less likely to be discharged home.
Our findings differ from other reports. In a large retrospective cohort (N = 236,379) using data from an electronic health records network [9], the estimated incidence of neurological or psychiatric diagnoses at 6 months following the acute phase of COVID‐19 was 33.6% (first diagnosis, 12.8%). The commonest neurological diseases were, in decreasing order, stroke (2.7%), dementia (0.7%) and parkinsonism (0.1%). Our higher rates can be explained by the source of our cases (80% hospitalized) and by patients seen in neurological consultation. However, in line with us, the incidence of stroke and dementia were significantly higher in patients with more severe disease.
In a prospective study of 4182 incident cases of COVID‐19 who self‐reported their symptoms using a mobile application, 558 participants (13.3%) experienced symptoms lasting ≥28 days, 189 (4.5%) for ≥8 weeks and 95 (2.3%) for ≥12 weeks [8]. The commonest were fatigue, headache, dyspnoea and anosmia and they were more frequent with increasing age, body mass index and female sex. The presence of more than five symptoms during the first week of illness was associated with prolonged complaints during follow‐up. This is in line with our study and suggests that the higher severity of the disease is the consequence of a multisystem involvement by the virus, as shown by others [18]
Post‐hospital persistent symptoms (including memory loss [34%], concentration and sleep disorders [28% and 30%]) were reported during phone calls by 279 patients who had COVID‐19 [6]. Although some study limitations (single centre, inclusion of patients without neurological complaints, high attrition rate) can explain the differences with our findings, the frequent report of cognitive impairment and sleep disorders indicated similarities.
In a study including 1733 of 2469 discharged patients with a median follow‐up of 6 months, fatigue or muscle weakness (63%) and sleep difficulties (26%) were the commonest persistent symptoms [13]. Twenty‐four per cent of cases reported a median 6‐min walking distance less than the lower limit of the normal range. Compared to our study, these higher rates might be explained by high attrition (736 patients, perhaps the least severe cases, did not attend follow‐up appointments).
In a population‐based cohort study including non‐hospitalized subjects, 938 subjects were invited to participate in a postal survey and 48% responded. Although the interviewees reported reduction of symptoms 1.5–6 months after the acute phase, 16% manifested persisting dyspnoea, 12% dysosmia and 10% dysgeusia [7]. The differences between this study and ours are reflected, on one side, by our longer follow‐up and, on the other side, by the possible under‐ascertainment of non‐neurological manifestations in our study or under‐ascertainment of neurological complaints in that study.
Sequelae at 6 months were reported in a prospective cohort study by 32.8% of 177 adults recovering from COVID‐19 [11]. The commonest persistent symptoms included fatigue and loss of smell or taste. The lower prevalence of sequelae in our study might reflect the focus on neurological manifestations.
Our study has strengths and limitations. The major strength is the large sample, which includes data from different countries and settings. Another strength is the accurate search and diagnostic assessment of neurological manifestations. All patients were examined by a neurologist and, to optimize inter‐rater agreement, diagnoses were guided by standard definitions. Although each neurological manifestation was investigated based on the findings available at the time of the interview, the e‐CRF included precise questions and clinical assessment of the patient was to be completed according to a detailed checklist (Appendix S1). The major limitation of our study is the lack of a population base. It was attempted to define the reference population to estimate incidence and prevalence of the various neurological manifestations. However, the differing catchment areas served by the participating sites did not consent precise calculations. Another important limitation is the focus on neurological manifestations. Eligible patients were those seen in neurological consultation. Although efforts were made to collect information on all comorbidities with impact on patients' health and the major complications of COVID‐19, our investigation of the full spectrum of the disease has been incomplete. Then, diagnostic accuracy was not always high as a more detailed assessment of registered patients (results of neuropsychological and imaging tests, treatments) was not required to avoid a time consuming data collection, given the emergency context in which neurological consultation was performed. It was also chosen not to collect data on treatments as they were rarely supported by evidence‐based recommendations. Finally, the use of mRS at discharge to predict functional disability at home or in residential or rehabilitation settings could be debated.
In conclusion, in a multinational cohort of patients with neuro‐COVID undergoing structured neurological consultation, a severe disease was found in a high proportion of patients. The presence of severe infection with complications predicted worse outcome at discharge, persistence of functional disability, and a number of sequelae at 6 months follow‐up, some of those occurring after the remission or stabilization of the acute phase of the disease. Patients with neurological manifestations during the acute phase of COVID‐19 infection should be carefully monitored to prevent the occurrence of long‐term complications and premature mortality.
CONFLICT OF INTEREST
Dr Beghi reports grants from the Italian Ministry of Health, grants from SOBI, personal fees from Arvelle Therapeutics, grants from American ALS Association, outside the submitted work. Dr Moro reports personal fees from Medtronic, personal fees from Abbott, grants from Boston, outside the submitted work. Dr Cavallieri reports personal fees from Zambon, outside the submitted work. Dr Kiteva‐Trenchevska reports personal fees from Roche, personal fees from Pliva, personal fees from Medis, outside the submitted work. Dr Aamodt reports research grants outside the submitted work from Medtronic and Boehringer Ingelheim and personal fees outside the submitted work from Bayer, Boehringer Ingelheim, Roche, Allergan, Novartis and Teva. Dr von Oertzen reports personal fees from Liva Nova, grants from Merck, personal fees from Indivior Austria GmbH, personal fees and non‐financial support from gtec GmbH Austria, grants, personal fees and non‐financial support from Boehringer Ingelheim, personal fees from Philips, personal fees from UCB Pharma, personal fees from Almirall, grants and personal feees from Eisai, personal fees from Arvelle Therapeuticos, personal fees from GW Pharma, personal fees from Zogenix GmbH, personal fees from Angelini Pharma Österreiche, personal fees from Novartis Pharma GmbH, outside the submitted work; and he is co‐chair of the Communication Committee, scientific panel for epilepsy, and COVID taskforce, all of the European Academy of Neurology, president of the Österreichische Gesellschaft für Epileptologie (Austrian ILAE chapter) and president of the upper Austrian MS society. The other authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Ettore Beghi: Conceptualization (lead); supervision (lead); writing—original draft (lead); writing—review and editing (lead). Raimund Helbok: Conceptualization (equal); supervision (equal); writing—original draft (equal). Serefnur Ozturk: Conceptualization (equal); writing—original draft (equal); writing—review and editing (equal). Oxana Grosu: Conceptualization (equal); writing—original draft (equal). Omer Karadas: Conceptualization (equal); writing—original draft (equal). Vitalie Lisnic: Conceptualization (equal); supervision (equal); writing—original draft (equal). Tibor Kovács: Conceptualization (equal); writing—original draft (equal). Levente Dobronyi: Conceptualization (equal); investigation (equal); writing—original draft (equal). Dániel Bereczki: Conceptualization (equal); investigation (equal); writing—original draft (equal). Maria Sofia Cotelli: Conceptualization (equal); supervision (equal); writing—original draft (equal). Eugenia Irene Davidescu: Conceptualization (equal); writing—original draft (equal). Bogdan Ovidiu Popescu: Conceptualization (equal); investigation (equal); writing—original draft (equal). Franco Valzania: Conceptualization (equal); writing—original draft (equal). Hanno Ulmer: Conceptualization (equal); data curation (equal); writing—original draft (equal). Francesco Cavallieri: Conceptualization (equal); supervision (equal); writing—original draft (equal). Luis Maia: Conceptualization (equal); data curation (equal); writing—original draft (equal). Anne Hege Aamodt: Conceptualization (equal); data curation (equal); writing—original draft (equal). Carmel Armon: Conceptualization (equal); data curation (equal); writing—original draft (equal). Anis Riahi: Conceptualization (equal); data curation (equal); writing—original draft (equal). Viktoria Gryb: Conceptualization (equal); data curation (equal); writing—original draft (equal). Waldemar Brola: Conceptualization (equal); data curation (equal); writing—original draft (equal). Ingomar Krehan: Conceptualization (equal); data curation (equal); writing—original draft (equal). Tim J von Oertzen: Conceptualization (equal); data curation (equal); supervision (equal). Mohammed Azab: Conceptualization (equal); data curation (equal); supervision (equal). Michael Crean: Conceptualization (equal); data curation (equal); supervision (equal); writing—original draft (equal). Maria Lolich: Conceptualization (equal); data curation (equal); supervision (equal); writing—original draft (equal). Maria João Lima: Conceptualization (equal); data curation (equal); supervision (equal). Johann Sellner: Conceptualization (equal); data curation (equal); supervision (equal); writing—original draft (equal); writing—review and editing (equal). Julian Perneczky: Conceptualization (equal); data curation (equal); supervision (equal); writing—original draft (equal). Thomas M Jenkins: Conceptualization (equal); data curation (equal); supervision (equal); writing—original draft (equal). Sara Meoni: Conceptualization (equal); data curation (equal); supervision (equal); writing—original draft (equal). Elisa Bianchi: Conceptualization (equal); data curation (equal); formal analysis (equal); software (equal); writing—original draft (equal); writing—review and editing (equal). Elena Moro: Conceptualization (equal); data curation (equal); supervision (equal); writing—original draft (equal); writing—review and editing (equal). Claudio L Bassetti: Conceptualization (equal); data curation (equal); funding acquisition (lead); supervision (equal); writing—original draft (equal).
Supporting information
ACKNOWLEDGEMENTS
The authors are indebted to Sherry H‐Y Chou MD and Molly McNett PhD for their valuable intellectual support to the organization of the ENERGY registry, to Lalit Kaltenbach for the preparation of the web database, to Ms Anja Sander for her assistance during the activation and conduct of the ENERGY registry, and all participating patients.
APPENDIX 1.
ENERGY Study Group: Onur Ural and Iskender Kara, Konya, Turkey; Bilgin Ozturk, Ankara, Turkey; Mihail Gavriliuc and Olesea Odainic, Chisinau, Moldova; Patrizia Civelli and Marta Bianchi, Esine‐Brescia, Italy; Teodora Bunea and Georgiana Sandu, Bucharest, Romania; Giulia Toschi, Reggio Emilia, Italy; Vanessa Oliveira and Alexandre Dias, Porto, Portugal; Simon Jung and Robert Hoepner, Bern, Switzerland; Marion Boldingh, Oslo, Norway; Netta Agajany and Sharon Wolfson, Zerifin, Israel; Lipowski Michał, Konskie, Poland; Lesiv Marjiana, Ivano‐Frankivsk, Ukraine; Hajer Derbali, Tunis, Tunisia; Mafalda Seabra, São João, Brazil; Vanessa Carvalho, Matosinhos, Portugal; Heidi Øyen Flemmen, MD, Skien, Norway; Clarissa Lin Yasuda, Campinas, SP, Brazil; Pille Taba, Tartu, Estonia; Osama Yassin, Alexandria, Egypt; Gordana Kiteva‐Trenchevska, Skopje, Macedonia.
Beghi E, Helbok R, Ozturk S, et al; the ENERGY Study Group . Short‐ and long‐term outcome and predictors in an international cohort of patients with neuro‐COVID‐19. Eur J Neurol. 2022;29:1663–1684. doi: 10.1111/ene.15293
Funding information
The study was supported by the European Academy of Neurology
[Correction added on 12 March 2022 after first online publication: Serefnur Ozturk's name and third affiliation was corrected.]
Contributor Information
Ettore Beghi, Email: ettore.beghi@marionegri.it.
the ENERGY Study Group:
Onur Ural, Iskender Kara, Bilgin Ozturk, Mihail Gavriliuc, Olesea Odainic, Patrizia Civelli, Marta Bianchi, Teodora Bunea, Georgiana Sandu, Giulia Toschi, Vanessa Oliveira, Alexandre Dias, Simon Jung, Robert Hoepner, Marion Boldingh, Netta Agajany, Sharon Wolfson, Lipowski Michał, Lesiv Marjiana, Hajer Derbali, Mafalda Seabra, Vanessa Carvalho, Heidi Øyen Flemmen, Clarissa Lin Yasuda, Pille Taba, Osama Yassin, and Gordana Kiteva‐Trenchevska
DATA AVAILABILITY STATEMENT
Data can only be shared with permission of individual countries.
REFERENCES
- 1. Chou SH, Beghi E, Helbok R, et al. Global incidence of neurological manifestations among patients hospitalized with COVID‐19—a report for the GCS‐NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. 2021;4(5):e2112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cagnazzo F, Arquizan C, Derraz I, et al. Neurological manifestations of patients infected with the SARS‐CoV‐2: a systematic review of the literature. J Neurol. 2020;268:1‐10. doi: 10.1007/s00415-020-10285-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Favas TT, Dev P, Chaurasia RN, et al. Neurological manifestations of COVID‐19: a systematic review and meta‐analysis of proportions. Neurol Sci. 2020;41(12):3437‐3470. doi: 10.1007/s10072-020-04801-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yassin A, Nawaiseh M, Shaban A, et al. Neurological manifestations and complications of coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. BMC Neurol. 2021;21(1):138. doi: 10.1186/s12883-021-02161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beghi E, Michael BD, Solomon T, Westenberg E, Winkler AS, COVID‐19 Neuro Research Coalition . Approaches to understanding COVID‐19 and its neurological associations. Ann Neurol. 2021;89:1059‐1067. doi: 10.1002/ana.26076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garrigues E, Janvier P, Kherabi Y, et al. Post‐discharge persistent symptoms and health‐related quality of life after hospitalization for COVID‐19. J Infect. 2020;81(6):e4‐e6. doi: 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Prevalence and determinants of fatigue after COVID‐19 in non‐hospitalized subjects: a population‐based study. Int J Environ Res Public Health. 2021;18(4):2030. doi: 10.3390/ijerph18042030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626‐631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6‐month neurological and psychiatric outcomes in 236 379 survivors of COVID‐19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416‐427. doi: 10.1016/S2215-0366(21)00084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS‐CoV‐2 infection: retrospective cohort study. BMJ. 2021;19(373):n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID‐19 infection. JAMA Netw Open. 2021;4(2):e210830. doi: 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moreno‐Pérez O, Merino E, Leon‐Ramirez JM, et al. Post‐acute COVID‐19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chopra V, Flanders SA, O'Malley M, Malani AN, Prescott HC. Sixty‐day outcomes among patients hospitalized with COVID‐19. Ann Intern Med. 2021;174(4):576‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220‐232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beghi E, Helbok R, Crean M, et al. The European Academy of Neurology COVID‐19 registry (ENERGY): an international instrument for surveillance of neurological complications in patients with COVID‐19. Eur J Neurol. 2020;28(10):3303‐3323. doi: 10.1111/ene.14652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Savio K, Pietra GL, Oddone E, Reggiani M, Leone MA. Reliability of the modified Rankin Scale applied by telephone. Neurol Int. 2013;5(1):e2. doi: 10.4081/ni.2013.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized patients with COVID‐19 in New York City. Neurology. 2021;96(4):e575‐e586. doi: 10.1212/WNL.0000000000010979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low‐risk individuals with post‐COVID‐19 syndrome: a prospective, community‐based study. BMJ Open. 2021;11(3):e048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can only be shared with permission of individual countries.


