Abstract
Currently the most powerful tool in combating the COVID‐19 pandemic is vaccination against SARS‐CoV‐2. A growing percentage of the world's population is being vaccinated. Various vaccines are worldwide on the market. Several adverse reactions have been reported as a part of post‐marketing surveillance of COVID‐19 vaccines. Among the possible adverse events, cutaneous vasculitis has occasionally been reported. We present a narrative review on cutaneous vasculitis related to COVID‐19‐vaccination to summarize clinical findings, histopathology, treatment and outcome. We searched for “COVID vaccine”, “COVID vaccination” AND “cutaneous vasculitis” in PUBMED. Articles in English have been selected, from inception to December 2021, and analyzed for patient's characteristics, type of vaccine, time of appearance of cutaneous vasculitis and clinico‐histopathologic type. Treatment and outcome have also been considered in this narrative review. Two new unpublished cases of ours were added. Cutaneous vasculitis is a rare adverse event to COVID‐19 vaccination. It has been observed with mRNA and adenovirus‐vector vaccines. IgA vasculitis, lymphocytic and ANCA‐associated vasculitis, leukocytoclastic and urticarial vasculitis have been reported. This adverse event can occur after first or second shot. Most cases run a mild to moderate course. Cornerstone of medical treatment are systemic corticosteroids. Complete remission could be achieved in most patients. Vasculitis may not be considered as a contraindication of vaccination, being uncommonly reported and shows a favorable prognosis. The benefit of the vaccination remains high especially for immunocompromised patients. COVID‐vaccine induced vasculitis is important in the differential diagnosis of purpuric and vasculitis disorders.
Keywords: adverse events, COVID‐19, cutaneous vasculitis, mRNA vaccine, SARS‐CoV‐2 vaccination, vector vaccine
1. INTRODUCTION
Vasculitis represent a heterogeneous group of disorders with blood vessels inflammation. The classification of vasculitis based upon vessels affected, dominant cells of the inflammatory infiltrate and clinical characteristics. The ideal classification has yet to be found. 1 Vascular affection is common in COVID‐19 disease. Thromboembolic and inflammatory reaction patterns have been observed. 2 Vaccination against SARS‐CoV2 is currently the most powerful medical approach against the pandemic. Although the various vaccines are in general well‐tolerated, adverse events have occasionally been reported including the appearance or re‐activation of cutaneous vasculitis. 3 In this narrative review, we analyzed the available data in the English medical literature to better characterize these adverse events and discuss the treatment options. Two new unpublished cases of ours were added (Figure 1).
FIGURE 1.
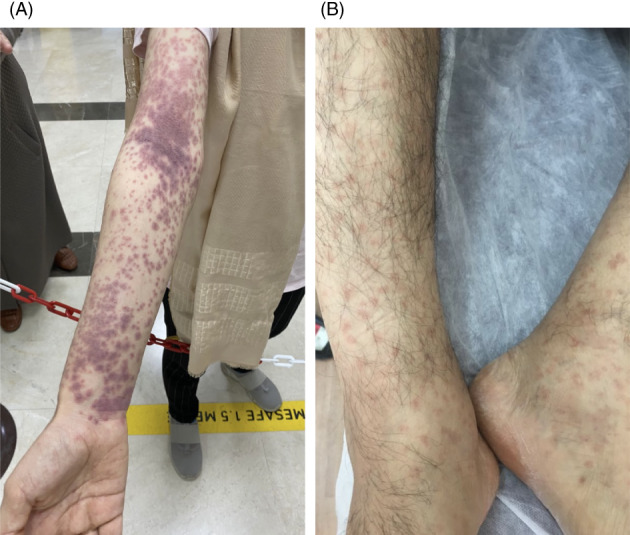
(A) A case of a 17‐year‐old female with IgA‐vasculitis 10 days after the first dose of Pfizer‐BioNTech BNT16B2b2 mRNA vaccine. (B) A case of 48‐year‐old man with LCV 4 days after the second dose of Pfizer‐BioNTech BNT16B2b2 mRNA vaccine
2. MATERIAL AND METHODS
We searched for “COVID vaccine”, “COVID vaccination” AND “cutaneous vasculitis” in PUBMED. Articles in English have been selected, from inception to December 2021, and analyzed for patient's characteristics, type of vaccine, time of appearance of cutaneous vasculitis and clinico‐histopathologic type. Treatment and outcome have also been considered in this narrative review. The results of our research have been classified according to the subtype of vasculitis.
3. RESULTS
Of the 38 cases, including ours, 52.6% (20 cases) had received the mRNA vaccine, 31.6% (12 cases) had received ChaAdOx1 nCoV‐19 vaccine (vector), and 15.8% (6 cases) had received the inactivated SARS‐CoV‐2 vaccine. Vasculitis developed in 63.2% (24 cases) after the first dose. The mean age of the cases was 53. 65.8% (25 cases) of the cases were female. 55.3% (20 cases) were LCV, 23.7% (9 cases) were IgA vasculitis, 7.9% (3 cases) were lymphocytic vasculitis, 5.3% (2 cases) were ANCA‐associated vasculitis, 5.3% (3 cases) was urticarial vasculitis, and 2.6% (1 cases) was Immune Complex Vasculitis. The average occurrences time was 6.2 days. The lesions in the cases disappeared in an average of 2.5 weeks. The average occurrences time was 6.2 days. The lesions in the cases disappeared in an average of 2.5 weeks. The following is an overview of the reviewed cases. Summary of the data is highlighted in Table 1.
TABLE 1.
Cutaneous vasculitis reports related to SARS‐CoV‐2 vaccine in the literature, and two new cases
| Author year | A/G | Clinical findings | Histopathology /Vasculitis type | Time to reaction | Time to resolution | Which dose | Outcome | Treatment | Vaccine type |
|---|---|---|---|---|---|---|---|---|---|
| Hines et al., 2021 4 | 40/F | Gluteal region rash | IgA Vasculitis | 20 days | 1 week | Second dose | Recovery | Only follow‐up |
Pfizer‐BioNTech BNT16B2b2 |
| Sirufo et al., 2021 5 | 76/F | Maculopapular purpuric rash on gluteal and leg regions | IgA Vasculitis | 7 days | 3 weeks | First dose | Recovery | Deflazacort 30 mg/kg for ten days | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Naitlho et al., 2021 6 |
62/M |
Petechial purpuric rashinvolving both legs | IgA Vasculitis | 8 days | 1 week | First dose | Recovery | Prednisone 40 mg per day for 7 days | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Vassallo et al., 2021 7 | 51/F | Maculopapular rash on upper limbs and trunk | Lymphocytic vasculitis | 1 day | 2 weeks | First dose | Recovery | Systemic antihistamine and local steroid |
Pfizer‐BioNTech BNT16B2b2 |
| Kharkar et al., 2021 8 | 31/F | Purpuric lesions on her legs | Lymphocytic vasculitis | 1 day | 2 weeks | Second dose | Recovery | Systemic antihistamine | Inactivated viral vaccineCOVAXIN® |
| Ungari et al., 2021 9 | 64/M | Maculopapular rash on the limbs and trunk | Lymphocytic vasculitis | 3 days | 2 weeks | Second dose | Recovery | Only follow‐up | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Badier et al., 2021 10 | 72/M | Maculopapular rashlower limbs | IgA Vasculitis | 15 days | 3–4 weeks | First dose | Recovery | Prednisone 40 mg per day for 3 weeks | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Bostan et al., 2021 11 | 33/M | Erythematous macules and palpable papules on the legs, forearms | Leukocytoclastic vasculitis | 3 days | 2 weeks | First dose | Partial resolution | Local steroid | Inactivated COVID‐19 vaccine (CoronaVac) |
| Maye et al., 2021 12 | 23/F | Palpable purpuric rash on the extremities and trunk | IgA Vasculitis | 2 days | 2 weeks | Second dose | Recovery | Prednisone 20 mg per day for 2 weeks |
Pfizer‐BioNTech BNT16B2b2 |
| Obeid et al., 2021 13 | 78/F | Palpable purpura on the hips and lower limbs | IgA Vasculitis | 7 days | 2 weeks | First dose | Recovery | Methylprednisolone 1 mg/kg for 2 weeks | mRNA‐1273 (Moderna) vaccine |
| Grossmanet al., 2021 14 | 94/M | Palpable purpura on the waist | IgA Vasculitis | 10 days | 3 weeks | Second dose | Recovery | Prednisone 60 mg/day for 3 weeks | mRNA‐1273 (Moderna) vaccine |
| Iwata et al., 2021 15 | 70/F | Palpable purpura on the feet | IgA Vasculitis | 2 days | 3 weeks | Second dose | Recovery | Only follow‐up |
Pfizer‐BioNTech BNT16B2b2 |
| HakroushandTampe., 2021 16 | 79/F | Upper thigh pain | ANCA‐associated vasculitis | 14 days | 4 weeks | Second dose | Partial resolution | Methylprednisolone 250 mg per day for 3 days |
Pfizer‐BioNTech BNT16B2b2 |
| Okuda et al., 2021 17 | 37/F | Erythema on her bilateral forearms, forehead, and thighs, and red and swollen left auricle | ANCA‐associated vasculitis | 12 days | 3 weeks | First dose | Partial resolution | Prednisolone 30 mg/day for 3 weeks |
Pfizer‐BioNTech BNT16B2b2 |
| Fritzen et al., 2021 18 | 60/F | Painful purpuric lesions and palpable papules on the lower limbs | Leukocytoclastic vasculitis | 13 days | 2 weeks | Second dose | Recovery | Prednisolone 60 mg/day for 2 weeks | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Cohen et al., 2021 19 | 46/F | Erythematous palpable papules on the legs | Leukocytoclastic vasculitis | 2 days | 2 weeks | Second dose | Recovery | Prednisolone 20 mg/day for 2 weeks |
Pfizer‐BioNTech BNT16B2b2 |
| Ball‐Burack et al., 2021 20 | 22/M | Palpable purpura on the dorsal feet and the both lower extremities | Leukocytoclastic vasculitis | 10 days | 3 weeks | First dose | Recovery | Systemic antihistamine and local steroid | Johnson & Johnson SARS‐CoV‐2 vaccine |
| Nastroet al., 2021 21 | 84/M | Palpable purpura on the legs | Leukocytoclastic vasculitis | 2 days | 2 weeks | First dose | Recovery | Systemic antihistamine and local steroid |
Pfizer‐BioNTech BNT16B2b2 |
| Sandhu et al., 2021 22 |
55/F 48/M |
Palpable purpura over both ankles, that progressed to the lower limb | Leukocytoclastic vasculitis | 5 days | 2 weeks | First dose | Recovery | Prednisolone 0.5 mg/kg/day, for 2 weeks | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Bostan et al., 2021 23 | 57/F | Widespread erythematous eruption involving the trunk and extremities | Leukocytoclastic vasculitis | 7 days | 1 week | First dose | Recovery | Prednisolone 20 mg/day for 1 week |
Pfizer‐BioNTech BNT16B2b2 |
| Dickset al., 2021 24 |
65/M |
Palpable purpuric lesions on the legs | Leukocytoclastic vasculitis | 2 days | 3 weeks | Third dose | Recovery | Prednisolone 60 mg/day for 1 week |
Pfizer‐BioNTech BNT16B2b2 |
| Bencharattanaphakhi et al., 2021 25 |
23/F 26/F |
Erythematous pinpoint purpura on the lower and upper extremities | Leukocytoclastic vasculitis | 2 days | 4 weeks | First dose | Recovery | Prednisolone 20 mg/day for 5 days in patient A and oral colchicine 0.6 mg twice a day in patient B | Inactivated COVID‐19 vaccine (CoronaVac) |
| Kar et al., 2021 26 |
46/F |
Purpuric papules on the legs | Leukocytoclastic vasculitis | 5 days | 2 weeks | First dose | Recovery | Systemic antihistamine and local steroid | Inactivated viral vaccine COVAXIN® |
| Jin et al., 2021 27 |
68/F |
Erythematous macules on both lower extremities | Leukocytoclastic vasculitis | 2 days | 3 weeks | First dose | Recovery | Oral colchicine 1.2 mg/day and topical steroid for 3 weeks | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Cavalliet al., 2021 28 |
57/M |
Erythematous macules on both lower extremities | Leukocytoclastic vasculitis | 6 days | 3 weeks | First dose | Recovery | Prednisolone 1 mg/kg/day for 3 weeks | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Liang et al., 2021 29 |
62/F |
Petechial rash on the bilateral lower limb | Leukocytoclastic vasculitis | 7 days | 3 weeks | First dose | Recovery | Prednisolone 1 mg/kg/day for 3 weeks | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Guzmán‐Pérez et al., 2021 30 |
57/F |
Palpable purpura lesions on the buttocks | Leukocytoclastic vasculitis | 1 day | 1 week | First dose | Recovery | Only follow‐up | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Shahrigharahkoshanet al., 2021 31 |
77/F |
Palpable papules on the lower limb and the hands | Leukocytoclastic vasculitis | 10 days | 5 weeks | First dose | Recovery | Dapsone 50 mg daily was prescribed for 60 days | Oxford‐AstraZenecaCOVID19 = ChaAdOx1 nCoV‐19 |
| Erler et al., 2021 32 |
42/F |
Progressive rash on the lower legs and the gluteal region | Leukocytoclastic vasculitis | 4 days | 1 week | First dose | Recovery | Only follow‐up |
Pfizer‐BioNTech BNT16B2b2 |
| Colia et al., 2021 33 |
22/F |
Purpuric rash on the legs | Leukocytoclastic vasculitis | 7 days | 3 weeks | Second dose | Recovery | Prednisolone 25 mg/day for 1 week |
Pfizer‐BioNTech BNT16B2b2 |
| Dash et al., 2021 34 |
27/M |
Urticarial plaques over the trunk and extremities | Urticarial vasculitis | 7 days | 1 week | Second dose | Recovery | Oral indomethacin 75 mg once daily | Inactivated COVID‐19 vaccine (CoronaVac) |
| Mückeet al., 2021 35 |
76/M |
Purpuric rash with palpable purpura on both hands, legs and thighs | Immune Complex Vasculitis | 12 days | 3 weeks | Second dose | Recovery | Prednisolone 40 mg/day for 2 weeks |
Pfizer‐BioNTech BNT16B2b2 |
| Larson et al., 2021 36 |
83/F |
Palpable purpura on both hands, legs and thighs | Leukocytoclastic vasculitis | 7 days | 2 weeks | Second dose | Recovery | Oral antibiotic and topical corticosteroids |
Pfizer‐BioNTech BNT16B2b2 |
| Larson et al., 2021 36 |
35/F |
Palpable purpura on both hands, legs and thighs | Urticarial vasculitis | 1 day | 4 weeks | First dose | Recovery | Systemic antihistamines, methylprednisolone, and dapsone | mRNA‐1273 (Moderna) vaccine |
| Altun et al., 2021 37 |
38/M |
Palpable purpura on legs | Leukocytoclastic vasculitis | 4 days | 2 weeks | First dose | Recovery | Systemic antihistamines, methylprednisolone |
Pfizer‐BioNTech BNT16B2b2 |
| Nazzaro et al., 2021 38 |
27/F |
Maculopapular rash on both hands, legs and trunk | Urticarial vasculitis | 10 days | 3 weeks | First dose | Recovery | Systemic methylprednisolone | mRNA‐1273 (Moderna) vaccine |
| Our Cases |
17/F 48/M |
Palpable purpura on both arms and legs Palpable purpura on both legs |
IgA Vasculitis Leukocytoclastic vasculitis |
10 days 4 days |
4 weeks 3 weeks |
First dose Second dose |
Recovery | Systemic antihistamine and local steroid |
Pfizer‐BioNTech BNT16B2b2 |
Abbreviations: A, Age; F, Female; G, Gender; M, Male.
3.1. IgA vasculitis
IgA vasculitis is an of small vessel vasculitis caused by perivascular deposition of IgA1 and activation of neutrophils. It may present as systemic vasculitis (Henoch‐Schönlein purpura) or as a skin‐limited variant. IgA‐vasculitis is the most common vasculitis type in infants. 39 IgA vasculitis has been observed after Pfizer‐BioNTechBNT16B2b2 mRNA vaccine and Vaxzevria (ChAdOx1 nCoV‐19 AZD1222) vaccine. 4 , 5 , 6 , 10 , 11 , 12 , 13 , 14 , 15 In case of appearance after first shot, a second shot of vaccination did not cause a relapse of the vasculitis symptoms. Treatment of choice was oral corticosteroids; however, spontaneous remission was occasionally reported. Interestingly, of the reviewed cases, two had history of COVID‐19 infection; two had history of IgA vasculitis. 6 , 10 , 11 , 12 , 13 , 14 , 15 A favorable risk–benefit profile of BNT162b2 in rheumatoid arthritis patients, including those on biologics, has been noted. 15 , 40
3.2. Lymphocytic vasculitis
Lymphocytic vasculitis is a histologic reaction pattern with a dominant lymphocytic inflammatory infiltrate that correlates with broad clinical differential diagnosis from lichenoid, infectious, neoplastic to autoimmune connective tissue disorders. 41 Only three cases have been reported so far. 7 , 8 , 9 Kharkar et al. 8 reported a 31‐year‐old woman with painful purpuric lesions on her legs with pedal edema, one day after her second dose of inactivated viral vaccine (COVAXIN®; Bharat Biotech, Hyderabad, India). RT‐PCR for SARS‐CoV‐2 infection was negative. The lesions improved over 10 days on antihistaminic. Eosinophils were reported in some of histology‐confirmed cutaneous manifestations associated with COVID‐19. Cinottiet al. 42 reported a case of eosinophilic cellulitis after the first dose of AstraZeneca vaccination in an elderly man. In a biopsy‐proven study on cutaneous reactions after mRNA‐based COVID‐19 vaccines, Tihyet al. 43 noted that most of the COVID‐19‐vaccine induced reactions cases have the features of drug reaction‐like such as epidermal keratinocyte necrosis associated with a perivascular lymphocytic infiltrate in the superficial and mid dermis, and a variable number of eosinophils and sometimes neutrophils. Interestingly, in a recent study, purpura on the lower legs was mostly seen in patients with severe “Drug‐induced hypersensitivity syndrome.” 44
3.3. ANCA‐associated vasculitis
Anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis is an autoimmune vasculitis type which affects small‐ to medium‐sized vessels. ANCA‐associated vasculitis includes granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis. 45 Only 2 cases of ANCA‐associated vasculitispresented with associated cutaneous findings post‐COVID‐19 vaccination. 16 , 17 Kidney biopsy of one cases confirmed severe acute tubular injury with pauci‐immune crescentic GN and interstitial nephritis. The kidney function then normalized following intravenous cyclophosphamide (initiated at 10 mg/kg). 16
3.4. Leukocytoclastic vasculitis
Leukocytoclastic vasculitis(LCV) represents a type of small vessel vasculitis, characterized by presence of neutrophil infiltrates, leukocytoclasis, fibrinoid necrosis, resulting in vessel walls damage. 46 Twenty cases of COVID‐19‐vaccine triggered‐LCV were noted; three with an elevated anti‐Spike SARS CoV‐2 Antibody titer, two of them had no history of COVID‐19.Oral mucosal involvement was noted in one case. Only one had cryoglobulinemia. 7 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 36 , 37 Nastro et al. 21 reported an 84‐year‐old woman, with history of chronic kidney disease and depressive disorder, developed burning pain on the distal part of right leg and foot, followed by ipsilateral multiple non‐confluent purpuric papules and vesicles few hours after she received the first dose of Pfizer‐BioNTech COVID‐19 vaccine. PCR of a skin swab for varicella‐zoster virus (VZV) resulted positive, and VZV IgM and IgG antibodies were positive. Skin biopsy of right lower leg consisted with LCV. Direct immunofluorescence test was negative. Atypical herpes zoster associated with cutaneous vasculitis was considered. Famciclovir 500 mg orally every 8 h for 10 days was started. Complete resolution of the lesion 2 weeks later with the persistence of local pain was noted. Because of the persistence of local pain and elevated liver enzymes, she refused the second dose of vaccine. Ibuchiet al. 47 recently reported a series of 14 patients with VZV reactivation after BNT162b2 vaccination. The majority of them where female, who developed herpes zoster (HZ) after the first dose. None had immunosuppressive or immunomodulatory treatment, except one. No recurrence was noted with the second dose.
3.5. Urticarial vasculitis
Urticarial vasculitis is an uncommon clinic‐pathologic entity that is characterized by chronic or recurrent episodes of urticarial lesions and LCV. 48 Three cases were published in association with COVID‐19 vaccination. Two after the first dose of Moderna vaccine. 34 , 38 , 48
3.6. Immune complex vasculitis
Mücke et al. 35 reporteda 76‐year old man, presented with a purpuric non‐blanchable rash both hands, legs and thighs, and lower abdomen 12 days after the second mRNA‐based vaccination for COVID‐19 with BNT162b2.He has a history of compensated alcoholic liver cirrhosis. However, he reported of melena and diarrhea the day before. Workup revealed; elevated inflammatory biomarkers, micro‐erythruria and leukocyturia, positive occult blood and moderately elevated stool calprotectin levels. Oral prednisolone, 40 mg, once daily, was started with resolution of all symptoms.
4. DISCUSSION
Vasculitis post‐COVID19 vaccination is a rare adverse event. The mean age of the patients was 53 years. 65.8% (25 cases) of the cases were female. Both induction and reactivation have been observed‐ either after first or second shot. Vasculitis developed in 63.2% (24 cases) after the first dose. In case of occurrence of vasculitis after first shot, the second vaccination was uncomplicated. Generally, vasculitis is not an absolute contraindication to receive the second dose of the vaccine. The most common subtypes of vasculitis reported are IgA and LCV; 55.3% (20 cases) were LCV, 23.7% (9 cases) were IgA vasculitis, 7.9% (3 cases) were lymphocytic vasculitis, 5.3% (2 cases) were ANCA‐associated vasculitis, 5.3% (3 cases) was urticarial vasculitis, and 2.6% (1 cases) was immune complex vasculitis. The average occurrence time was 6.2 days after vaccination. The lesions disappeared on average after 2.5 weeks. Most cases have been reported with mRNA vaccines being the most worldwide‐distributed COVID‐19 vaccines. However, vasculitis was also reported after inactivated virus vaccines and others. Of the 38 cases, including ours, 52.6% (20 cases) had received the mRNA vaccine, 31.6% (12 cases) had received ChaAdOx1 nCoV‐19 vaccine (vector), and 15.8% (6 cases) had received the inactivated SARS‐CoV‐2 vaccine. The most frequent causes of vasculitis in the reviewed cases have been reasonably ruled out, that means exposure to the vaccines could be the potential trigger. An inflammatory response to vaccine component/antigen encoding SARS‐CoV‐2 spike glycoprotein, depositing in micro‐vessels, targeting vascular endothelium and resulting in a neutrophil‐rich inflammatory reaction, could be hypothesized. Type I adverse events have in part be related to non‐viral vaccine constituents, such as polyethylene glycol. However, without testing the various components of the vaccines, it would be difficult to elucidate the antigenic trigger. Molecular mimicry between SARS‐CoV‐2 and vaccine components such the spike‐protein sequences may contribute to adverse reactions to COVID‐19 vaccination. 49 Nevertheless, seems unlikely, that only one pathway is responsible. Further studies are warranted to reach the precise pathogenesis of these reactions. Treatment of vasculitis depends on type of the disease, severity, age, and comorbidities. First line therapy in more advanced cases is oral prednisolone. Mild cases may show a spontaneous remission. It is important to exclude involvement of internal organs such as kidney, intestine, and central nervous system, but fortunately, most patients had a cutaneous vasculitis only.
5. CONCLUSIONS
Vasculitis are a heterogeneous group of diseases, which share the common feature of endothelial damage secondary to inflammation. Cutaneous vasculitis is a possible adverse event associated with vaccination against SARS‐CoV‐2. Hypersensitivity vasculitis and antigen–antibody complex deposition (type III hypersensitivity reaction) in the small vessels with potential internal organs involvement, such as the kidney and intestines, are the most common subtypes. However, as most of other post‐COVID‐19 vaccination cutaneous reactions, cutaneous vasculitis is not part of a multiorgan adverse hypersensitivity response triggered by the vaccine but mostlya skin‐limited inflammation. The pathogenesis of vaccination‐associated vasculitis is not well understood. There seems to be regional differences in frequency and presentation of vasculitis. The knowledge of (cutaneous) vasculitis adverse events is important for patient safety and in the differential diagnosis of vasculitis and purpuric skin disorders in general. We think this review will aid the dermatologists in daily practice. It also provides an overlook for international agencies to guide the manufacturers about vasculitis as one of the important reactions to COVID‐19 vaccines.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Ayman Abdelmaksoud reviewed the literature and submitted the final draft. Uwe Wollina shared in reviewing the literature, reviewed and edited the final draft. Selami Aykut Temiz shared in literature review and data analysis. Abdulkarim Hasan reviewed the pathology of our cases. All the authors approved the final draft for submission.
Abdelmaksoud A, Wollina U, Temiz SA, Hasan A. SARS‐CoV‐2 vaccination‐induced cutaneous vasculitis: Report of two new cases and literature review. Dermatologic Therapy. 2022;35(6):e15458. doi: 10.1111/dth.15458
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Wollina U, Unger L, Haroske G, et al. Classification of vascular disorders in the skin and selected data on new evaluation and treatment. Dermatol Ther. 2012;25(4):287‐296. [DOI] [PubMed] [Google Scholar]
- 2. Wollina U, Karadağ AS, Rowland‐Payne C, et al. Cutaneous signs in COVID‐19 patients: a review. Dermatol Ther. 2020;33(5):e13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Temiz SA, Abdelmaksoud A, Wollina U, et al. Cutaneous and allergic reactions due to COVID‐19 vaccinations: a review. J Cosmet Dermatol. 2022;21(1):4‐12. doi: 10.1111/jocd.14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hines AM, Murphy N, Mullin C, et al. Henoch‐Schönlein purpura presenting post COVID‐19 vaccination. Vaccine. 2021;39(33):4571‐4572. doi: 10.1016/j.vaccine.2021.06.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sirufo MM, Raggiunti M, Magnanimi LM, et al. Henoch‐Schönlein purpura following the first dose of COVID‐19 viral vector vaccine: a case report. Vaccines (Basel). 2021;9(10):1078. doi: 10.3390/vaccines9101078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naitlho A, Lahlou W, Bourial A, et al. A rare case of Henoch‐Schönlein purpura following a COVID‐19 vaccine‐case report. SN Compr Clin Med. 2021;1‐4. Epub ahead of print. doi: 10.1007/s42399-021-01025-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vassallo C, Boveri E, Brazzelli V, et al. Cutaneous lymphocytic vasculitis after administration of COVID‐19 mRNA vaccine. Dermatol Ther. 2021;34(5):e15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kharkar V, Vishwanath T, Mahajan S, et al. Asymmetrical cutaneous vasculitis following COVID‐19 vaccination with unusual eosinophil preponderance. Clin Exp Dermatol. 2021. Dec;46(8):1596‐1597. doi: 10.1111/ced.14797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ungari M, Pezzarossa E. Cutaneous lymphocytic Vasculitis after Administration of the Second Dose of AZD1222 (Oxford‐AstraZeneca) severe acute respiratory syndrome coronavirus 2 vaccination: Casuality or causality? Am J Dermatopathol. 2022;44(1):80‐82. doi: 10.1097/DAD.0000000000002104 [DOI] [PubMed] [Google Scholar]
- 10. Badier L, Toledano A, Porel T, et al. IgA vasculitis in adult patient following vaccination by ChadOx1 nCoV‐19. Autoimmun Rev. 2021;20(11):102951. doi: 10.1016/j.autrev.2021.102951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bostan E, Gulseren D, Gokoz O. New‐onset leukocytoclastic vasculitis after COVID‐19 vaccine. Int J Dermatol. 2021;60(10):1305‐1306. doi: 10.1111/ijd.15777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maye JA, Chong HP, Rajagopal V, et al. Reactivation of IgA vasculitis following COVID‐19 vaccination. BMJ Case Rep. 2021;14(11):e247188. doi: 10.1136/bcr-2021-247188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Obeid M, Fenwick C, Pantaleo G. Reactivation of IgA vasculitis after COVID‐19 vaccination. Lancet Rheumatol. 2021. Sep;3(9):e617. doi: 10.1016/S2665-9913(21)00211-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grossman ME, Appel G, Little AJ, et al. Post‐COVID‐19 vaccination IgA vasculitis in an adult. J Cutan Pathol. 2022;49(4):385‐387. doi: 10.1111/cup.14168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iwata H, Kamiya K, Kado S, et al. Case of immunoglobulin a vasculitis following coronavirus disease 2019 vaccination. J Dermatol. 2021. Dec;48(12):e598‐e599. doi: 10.1111/1346-8138.16167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hakroush S, Tampe B. Case report: ANCA‐associated Vasculitis presenting with rhabdomyolysis and Pauci‐immune crescentic glomerulonephritis after Pfizer‐BioNTech COVID‐19 mRNA vaccination. Front Immunol. 2021;12:762006. doi: 10.3389/fimmu.2021.762006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okuda S, Hirooka Y, Sugiyama M. Propylthiouracil‐induced Antineutrophil cytoplasmic antibody‐associated Vasculitis after COVID‐19 vaccination. Vaccines (Basel). 2021;9(8):842. doi: 10.3390/vaccines9080842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fritzen M, Funchal GDG, Luiz MO, et al. Leukocytoclastic vasculitis after exposure to COVID‐19 vaccine. An Bras Dermatol. 2022;97(1):118‐121. doi: 10.1016/j.abd.2021.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen SR, Prussick L, Kahn JS, et al. Leukocytoclastic vasculitis flare following the COVID‐19 vaccine. Int J Dermatol. 2021;60(8):1032‐1033. doi: 10.1111/ijd.15623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ball‐Burack MR, Kosowsky JM. A case of Leukocytoclastic Vasculitis following SARS‐CoV‐2 vaccination. J Emerg Med. 2021. doi: 10.1016/j.jemermed.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nastro F, Fabbrocini G, di Vico F, et al. Small vessel vasculitis related to varicella‐zoster virus after Pfizer‐BioNTech COVID‐19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(11):e745‐e747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandhu S, Bhatnagar A, Kumar H, et al. Leukocytoclastic vasculitis as a cutaneous manifestation of ChAdOx1 nCoV‐19 corona virus vaccine (recombinant). Dermatol Ther. 2021;34(6):e15141. doi: 10.1111/dth.15141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bostan E, Zaid F, Akdogan N, et al. Possible case of mRNA COVID‐19 vaccine‐induced small‐vessel vasculitis. J Cosmet Dermatol. 2022;21(1):51‐53. doi: 10.1111/jocd.14568 [DOI] [PubMed] [Google Scholar]
- 24. Dicks AB, Gray BH. Images in vascular medicine: Leukocytoclastic vasculitis after COVID‐19 vaccine booster. Vasc Med. 2022;27(1):100‐101. doi: 10.1177/1358863X211055507 [DOI] [PubMed] [Google Scholar]
- 25. Bencharattanaphakhi R, Rerknimitr P. Sinovac COVID‐19 vaccine‐induced cutaneous leukocytoclastic vasculitis. JAAD Case Rep. 2021;18:1‐3. doi: 10.1016/j.jdcr.2021.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kar BR, Singh BS, Mohapatra L, et al. Cutaneous small‐vessel vasculitis following COVID‐19 vaccine. J Cosmet Dermatol. 2021;20(11):3382‐3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin WJ, Ahn SW, Jang SH, et al. Leukocytoclastic vasculitis after coronavirus disease 2019 vaccination. J Dermatol. 2022;49(1):e34‐e35. doi: 10.1111/1346-8138.16212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cavalli G, Colafrancesco S, De Luca G, et al. Cutaneous vasculitis following COVID‐19 vaccination. Lancet Rheumatol. 2021;3(11):e743‐e744. doi: 10.1016/S2665-9913(21)00309-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liang I, Swaminathan S, Lee AYS. Emergence of de novo cutaneous vasculitis post coronavirus disease (COVID‐19) vaccination. Clin Rheumatol. 2021;1‐2. Online ahead of print. doi: 10.1007/s10067-021-05948-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guzmán‐Pérez L, Puerta‐Peña M, Falkenhain‐López D, et al. Small‐vessel vasculitis following Oxford‐AstraZeneca vaccination against SARS‐CoV‐2. J Eur Acad Dermatol Venereol. 2021. Nov;35(11):e741‐e743. doi: 10.1111/jdv.17547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shahrigharahkoshan S, Gagnon LP, Mathieu S. Cutaneous Leukocytoclastic Vasculitis induction following ChAdOx1 nCoV‐19 vaccine. Cureus. 2021;13(10):e19005. doi: 10.7759/cureus.19005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erler A, Fiedler J, Koch A, et al. Clinical images: Leukocytoclastic vasculitis after vaccination with a SARS‐CoV‐2 vaccine. Arthritis Rheumatol. 2021;73(12):2188. doi: 10.1002/art.41910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colia R, Rotondo C, Corrado A, et al. Cutaneous vasculitis after severe acute respiratory syndrome coronavirus 2 vaccine. Rheumatol Adv Pract. 2021;5(3):rkab050. doi: 10.1093/rap/rkab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dash S, Behera B, Sethy M, et al. COVID‐19 vaccine‐induced urticarial vasculitis. Dermatol Ther. 2021;34(5):e15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mücke VT, Knop V, Mücke MM, et al. First description of immune complex vasculitis after COVID‐19 vaccination with BNT162b2: a case report. BMC Infect Dis. 2021;21(1):958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Larson V, Seidenberg R, Caplan A, et al. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID‐19 vaccination. J Cutan Pathol. 2022;49(1):34‐41. doi: 10.1111/cup.14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID‐19 vaccination. Dermatol Ther. 2021. Dec;20:e15279. doi: 10.1111/dth.15279 [DOI] [PubMed] [Google Scholar]
- 38. Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti‐COVID‐19 vaccine. Dermatol Ther. 2021. Dec;20:e15282. doi: 10.1111/dth.15282 [DOI] [PubMed] [Google Scholar]
- 39. Pillebout E, Sunderkötter C. IgA vasculitis. Semin Immunopathol. 2021;43(5):729‐738. [DOI] [PubMed] [Google Scholar]
- 40. Cristaudo A, Graceffa D, Pimpinelli F, et al. Immunogenicity and safety of anti‐SARS‐CoV‐2 BNT162b2 vaccine in psoriasis patients treated with biologic drugs. J Eur Acad Dermatol Venereol. 2022;36(4):e266‐e268. doi: 10.1111/jdv.17861 [DOI] [PubMed] [Google Scholar]
- 41. Carlson JA, Chen KR. Cutaneous vasculitis update: neutrophilic muscular vessel and eosinophilic, granulomatous, and lymphocytic vasculitis syndromes. Am J Dermatopathol. 2007;29(1):32‐43. doi: 10.1097/01.dad.0000245198.80847.ff [DOI] [PubMed] [Google Scholar]
- 42. Cinotti E, Perrot JL, Bruzziches F, Tognetti L, et al. Eosinophilic dermatosis after AstraZeneca COVID‐19 vaccination. J Eur Acad Dermatol Venereol. 2022;36(3):e171‐e172. doi: 10.1111/jdv.17806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tihy M, Menzinger S, André R, et al. Clinicopathological features of cutaneous reactions after mRNA‐based COVID‐19 vaccines. J Eur Acad Dermatol Venereol. 2021. Dec;35(12):2456‐2461. doi: 10.1111/jdv.17633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takei S, Hama N, Mizukawa Y, et al. Purpura as an indicator of severity in drug‐induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: evidence from a 49‐case series. J Eur Acad Dermatol Venereol. 2022;36(4):e310‐e313. doi: 10.1111/jdv.17838 [DOI] [PubMed] [Google Scholar]
- 45. Kronbichler A, Lee KH, Denicolò S, et al. Immunopathogenesis of ANCA‐associated Vasculitis. Int J Mol Sci. 2020;21(19):7319. doi: 10.3390/ijms21197319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yap BJM, Lai‐Foenander AS, Goh BH, et al. Unraveling the Immunopathogenesis and genetic variants in Vasculitis toward development of personalized medicine. Front Cardiovasc Med. 2021;8:732369. doi: 10.3389/fcvm.2021.732369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ibuchi Y, Tamayose F, Katayama H, et al. Varicella zoster virus reactivation after coronavirus disease 2019 vaccination in Japanese patients: a series of 14 cases. J Dermatol. 2021. Online ahead of print. doi: 10.1111/1346-8138.16287 [DOI] [PubMed] [Google Scholar]
- 48. Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. 2021;7(3):290‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Magro C, Crowson AN, Franks L, Schaffer PR, Whelan P, Nuovo G. The histologic and molecular correlates of COVID‐19 vaccine‐induced changes in the skin. Clin Dermatol. 2021;39(6):966‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


