Abstract
Reduced COVID‐19 vaccine effectiveness (VE) has been observed with increasing predominance of SARS‐CoV‐2 Delta (B.1.617.2) variant. Two‐dose VE against laboratory‐confirmed SARS‐CoV‐2 infection (symptomatic and asymptomatic) was estimated using Cox proportional hazards models with time‐varying vaccination status in a prospective rural community cohort of 1266 participants aged ≥12 years. Between November 3, 2020 and December 7, 2021, VE was 56% for mRNA COVID‐19 vaccines overall, 65% for Moderna, and 50% for Pfizer‐BioNTech. VE when Delta predominated (June to December 2021) was 54% for mRNA COVID‐19 vaccines overall, 59% for Moderna, and 52% for Pfizer‐BioNTech.
Keywords: COVID‐19, SARS‐CoV‐2, vaccine effectiveness
1. BACKGROUND
Multiple studies have demonstrated high effectiveness of coronavirus disease (COVID‐19) vaccines in real‐world settings. 1 However, some studies have found reduced vaccine effectiveness (VE) against severe COVID‐19 caused by acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) Delta (B.1.617.2) variant among persons who are immunocompromised, and against symptomatic infection at longer time since vaccination. 1 , 2 , 3 , 4 , 5 , 6 Furthermore, most evaluations of COVID‐19 VE have focused on prevention of medically attended SARS‐CoV‐2 infection or on effectiveness in high‐risk populations, such as healthcare workers. We conducted active surveillance in a well‐defined rural community cohort to estimate the effectiveness of messenger RNA (mRNA) COVID‐19 vaccines against symptomatic and asymptomatic laboratory‐confirmed SARS‐CoV‐2 infection.
2. METHODS
This analysis used data collected from an ongoing prospective community cohort study to assess SARS‐CoV‐2 infection in rural central Wisconsin, United States. Participants were enrolled November 2020 to March 2021, and actively monitored weekly (up to 52 weeks) to ascertain symptoms and identify new SARS‐CoV‐2 infections.
2.1. Study population
Participants were randomly sampled community‐dwelling individuals living in the Marshfield Epidemiologic Study Area (central region), a 14 zip code region in central Wisconsin that includes Marshfield and surrounding area. The population is ~53,000, and 89% receive most of their care from Marshfield Clinic Health System (MCHS). 7 Further description of cohort eligibility are in the supporting information.
2.2. Weekly illness surveillance
Each week, all participants reported the absence or presence of specific symptoms. Anterior nasal swabs were self‐collected (or parent‐collected) for each qualifying illness episode. In addition, approximately half of the cohort was assigned to collect swabs weekly for the first 26 weeks. Further details on assignment to symptomatic versus weekly nasal swab collection are provided in the supporting information. A qualifying illness was a new illness onset in the past 7 days and at least one of the following symptoms: cough, fever or chills, sore throat, muscle or body aches, loss of smell or taste, shortness of breath, or diarrhea. Participants were instructed to also report any new respiratory symptoms by phone as soon as symptoms developed.
2.3. Defintions and data collection
Participants had laboratory‐confirmed SARS‐CoV‐2 infection if a specimen collected during surveillance was positive for SARS‐CoV‐2 by real‐time reverse transcription polymerase chain reaction (rRT‐PCR) 8 or if they tested positive from a clinical PCR‐based test at MCHS. Dates and results of clinical SARS‐CoV‐2 tests and vaccinations were extracted from MCHS electronic health records and obtained from self‐report. Additional information regarding vaccinations, vaccine eligibility in Wisconsin, collection of demographic information and serum samples, and laboratory methods are described in the supporting information.
2.4. Analysis
Participant characteristics were compared across groups using chi‐square or Wilcoxon rank‐sum tests. VE against laboratory‐confirmed SARS‐CoV‐2 infection was estimated using Cox proportional hazards models with time‐varying vaccination status, respiratory sample collection frequency, and age. Person‐time at risk began at enrollment for persons aged ≥16 years or when participants aged 12–15 years became age‐eligible for vaccination (May 13, 2021 or 12th birthday after May 13, 2021), and ended December 7, 2021, date of positive SARS‐CoV‐2 infection, date of withdrawal from the study, or date of last weekly survey (study week 52), whichever occurred first. Unvaccinated person‐time was defined as time before receipt of the first dose. Vaccinated person‐time began ≥ 14 days after receipt of the second dose. Person‐time from receipt of the first dose through 13 days after the second dose, and after receipt of vaccine off‐label (before age‐eligible or mixed‐product series) was excluded, as were days following receipt of a third dose. In addition, person‐time was excluded after receipt of Johnson & Johnson (Janssen) vaccine due to low uptake in the population.
VE for any mRNA vaccine and for each mRNA vaccine product was calculated as (1–hazard ratio) × 100%; the hazard ratio represented the ratio of SARS‐CoV‐2 infections in two‐dose vaccinated to unvaccinated person‐time. VE against symptomatic SARS‐CoV‐2 infection was estimated by excluding participants with infection with no reported symptoms during the 2 weeks before and after the positive test result (asymptomatic). VE against the Delta variant was estimated by restricting person‐time at risk to the period after June 21, 2021, when > 50% of sequenced viruses in Wisconsin were Delta. 9 Sensitivity analyses excluded persons who self‐reported or had serologic evidence of prior SARS‐CoV‐2 infection. Analyses were conducted using SAS (Version 9.4; SAS institute).
Marshfield Clinic Research Institute (MCRI)'s Institutional Review Board reviewed and approved the study. The Centers for Disease Control and Prevention (CDC) ceded research oversight to MCRI (45 C.F.R. part 46; 21 C.F.R. part 56).
3. RESULTS
Of 1518 cohort participants, 1266 (83%) were aged ≥ 12 years and included in this VE analysis. By the end of follow‐up, almost half (48%) received two doses of Pfizer‐BioNTech vaccine, 26% received two doses of Moderna vaccine, and 26% were unvaccinated. Older adults, females, Non‐Hispanic White participants, those who received the 2020–2021 influenza vaccine, and those who work in healthcare were more likely to be vaccinated (Table 1). Most (76%) vaccinated participants received a second dose in January to April 2021 (Figure S1). Moderna recipients tended to be older, have a preexisting medical condition, have public insurance, and longer median time since receipt of the second dose (238 [interquartile range (IQR) 223–256] days vs. 217 [IQR 182–247] days, Wilcoxon P < 0.001) compared with Pfizer‐BioNTech recipients.
TABLE 1.
Characteristics of the rural central Wisconsin community cohort, aged ≥ 12 years
| Vaccination status a | |||||
|---|---|---|---|---|---|
| All (n = 1266) | Received Moderna COVID‐19 vaccine (n = 329) | Received Pfizer‐BioNTech COVID‐19 vaccine (n = 608) | Not vaccinated (n = 329) | SARS‐CoV‐2 infection b (n = 118) | |
| Age group c | |||||
| 12–17 years | 129 (10) | 0 | 84 (14) | 45 (14) | 11 (9) |
| 18–49 years | 470 (37) | 91 (28) | 219 (36) | 160 (49) | 58 (49) |
| 50–64 years | 272 (21) | 76 (23) | 133 (22) | 63 (19) | 23 (19) |
| ≥ 65 years | 395 (31) | 162 (49) | 172 (28) | 61 (19) | 26 (22) |
| Sex | |||||
| Female | 747 (59) | 188 (57) | 383 (63) | 176 (54) | 70 (59) |
| Male | 519 (41) | 141 (43) | 225 (37) | 153 (46) | 48 (41) |
| Race/ethnicity d | |||||
| Non‐Hispanic White | 1203 (95) | 321 (98) | 576 (95) | 306 (93) | 111 (94) |
| Hispanic | 39 (3) | 4 (1) | 18 (3) | 17 (5) | 5 (4) |
| Non‐Hispanic non‐White | 20 (2) | 3 (1) | 12 (2) | 5 (2) | 2 (2) |
| Preexisting medical condition e | |||||
| No | 547 (43) | 110 (33) | 281 (46) | 156 (47) | 55 (47) |
| Yes | 719 (57) | 219 (67) | 327 (54) | 173 (53) | 63 (53) |
| Asthma | 109 (9) | 27 (8) | 46 (8) | 36 (11) | 6 (5) |
| Cancer | 31 (2) | 15 (5) | 10 (2) | 6 (2) | 3 (3) |
| Chronic kidney disease | 26 (2) | 7 (2) | 13 (2) | 6 (2) | 1 (1) |
| COPD | 31 (2) | 11 (3) | 13 (2) | 7 (2) | 3 (3) |
| Hypertension | 307 (24) | 115 (35) | 142 (23) | 50 (15) | 17 (14) |
| Immunocompromised | 47 (4) | 15 (5) | 20 (3) | 12 (4) | 5 (4) |
| Serious heart condition | 72 (6) | 34 (10) | 24 (4) | 14 (4) | 5 (4) |
| Type 2 diabetes | 108 (9) | 41 (12) | 48 (8) | 19 (6) | 9 (8) |
| Obese | 471 (37) | 136 (41) | 208 (34) | 127 (39) | 51 (43) |
| Receipt of 2020–2021 influenza vaccine | |||||
| No | 382 (30) | 63 (19) | 125 (21) | 194 (59) | 46 (39) |
| Yes | 884 (70) | 266 (81) | 483 (79) | 135 (41) | 72 (61) |
| Health insurance type d | |||||
| Private | 804 (64) | 169 (51) | 425 (70) | 210 (64) | 83 (71) |
| Public | 435 (34) | 156 (48) | 173 (29) | 106 (33) | 31 (27) |
| None | 21 (2) | 4 (1) | 7 (1) | 10 (3) | 3 (3) |
| Occupation/Industry f | |||||
| Healthcare/Social services | 241 (36) | 45 (30) | 166 (51) | 30 (16) | 20 (25) |
| Manufacturing | 78 (12) | 15 (10) | 25 (8) | 38 (20) | 7 (9) |
| Education | 75 (11) | 33 (22) | 23 (7) | 19 (10) | 13 (16) |
| Retail | 49 (7) | 5 (3) | 22 (7) | 22 (12) | 6 (8) |
| Other service | 38 (6) | 8 (5) | 21 (6) | 9 (5) | 3 (4) |
| Other industry | 186 (28) | 46 (30) | 68 (21) | 72 (38) | 30 (38) |
| Frequency of respiratory sample collection g | |||||
| Weekly swabbing | 635 (50) | 174 (53) | 295 (49) | 166 (50) | 67 (57) |
| Swabbing with qualifying illness | 631 (50) | 155 (47) | 313 (51) | 163 (50) | 51 (43) |
| Prior SARS‐CoV‐2 infection h | |||||
| No | 1088 (86) | 292 (89) | 523 (86) | 273 (83) | 114 (97) |
| Yes | 178 (14) | 37 (11) | 85 (14) | 56 (17) | 4 (3) |
Note: Data are no. (%) of participants.
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Documented receipt of two doses before the time of censoring.
New laboratory‐confirmed SARS‐CoV‐2 infections occurring during the follow‐up period (November 3, 2020 to December 7, 2021) among two‐dose vaccinated or unvaccinated participants.
Age as of end of follow‐up (or censoring).
Data missing or unknown; % based on those with data.
Preexisting medical condition includes self‐report of asthma, cancer, chronic kidney disease, COPD, hypertension, immunocompromised state, serious heart condition, and type 2 diabetes, and obesity defined as BMI ≥ 30 calculated from self‐reported height and weight.
Percent among employed participants; based on the North American Industry Classification System (NAICS) grouping of 20 large industry sectors (https://www.census.gov/naics/).
Participants assigned to swab weekly for the first 26 weeks regardless of symptoms and with a qualifying illness after study week 27 or to swab with a qualifying illness for the duration of follow‐up. A qualifying illness was an illness with new onset in the past 7 days and at least one of the following symptoms: cough, fever or chills, sore throat, muscle or body aches, loss of smell or taste, shortness of breath, or diarrhea.
Evidence from enzyme‐linked immunosorbent assays (ELISA) targeting SARS‐CoV‐2 receptor‐binding domain and spike protein conducted at the Influenza Research Institute at University of Wisconsin‐Madison on serum samples collected at enrollment, molecular SARS‐CoV‐2 test results from study or clinic testing, and self‐report at study enrollment.
Between November 3, 2020 and December 7, 2021, 118 (9%) SARS‐CoV‐2 confirmed infections were documented during follow‐up; 6 were asymptomatic (2 received Pfizer‐BioNTech), 4 were previously infected (all unvaccinated) and 51 (43%) were vaccinated (29% received Pfizer‐BioNTech, 14% received Moderna). Mean age of those infected was 47.7 years, and 59% were female (Table 1). Among unvaccinated participants with infection, 11 (16%) sought care, and 4 (6%) were hospitalized. Among vaccinated participants with infection, 4 (8%) sought care, and 1 (2%) was hospitalized. Median time from receipt of the second dose to infection was 215 (IQR 163–241) days.
VE of mRNA vaccines against laboratory‐confirmed infection (symptomatic and asymptomatic) was 56% (95% confidence interval [CI] 31–71), 65% (95% CI 37–81) for Moderna, and 50% (95% CI 21–69) for Pfizer‐BioNTech (Figure 1). VE estimates were similar against symptomatic infections, when prior infections were excluded, and when restricted to the period when Delta predominated (Figure 1).
FIGURE 1.
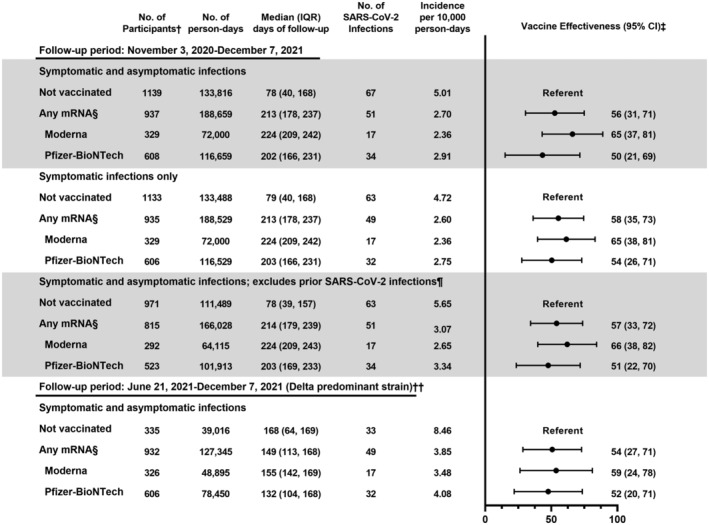
Effectiveness of COVID‐19 vaccines against SARS‐CoV‐2 infection in a rural community, Wisconsin, November 3, 2020 to December 7, 2021. †Participants can contribute both unvaccinated and vaccinated time during the follow‐up period. ‡Estimated from Cox proportional hazards model with time‐varying vaccination status, respiratory sample collection frequency, and age (modeled as natural cubic spline with five knots based on percentiles). §Vaccinated defined as ≥ 14 days after receipt of the second dose of any mRNA vaccine. ¶Defined based on evidence from enzyme‐linked immunosorbent assays (ELISA) targeting SARS‐CoV‐2 receptor‐binding domain and spike protein conducted at the Influenza Research Institute at University of Wisconsin‐Madison on serum samples collected before enrollment, molecular SARS‐CoV‐2 test results from clinic testing before enrollment, and self‐report at study enrollment. ††Delta variant identified in > 50% of samples sequenced in Wisconsin after June 21, 2021; 97% of 29 viruses sequenced from samples collected from study participants between June 21, 2021 and October 7, 2021 were Delta variant. Participants with SARS‐CoV‐2 infection between November 3, 2020 and June 20, 2021 were excluded
4. DISCUSSION
In this prospective rural community cohort with active illness surveillance, mRNA vaccines were 56% effective against symptomatic and asymptomatic SARS‐CoV‐2 infection. VE estimates were lower than reported estimates from clinical trials and observational studies based on clinical testing conducted soon after vaccines became available. 6 , 10 , 11
Our findings of lower VE against SARS‐CoV‐2 infection during a period with increased circulation of Delta is consistent with previous studies among healthcare workers, nursing home residents, and in retrospective, population‐based cohort studies. 2 , 3 , 4 , 5 , 12 Waning of vaccine protection is also possible, as lower VE was observed with longer time since vaccination in several studies. 2 , 3 , 13 However, assessment of the contribution of waning immunity and the Delta variant to the observed reduced vaccine protection is complicated by the local increase in Delta circulation coinciding with the period when most cohort members were > 5 months from receipt of their second dose. Further studies with additional follow‐up time after vaccination for all ages are needed to better understand the impact of waning protection and the importance of booster doses.
This study had several limitations. Relatively few cases occurred during the follow‐up period with most vaccinated cases occurring when Delta predominated. The small sample size led to wide CIs and limited our ability to control for potential confounding factors in VE estimates such as preexisting conditions, occupation, and behaviors, which may be associated with vaccination status, vaccine product received, and infection risk. Finally, the study population is largely non‐Hispanic White and from a single rural community in central Wisconsin so findings may not be generalizable to other rural communities or other racial and ethnic groups.
Strengths of this study include active follow‐up of participants for new illness that included weekly respiratory samples collection for SARS‐CoV‐2 testing for half of the participants during most of the follow‐up period. Weekly surveillance combined with clinical SARS‐CoV‐2 test results available from linked health records allowed comprehensive capture of SARS‐CoV‐2 infections. Second, MCHS's data exchange with the Wisconsin Immunization Registry allowed more accurate classification of vaccination status over time and product received. Third, our analysis included adolescents and rural community members, who have been underrepresented to date. Finally, prior SARS‐CoV‐2 infections were captured by self‐report and serologic testing, reducing the potential for biased VE estimates.
This study demonstrates that two doses of mRNA vaccine reduce the risk of SARS‐CoV‐2 infection. However, vaccinated persons continue to be at risk for infection in the community, serving as a reminder of the importance of layered prevention measures to break chains of transmission. A booster dose may help increase protection among vaccinated persons, but efforts to increase uptake are essential. Increasing uptake of the primary vaccine series in rural areas, where there is greater hesitancy to receive COVID‐19 vaccine and the burden of COVID‐19 and associated mortality has been higher than in urban areas, should be a priority. 14 , 15 As SARS‐CoV‐2 continues to circulate and evolve and the COVID‐19 vaccination program matures, continued monitoring of COVID‐19 VE in the general population is needed.
AUTHOR CONTRIBUTIONS
David L McClure: Conceptualization; formal analysis; methodology. Jennifer P King: Conceptualization; methodology; project administration. Jennifer K Meece: Conceptualization; methodology; project administration; supervision. David Pattinson: Conceptualization; data curation; methodology. Gabriele Neumann: Conceptualization; methodology; project administration; supervision. Yoshihiro Kawaoka: Conceptualization; funding acquisition; methodology; supervision. Melissa A Rolfes: Conceptualization; investigation; methodology; project administration; supervision. Edward A Belongia: Conceptualization; methodology; supervision.
CONFLICT OF INTEREST
All authors report no potential conflicts.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/irv.12970.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGMENTS
We thank the following for their contributions to the study: Roxy Eibergen, Lynn Ivacic, Diane Kohnhorst, Erik Kronholm, DeeAnn Polacek, Carla Rottscheit, Elizabeth Armagost, Bobbi Bradley, Hannah Berger, Adam Bissonnette, Joshua Blake, Thomas G. Boyce, Keegan Brighton, Gina Burbey, Deanna Cole, Leila L. Deering, Cody DeHamer, Rachel Fernandez, Sherri Guzinski, Kayla Hanson, Erin Higdon, Jacob P. Johnston, Julie M. Karl, Taylor Kent, Burney A. Kieke Jr., Sarah Kohn, Sarah L. Kopitzke, Tamara Kronenwetter Koepel, Stacey Kyle, Eric LaRose, Kate Lassa, Carrie Marcis, Karen McGreevey, Nidhi Mehta, Daniel Miesbauer, Jennifer Moran, Pamela Mundt, Lisa Ott, Nan Pan, Cory Pike, Rebecca Pilsner, Martha Presson, Nicole Price, Mark Riley, Jacklyn L. E. Salzwedel, Juan Saucedo, Kristin Seyfert, Alex Slenczka, Elisha Stefanski, Robert Strenn, Sandra K. Strey, Melissa Strupp, and Krishna C. Upadhyay at Marshfield Clinic Research Institute; Lizheng Guan and Peter Jester at University of Wisconsin‐Madison; Fatimah Dawood, Constance Ogokeh, Carrie Reed, and Douglas Slaughter at Centers for Disease Control and Prevention. This work was supported by the US Centers for Disease Control and Prevention (Grant 75D30120C09259). Investigators at the Centers for Disease Control and Prevention contributed to the design and conduct of the study.
McLean HQ, McClure DL, King JP, et al. mRNA COVID‐19 vaccine effectiveness against SARS‐CoV‐2 infection in a prospective community cohort, rural Wisconsin, November 2020 to December 2021. Influenza Other Respi Viruses. 2022;16(4):607-612. doi: 10.1111/irv.12970
Funding information US Centers for Disease Control and Prevention, Grant/Award Number: 75D30120C09259
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. International Vaccine Access Center (IVAC) , Results of COVID‐19 vaccine effectiveness studies: an ongoing systematic review, weekly summary tables. 2021, Johns Hopkins Bloomberg School of Public Health and World Health Organization. Accessed December 9, 2021. www.view-hub.org
- 2. Fowlkes A, Gaglani M, Groover K, et al. Effectiveness of COVID‐19 vaccines in preventing SARS‐CoV‐2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance—eight U.S. locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1167‐1169. doi: 10.15585/mmwr.mm7034e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tartof SY, Slezak JM, Fischer H, et al. Six‐month effectiveness of BNT162B2 mRNA COVID‐19 vaccine in a large US integrated health system: a retrospective cohort study. 2021. [DOI] [PMC free article] [PubMed]
- 4. Keehner J, Horton LE, Binkin NJ, et al. Resurgence of SARS‐CoV‐2 infection in a highly vaccinated health system workforce. N Engl J Med. 2021;385(14):1330‐1332. doi: 10.1056/NEJMc2112981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seppala E, Veneti L, Starrfelt J, et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill. 2021;26(35). doi: 10.2807/1560-7917.ES.2021.26.35.2100793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chodick G, Tene L, Rotem RS, et al. The effectiveness of the two‐dose BNT162b2 vaccine: analysis of real‐world data. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kieke AL, Kieke BA Jr, Kopitzke SL, et al. Validation of health event capture in the Marshfield Epidemiologic Study Area. Clin Med Res. 2015;13(3–4):103‐111. doi: 10.3121/cmr.2014.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ThermoFisher Scientific . TaqPath™ COVID‐19 combo kit and TaqPath™ COVID‐19 combo kit advanced* instructions for use. 2020.
- 9. Wisconsin State Laboratory of Hygiene . Wisconsin SARS‐CoV‐2 (hCOV‐19) genomic dashboard. 2021. Accessed October 8, 2021. https://dataportal.slh.wisc.edu/sc2dashboard#tab-2341-2
- 10. Pilishvili T, Gierke R, Fleming‐Dutra KE, et al. Effectiveness of mRNA Covid‐19 vaccine among U.S. health care personnel. N Engl J Med. 2021;385(25):e90. doi: 10.1056/NEJMoa2106599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA‐1273 COVID‐19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID‐19 disease in Qatar. Nat Med. 2021;27(9):1614‐1621. doi: 10.1038/s41591-021-01446-y [DOI] [PubMed] [Google Scholar]
- 12. Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer‐BioNTech and Moderna vaccines in preventing SARS‐CoV‐2 infection among nursing home residents before and during widespread circulation of the SARS‐CoV‐2 B.1.617.2 (Delta) variant—National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1163‐1166. doi: 10.15585/mmwr.mm7034e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS‐CoV‐2 infection in Qatar. N Engl J Med. 2021;385(24):e83. doi: 10.1056/NEJMoa2114114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ullrich F, Mueller K. COVID‐19 cases and deaths, metropolitan and nonmetropolitan counties over time (update). 2021, RUPRI Center for Rural Health Policy Analysis, University of Iowa College of Public Health. Accessed October 11, 2020. https://rupri.public-health.uiowa.edu/publications/policybriefs/2021/COVID%20Vaccinations%20and%20Cases.pdf
- 15. Andrejko KL, Pry J, Myers JF, et al. Prevention of COVID‐19 by mRNA‐based vaccines within the general population of California. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab640 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


