Abstract
Background and purpose
Anti‐myelin oligodendrocyte glycoprotein antibodies (MOG‐Abs) distinguish a group of inflammatory disorders which can be preceded by specific or non‐specific infections. A few single cases have been reported in association with SARS‐CoV‐2 infection, but a specific study on the correlation between COVID‐19 and myelin oligodendrocyte glycoprotein (MOG)‐associated disorder (MOGAD) has not yet been performed. The aim of this study was to determine the impact of the pandemic on this condition.
Methods
We analysed SARS‐CoV‐2 serology in patients newly diagnosed with MOGAD (1 August 2020 to 31 May 2021). MOG‐Ab‐seronegative age‐ and time‐matched subjects were used as controls. SARS‐CoV‐2 immunoglobulin G (IgG) levels were analysed using an anti‐SARS‐CoV‐2 US Food and Drug Administration‐approved ELISA assay and confirmed with a trimeric anti‐SARS‐CoV‐2 S1/S2 IgG immunochemiluminescent test, concomitantly assaying the anti‐receptor binding domain (RBD) of spike protein IgG and anti‐RBD total Ig. We actually compared the number of cases referred in each of the last 3 years.
Results
Presence of SARS‐CoV‐2 IgG antibodies was more common (12/30, 40%) in MOGAD patients than in controls (6/30, 20%), although the difference was not significant (p = 0.16; odds ratio 2.67, 95% confidence interval 0.85–9.17). The most common clinical presentations of MOGAD SARS‐CoV‐2‐seropositive patients included optic neuritis (n = 6) and myelitis (n = 3). The number of diagnosed cases increased over the last 3 years, in particular, when including cases referred to us before the COVID‐19 pandemic, in the initial phase of the first wave and in the late phase of the second wave (n = 9, rate 10.6% in 2019; n = 13, rate 12.3% in 2020; n = 15, rate 14.7% in 2021).
Conclusion
Our findings provide preliminary data on SARS‐CoV‐2 as a potential trigger of MOGAD.
Keywords: COVID‐19, MOG, myelitis, optic neuritis, SARS‐CoV‐2
The increase and monthly distribution of anti‐myelin oligodendrocyte glycoprotein antibody (MOG‐Ab)‐positive cases diagnosed, in particular in 2021, may suggest that SARS‐CoV‐2 has a role in triggering this condition.

INTRODUCTION
Anti‐myelin oligodendrocyte glycoprotein antibodies (MOG‐Abs) define a distinct group of inflammatory central nervous system disorders of both adults and children with a clinical phenotype compatible with acute disseminated encephalomyelitis (ADEM), optic neuritis, myelitis, brainstem syndrome and encephalitis, which evolve in a monophasic or relapsing course [1, 2]. Attacks can be preceded by infections which have been proposed as potential triggers of MOG‐associated disorder (MOGAD) [3]. Antecedent symptoms are usually referred to as non‐specified respiratory or feverish infection, but in some patients a specific infectious agent can be identified, supporting the occurrence of infection‐associated immune response [3].
A few case reports of MOGAD occurring concomitantly or after SARS‐CoV‐2 infection have been described [4, 5, 6, 7, 8, 9, 10], however, the possible correlation between these two conditions has not yet been investigated systematically.
The aim of this study was to analyse the impact of SARS‐CoV‐2 infection on MOGAD by (i) determining the frequency of SARS‐CoV‐2 infection in newly diagnosed MOGAD through extensive serological analyses and (ii) analysing the number of MOGAD cases referred to our tertiary center during the COVID‐19 pandemic in comparison to the pre‐pandemic period.
METHODS
Study subjects
We retrospectively identified consecutive treatment‐naive patients who tested MOG‐IgG positive for the first time at our Laboratory of Neuropathology, University Hospital of Verona, Italy between 1 August 2020 and 31 May 2021. An equal number of MOG‐Ab‐seronegative age‐ and time‐matched controls were then selected for comparison purposes.
Comparison of relevant clinical variables was performed using the Mann–Whitney U‐test and Fisher's exact test, as appropriate (IBM SPSS 26).
SARS‐CoV‐2 IgA/IgG testing
Sera were tested for anti‐SARS‐COV‐2 IgA and IgG (using a US Food and Drug Administration‐approved ELISA assay; Euroimmun, Luebeck, Germany). Positive results were validated using a trimeric anti‐SARS‐CoV‐2 S1/S2 IgG test (DiaSorin, Saluggia, Italy), an anti‐SARS‐CoV‐2 receptor‐binding domain (RBD) IgG test (Beckman‐Coulter, Brea, CA, USA), and an anti‐SARS‐CoV‐2 RBD total antibodies test (Roche Diagnostics, Basel, Switzerland).
Testing for MOG‐Abs
The presence of MOG‐Abs was analysed by two independent investigators (S.F., S.M.) at the Verona Neuropathology Laboratory using a recombinant live cell‐based immunofluorescence assay with HEK293A cells transfected with full‐length MOG (human MOG alpha‐1 enhanced green fluorescent protein fusion protein), as previously described [2].
Evaluation of MOGAD cases
We analysed the number of MOGAD cases diagnosed in the last 3 years and their monthly distribution (between 1 August and 31 May). In addition, we compared the number of MOG‐Ab‐seropositive cases, together with the rate of MOG‐Ab positivity among patients with a compatible clinical phenotype, that were referred to us in the pre‐pandemic period (2019), in the initial phase of the pandemic (2020), and in the late pandemic phase (2021; all between 1 February and 31 May).
Standard protocol approval
The study was approved by the local Bioethics Committee (Comitato Etico per la Sperimentazione Clinica, Azienda Ospedaliera Universitaria Integrata di Verona) (BIOB‐NEU‐DNA‐2014, protocol 13582). All patients consented to diagnostic procedures and biological sample storage at the referring laboratory.
RESULTS
SARS‐CoV‐2 IgG positivity was higher in MOG‐Ab‐seropositive patients (12/30, 40%) than in the MOG‐Ab‐seronegative controls (6/30, 20%), although the difference was not significant (p = 0.16; odds ratio 2.67, 95% confidence interval 0.85–9.17). The monthly distribution of SARS‐CoV‐2‐seropositive cases in relation to MOG‐Ab status is reported in Figure 1a.
FIGURE 1.
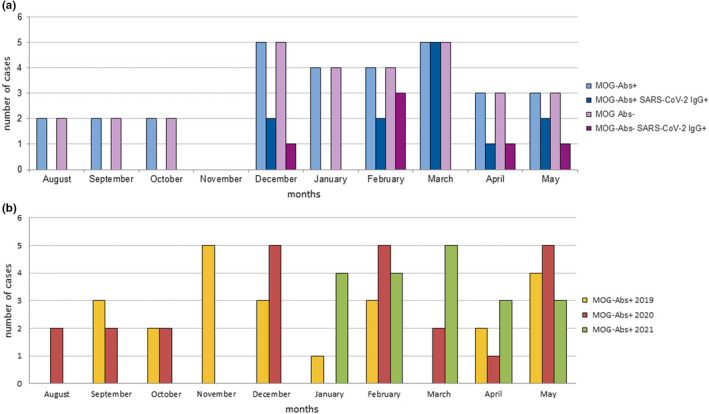
Distribution of anti‐myelin oligodendrocyte glycoprotein antibody (MOG‐Ab)‐positive patients in the last 3 years with a focus on SARS‐CoV‐2 serostatus (August 2020 to May 2021). (a) Monthly distribution of MOG‐Abs‐seropositive (light blue) and ‐seronegative patients (light purple) referred to our tertiary center between August 2020 and May 2021 in comparison with SARS‐CoV‐2 serostatus (blue and purple, respectively). (b) Newly diagnosed MOG‐associated disorder (MOGAD) cases per month (August to May) during the last 3 years [Colour figure can be viewed at wileyonlinelibrary.com]
The median (range) age of MOGAD SARS‐CoV‐2 seropositive patients was 43 (23–86) years and, for MOGAD SARS‐CoV‐2‐seronegative controls, it was 49 (5–78) years (p = 0.710), while sex was equally distributed within the two groups (p = 0.645). The clinical presentation of MOGAD SARS‐CoV‐2‐seropositive patients included monolateral optic neuritis (n = 6), myelitis (n = 3), encephalitis (n = 2), and V cranial neuropathy (n = 1). Among MOGAD SARS‐CoV‐2‐seronegative patients, myelitis was the most common presentation (n = 6), followed by encephalitis (n = 4, including one patient with brainstem encephalitis and one with unilateral cortical FLAIR‐hyperintense lesions with seizures [FLAMES]), encephalomyelitis (n = 4, including one patient with ADEM), optic neuritis (n = 2), neuromyelitis optica spectrum disorder (n = 1), and brainstem encephalitis associated with myelitis and bilateral optic neuritis (n = 1). All serum samples were obtained before administering any treatment that could potentially influence antibody status. Among the MOGAD SARS‐CoV‐2‐seropositive patients, seven had mild symptomatic COVID‐19 not requiring hospitalization and five had an asymptomatic infection.
Of the 66 MOGAD patients diagnosed during the analysed timeframe of the last 3 years, 23 were referred to our center in 2019, 24 in 2020 (over 10 months; Figure 1b) and 19 in 2021 (over 5 months; Figure 1b). When including patients referred to us before the pandemic, in the initial phase of the first wave and in the late phase of the second wave (all between 1 February and 31 May) we detected nine MOGAD cases in 2019, 13 in 2020, and 15 in 2021 (Figure 1b). The rate of MOG‐Ab positivity among patients with a compatible phenotype (i.e., optic neuritis, myelitis, encephalitis, encephalomyelitis, or a combination of these syndromes) referred to us over this period was 10.6% in 2019, 12.3% in 2020, and 14.7% in 2021.
DISCUSSION
In this study, we analysed the possible correlation between SARS‐CoV‐2 infection and MOGAD by studying SARS‐CoV‐2 IgG serology in this condition and by comparing its incidence in different periods over the last years. The incidence of SARS‐CoV‐2 infection was higher but not significantly different in newly diagnosed MOG‐Ab‐seropositive cases in comparison with age‐ and time‐matched controls, calling into question the role of SARS‐CoV‐2 infection as a potential trigger for MOGAD. However, on a larger scale, the increase in and monthly distribution of MOG‐Ab‐ positive cases diagnosed, in particular in 2021, may suggest a role of SARS‐CoV‐2 in triggering this condition, and this is supported also by the post‐infectious nature of some MOGAD cases previously reported [3]. Confounding factors must be taken into account, such as the pandemic effect, an improvement in diagnostic techniques, and the better preselection of patients by trained neurologists. Of note, we have used the same cell‐based assay to analyse patients over the last years and noted an increase in the percentage of seropositive cases among those with a compatible phenotype. In addition, the pandemic effect should have decreased the number of referred patients, especially those with a mild phenotype. All these data reinforce the possibility that SARS‐CoV‐2 might be a trigger for this condition.
Regarding data on individual patients, our findings expand those recently described for single cases of MOGAD occurring during/after SARS‐CoV‐2 infection [4, 5, 6, 7, 8, 9, 10]. Of note, cerebrospinal fluid SARS‐CoV‐2 PCR tests are usually negative in these cases, further supporting the role of immune‐mediated processes rather than direct viral damage [4, 6, 7, 8]. In accordance with our findings, previously reported cases [5, 6, 7, 8, 9, 10] occurred during/after mild or asymptomatic SARS‐CoV‐2 infection, which supports SARS‐CoV‐2 serology testing to recognize an antecedent infection. The clinical presentation of MOGAD SARS‐CoV‐2‐seropositive patients observed in this study was compatible with the phenotypes previously described [1, 2], with most of the patients presenting with optic neuritis and/or myelitis. By contrast, patients presenting with SARS‐CoV‐2‐associated ADEM are unlikely to harbor MOG‐Abs [11]. The predominance of encephalitis/encephalopathies recently reported might be the effect of a selection bias, since SARS‐CoV‐2 infection might be investigated predominantly in these conditions [4, 5, 6, 8, 10].
Our study has some relevant limitations including: (i) the small sample size of the analysed cohort, which might have influenced our findings, in particular the comparison between MOGAD SARS‐CoV‐2 IgG‐seropositive and ‐seronegative cases (according to our data, the required sample size for further investigation, accounting for a 5% precision and 95% confidence interval, would be 369 MOG‐Ab‐positive and 246 MOG‐Ab‐negative patients, which would require a multicenter epidemiology‐based study); (ii) the monocentric nature of our study; (iii) the inability to analyse other possible infectious triggers, such as influenza, the incidence of which nevertheless decreased during the pandemic [12], because of the diagnostic restrictions that were in place during the pandemic; and (iv) the short follow‐up, which prevents us from determining the impact of SARS‐CoV‐2 infection on the MOGAD disease course.
To conclude, according to our data, SARS‐CoV‐2 infection might have a relationship with MOGAD, even in paucisymptomatic cases. Further multicenter prospective studies are necessary to confirm our findings and to determine the disease course of these patients.
CONFLICT OF INTEREST
S. Ferrari received support for attending scientific meetings from Shire, Sanofi Genzyme and Euroimmun. S. Mariotto received support for attending scientific meetings from Merck and Euroimmun, and received speaker honoraria from Biogen. The other authors report no competing interests.
AUTHOR CONTRIBUTIONS
Sara Mariotto: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (lead); Methodology (lead); Writing – original draft (lead); Writing – review and editing (equal). Sara Carta: Data curation (lead); Formal analysis (lead); Investigation (lead); Writing – original draft (lead). Alessandro Dinoto: Data curation (equal); Formal analysis (equal); Methodology (equal); Writing – review and editing (equal). Giuseppe Lippi: Formal analysis (equal); Investigation (equal); Writing – review and editing (equal). Gian Luca Salvagno: Formal analysis (equal); Methodology (equal); Writing – review and editing (equal). Laura Masin: Data curation (equal); Formal analysis (equal); Methodology (equal). Daniela Alberti: Formal analysis (equal); Methodology (equal); Writing – review and editing (equal). Romain Marignier: Data curation (equal); Writing – review and editing (equal). Sergio Ferrari: Conceptualization (lead); Data curation (lead); Writing – review and editing (lead).
Mariotto S, Carta S, Dinoto A, et al. Is there a correlation between MOG‐associated disorder and SARS‐CoV‐2 infection? Eur J Neurol. 2022;29:1855–1858. doi: 10.1111/ene.15304
See commentary by R. Tanasescu and M. Reindl on page 1569.
DATA AVAILABILITY STATEMENT
The data that support this study are available for sharing and further examination from the corresponding authors (S.M.) on reasonable requests. The data are not publicly available because they contain information that could compromise patients' consent.
REFERENCES
- 1. Cobo‐Calvo A, Vukusic S, Marignier R. Clinical spectrum of central nervous system myelin oligodendrocyte glycoprotein autoimmunity in adults. Curr Opin Neurol. 2019;32:459‐466. [DOI] [PubMed] [Google Scholar]
- 2. Mariotto S, Ferrari S, Monaco S, et al. Clinical spectrum and IgG subclass analysis of anti‐myelin oligodendrocyte glycoprotein antibody‐associated syndromes: a multicenter study. J Neurol. 2017;264:2420‐2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jarius S, Ruprecht K, Kleiter I, et al. MOG‐IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long‐term outcome. J Neuroinflammation. 2016;13:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vraka K, Ram D, West S, et al. Two paediatric patients with encephalopathy and concurrent COVID‐19 infection: two sides of the same coin? Case Rep Neurol Med. 2021;2021:6658000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim JW, Abdullayev N, Neuneier J, Fink GR, Lehmann HC. Post‐COVID‐19 encephalomyelitis. Neurol Res Pract. 2021;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters J, Alhasan S, Vogels CBF, Grubaugh ND, Farhadian S, Longbrake EE. MOG‐associated encephalitis following SARS‐COV‐2 infection. Mult Scler Relat Disord. 2021;50:102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou S, Jones‐Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody‐associated optic neuritis and myelitis in COVID‐19. J Neuroophthalmol. 2020;40:398‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahsan N, Jafarpour S, Santoro JD. Myelin oligodendrocyte glycoprotein antibody encephalitis following severe acute respiratory syndrome coronavirus 2 in a pediatric patient. Clin Exp Pediatr. 2021;64:310‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodhall M, Mitchell JW, Gibbons E, Healy S, Waters P, Huda S. Case report: myelin oligodendrocyte glycoprotein antibody‐associated relapse with COVID‐19. Front Neurol. 2020;11:598531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pinto AA, Carroll LS, Nar V, Varatharaj A, Galea I. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID‐19. Neurol Neuroimmunol Neuroinflamm. 2020;7:e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manzano GS, McEntire CRS, Martinez‐Lage M, Mateen FJ, Hutto SK. Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis following COVID‐19: systematic review and meta‐synthesis. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olsen SJ, Azziz‐Baumgartner E, Budd AP, et al. Decreased influenza activity during the COVID‐19 pandemic — United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1305‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support this study are available for sharing and further examination from the corresponding authors (S.M.) on reasonable requests. The data are not publicly available because they contain information that could compromise patients' consent.


