Abstract
Aim
The COVID‐19 pandemic has reduced the capacity to diagnose and treat cancer worldwide due to the prioritization of COVID‐19 treatment. The aim of this study was to investigate treatment and outcomes of colon cancer in Sweden before and during the COVID‐19 pandemic.
Methods
In an observational study, using the Swedish Colorectal Cancer Registry, we included (i) all Swedish patients diagnosed with colon cancer, and (ii) all patients undergoing surgery for colon cancer, in 2016–2020. Incidence of colon cancer, treatments and outcomes in 2020 were compared with 2019.
Results
The number of colon cancer cases in Sweden in April–May 2020 was 27% lower than the previous year, whereas no difference was observed on an annual level (4,589 vs. 4,763 patients [−4%]). Among patients with colon cancer undergoing surgery in 2020, the proportion of resections was 93 vs. 94% in 2019, with no increase in acute resections. Time from diagnosis to elective surgery decreased (29 days vs. 33 days in 2020 vs. 2019). In 2020, more patients underwent a two‐stage procedure with a diverting stoma as first surgery (6.1%) vs. (4.4%) in 2019 (p = 0.0020) and more patients were treated with preoperative chemotherapy (5.1%) vs. (3,5%) 2019 (p = 0.0016). The proportion of patients that underwent laparoscopic surgery increased from 54% to 58% (p = 0.0017) There were no differences in length of stay, surgical complications, reoperation, ICU‐stay or 30‐day mortality between the years.
Conclusion
Based on nationwide annual data, we did not observe adverse effects of the COVID‐19 pandemic on colon cancer treatment and short time outcomes in Sweden.
Keywords: colorectal cancer, colorectal surgery, covid‐19, ostomy, surgery pandemic
What does this paper add to the literature?
The COVID‐19 pandemic has reduced the capacity to diagnose and treat cancer. How the treatment and outcomes of colon cancer in Sweden before and during the COVID‐19 pandemic was affected has been analysed, using the Swedish Colorectal Cancer Registry. Colon cancer management and treatment was maintatined in Sweden during 2020.
INTRODUCTION
The COVID‐19 pandemic has impacted health care systems worldwide due to the prioritization of COVID‐19 treatment over other diseases. Modelling studies have raised concerns that diagnostic delay and decreased access to treatments will lead to increased mortality from cancer, possibly even exceeding that from SARS‐Cov‐2 [1, 2]. Colorectal cancer has an excellent prognosis when diagnosed and treated in early stages. Reduced access to colonoscopy and surgery may lead to stage migration and reduced survival for the patients.
There is a scarcity in data regarding factual outcomes for colorectal cancer patients during the pandemic, with the majority of publications being recommendations [3, 4, 5], audits [6], surveys [3, 7] and case reports [8]. A few population‐based studies have been published, reporting disruptions in the management of colorectal cancer in England [9], Korea [10], and Spain [11]. Also in Sweden, the pandemic put the healthcare system under severe strain, with tens of thousands of operations being postponed [12].
The Swedish Cancer Register has reported a 12% reduction of cancer cases March–August 2020 compared to 2019 [13] but there are, to date, no reports on how colon cancer treatment was affected by the pandemic. The aim of this study was to assess colon cancer incidence, treatment and outcomes for year 2020 compared to the preceeding 4 years, with focus on differences between 2019 and 2020.
METHODS
Study design
In this observational population‐based cohort study we report incidence, stage, treatment, and short‐term outcomes for colon cancer in Sweden 2016–2020. We used the RECORD checklist [14] for the report.
Setting
Sweden is a high‐income country with a population of 10 millions in 2020. Healthcare is mainly tax‐funded with universal access to care [15]. The first wave of the COVID‐19 pandemic peaked in April–May 2020, and the second in October–December the same year. Screening for colorectal cancer with stool test, applied in some regions, was paused from May to August [16], and surgery for premalignant and benign conditions postponed. Temporary guidelines for colorectal cancer surgery were established 3 April 2020 with recommendations in three different scenarios of escalating COVID‐19 [17].
Data sources and participants
The Swedish Colorectal Cancer Registry (SCRCR) was used as data source. This register has extensive information on patient characteristics, pre‐and postoperative tumour staging, tumour location, surgical and oncological treatment and recurrences for colon cancer nationwide since 2007 [18, 19]. Data was extracted 20 May 2021. The coverage for cancer incidence was estimated at 100% for all years, and the coverage of surgical treatments at 97% for year 2020.
Inclusion criteria
We constructed two different cohorts; (1) all incident colon tumours diagnosed 2016–2020, and (2]) all surgical procedures performed for colon cancer 2016–2020.
Variables
Baseline characteristics included age, sex, tumour localization, tumour stage, and participation in colorectal cancer multidisciplinary conference (CRC‐MDC). Operation data included type of surgical intervention, acute/elective surgery, ostomy formation, neoadjuvant treatment, time from colon cancer diagnosis to surgery, hospital stay, 30‐day complications (defined as Clavien‐Dindo ≥2) [20], ICU‐care, reoperation, readmission, and mortality.
Bias
Misclassification due to registration errors and underreporting are assumed to be similar between the years.
Study size
All patients in Sweden with colon cancer diagnosed during the study period were included.
Missing data
Analyses were performed with an available case strategy.
Statistical analysis
Categorial variables are presented as number and percent and continuous variables as median and interquartile range (IQR). As each tumour is registered separately, and some patients had two tumours, results are reported both as number of diagnosed patients and as number of diagnosed tumours. Categorial variables were compared using χ2 test and continuous variables with the Wilcoxon signed rank test. The incidence proportion of colon cancer per year was estimated by dividing the number of patients diagnosed with colon cancer by the number of Swedish inhabitants on 31 December the year before (as reported by Statistics Sweden). All statistical tests were two‐sided, and p < 0.05 was considered statistically significant.
RESULTS
Incidence and stage at diagnosis
The number of diagnosed cases of colon cancer in April and May was 27% lower in 2020 than in 2019, but increased over the following months (Figure 1). In 2020, 4589 patients were diagnosed with colon cancer, corresponding to an incidence proportion of 0.044% (0.044%–0.047% in 2016–2019; Table 1). The patients' age, sex, and tumour locations were similar between the years. The incidence of cT4 tumours increased from 16% in 2016 to 23% in 2020, but with no significant increase between 2019 and 2020 (21%–23%, p = 0.11). During the same period the incidence of cTx tumours decreased, but with no change between 2019 and 2020. Similarly, cN1–N2 increased from 37% to 41% in 2016 to 2019 and further to 43% in 2020, and cNx decreased (p = 0.02). The proportion of patients with synchronous metastases increased from 21% in 2019 to 22% in 2020 (p = 0.01). No change in the number of patients assessed in CRC‐MDC was seen between 2019 and 2020 and the proportion of patients undergoing surgical intervention during the year of diagnosis was the same in 2020 and 2019 (74%).
FIGURE 1.
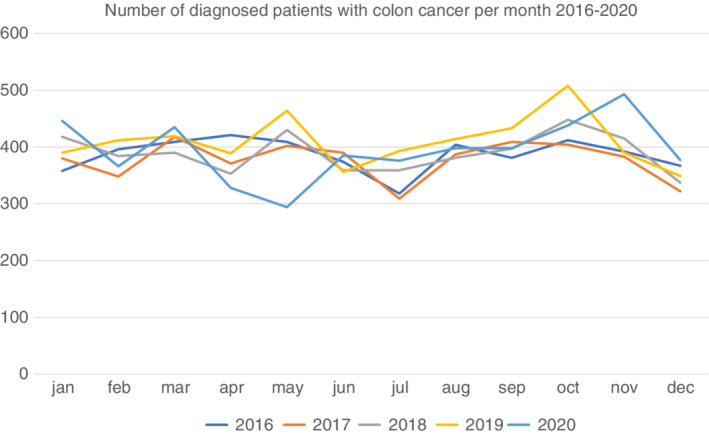
Number of diagnosed patients with colon cancer per month 2016–2020
TABLE 1.
Characteristics of colon cancer tumours diagnosed in Sweden in 2016–2020
| 2016 | 2017 | 2018 | 2019 | 2020 | p‐value for difference 2019–2020 | |
|---|---|---|---|---|---|---|
| Patients | ||||||
| Number of diagnosed patients | 4508 | 4407 | 4554 | 4763 | 4589 | N.A. |
| Incidence proportion a | 0.046 | 0.044 | 0.045 | 0.047 | 0.044 | N.A. |
| Female sex, number (%) | 2353 (51) | 2231 (49) | 2360 (49) | 2500 (51) | 2406 (51) | 1.0 |
| Age, mean (SD) median (IQR) | 73 (11) 74 (66–81) | 73 (11) 74 (66–81) | 73 (12) 74 (67–81) | 73 (12) 75 (67–81) | 73 (11) 75 (67–81) | 0.37 |
| Tumours | ||||||
| Number of diagnosed tumours | 4641 | 4523 | 4671 | 4917 | 4734 | |
| Location | ||||||
| Right colon b | 2202 (47) | 2116 (47) | 2279 (49) | 2445 (50) | 2303 (49) | 0.41 |
| Left colon c | 2419 (52) | 2390 (53) | 2375 (51) | 2459 (50) | 2422 (51) | |
| Unknown | 20 (0.43) | 17 (0.38) | 17 (0.36) | 13 (0.26) | 9 (0.19) | |
| cT stage | ||||||
| T1–T2 | 849 (18) | 1002 (22) | 1083 (23) | 1210 (25) | 1091 (23) | 0.11 |
| T3 | 1672 (36) | 1575 (35) | 1646 (35) | 1657 (34) | 1576 (33) | |
| T4 | 723 (16) | 780 (17) | 876 (19) | 1024 (21) | 1067 (23) | |
| Tx | 1397 (30) | 1166 (26) | 1066 (23) | 1026 (21) | 1000 (21) | |
| cN stage | ||||||
| N0 | 1976 (43) | 2124 (47) | 2212 (47) | 2254 (46) | 2037 (43) | 0.02 |
| N1‐2 | 1713 (37) | 1686 (37) | 1828 (39) | 2008 (41) | 2035 (43) | |
| Nx | 952 (21) | 713 (16) | 631 (14) | 655 (13) | 662 (14) | |
| cM stage | ||||||
| M0 | 3713 (80) | 3408 (75) | 3584 (77) | 3762 (77) | 3616 (76) | 0.01 |
| M1 | 911 (20) | 927 (21) | 929 (20) | 1023 (21) | 1031 (22) | |
| Mx | 17 (0.37) | 188 (4.2) | 158 (3.4) | 132 (2.7) | 87 (1.8) | |
| CRC‐MDC | 3841 (83) | 3748 (83) | 3913 (84) | 4155 (85) | 4017 (85) | 0.63 |
| Any surgical intervention same year | 3597 (78) | 3395 (75) | 3489 (75) | 3614 (74) | 3512 (74) | 0.44 |
Abbreviations: CRC‐MDC, colorectal cancer multidisciplinary conference; IQR, interquartile range; N.A., not applicable; SD, standard deviation.
Number of new cases/number of Swedish inhabitants 31 December the year before.
Appendix/caecum/ascendens/flexura hepatica.
Transverse colon/flexura lienalis/descending/sigmoid colon.
Treatment and short‐term outcomes
The number of interventions for colon cancer (including all procedures, e.g., polypectomy and nonresection surgery) was at its lowest point in May 2020 but the annual number was similar to previous years (Figure 2). The number of operations was 3,964 for year 2020 and 4,016 for year 2019 with similar proportions of resection surgery over calendar years (93% in 2020 vs. 94% 2019; Table 2 ). We did not observe any increase in acute surgery during year 2020, nor any difference in types of resections. The time from diagnosis to surgery in elective cases was shorter in 2020 (29 days, IQR: 22–41) than in 2019 (33 days, IQR: 22–43; p < 0.0001). In 2020, a larger proportion of patients underwent a 2‐stage procedure with a defunctioning stoma as first surgery (6.1%) compared to 2019 (4.4%; p = 0.0020). These patients had predominantly left‐sided tumours (80% in 2020 vs. 82% in 2019, p = 0.69). The proportion of patients receiving a stoma at resection surgery was 19% in 2020 vs. 18% in 2019, p = 0.09, data not shown). Similarly, preoperative chemotherapy was more common in 2020 compared with 2019 (5.1% vs. 3,5%, p = 0.0016). The proportion of patients treated with laparoscopic surgery continuously increased over the years from 39% in 2016 to 54% in 2019, and further to 58% in 2020 (p = 0.0017). The length of hospital stay was 5 (3–8) days both for 2019 and 2020. There were no statistically significant differences in surgical complications, ICU‐care, reoperation or 30‐day mortality between 2019 and 2020.
FIGURE 2.
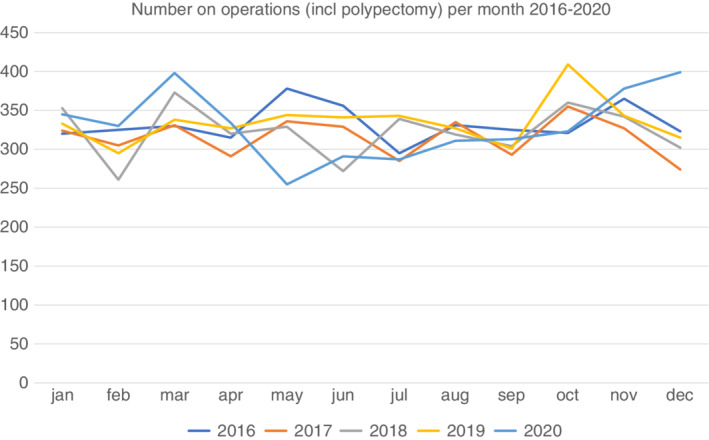
Number of operations (including polypectomy and nonresectional surgery) per month in Sweden in 2016–2020
TABLE 2.
Surgical treatment of colon tumours in Sweden 2016–2020
| 2016 | 2017 | 2018 | 2019 | 2020 | p‐value for difference 2019–2020 | |
|---|---|---|---|---|---|---|
|
All surgical treatments n (%) |
3984 | 3785 | 3874 | 4016 | 3964 | N.A. |
| Appendectomy | 17 (0.43) | 9 (0.24) | 18 (0.46) | 8 (0.20) | 8 (0.20) | 0.29 |
| Polypectomy/local resection | 157 (3.9) | 154 (4.1) | 123 (3.2) | 113 (2.8) | 124 (3.1) | |
| Stent | 40 (1.0) | 30 (0.79) | 24 (0.62) | 29 (0.72) | 28 (0.71) | |
| Laparotomy/laparoscopy without resection | 139 (3.5) | 93 (2.5) | 97 (2.5) | 91 (2.3) | 109 (2.8) | |
| Other operation | 50 (1.3) | 6 (0.16) | 2 (0.05) | 1 (0.02) | 6 (0.15) | |
| Resection surgery | 3579 (90) | 3491 (92) | 3610 (93) | 3774 (94) | 3688 (93) | |
| Type of treatment missing | 2 (0.05) | 2 (0.05 | 0 (0) | 0 (0) | 1 (0.03 | |
| Total number of ostomy constructions a | 734 (18) | 692 (18) | 691 (18) | 745 (19) | 800 (20) | 0.07 |
| All resection surgery | 3579 | 3491 | 3610 | 3774 | 3688 | N.A. |
| Acute resection surgery b | 544 (15) | 500 (14) | 508 (14) | 511 (14) | 515 (14) | 0.46 |
| Type of resection surgery c | ||||||
| Ileocecal resection | 37 (1.0) | 23 (0.66) | 26 (0.72) | 23 (0.61) | 24 (0.65) | 1.0 |
| Right‐sided hemicolectomy | 1982 (55) | 1838 (53) | 1962 (54) | 2111 (56) | 2039 (55) | |
| Transverse colon resection | 58 (1.6) | 57 (1.6) | 60 (1.7) | 54 (1.4) | 54 (1.5) | |
| Left‐sided resection | 334 (9.3) | 367 (11) | 370 (10) | 378 (10) | 359 (9.7) | |
| Sigmoid sesection | 756 (21) | 748 (21) | 725 (20) | 747 (20) | 757 (21) | |
| Hartmann resection | 109 (3.0) | 126 (3.6) | 89 (2.5) | 117 (3.1) | 120 (3.3) | |
| Anterior resection | 121 (3.4) | 133 (3.8) | 147 (4.1) | 135 (3.6) | 134 (3.6) | |
| Colectomy | 178 (5.0) | 189 (5.4) | 222 (6.2) | 196 (5.2) | 191 (5.2) | |
| Abdominoperineal resection | 4 (0.11) | 10 (0.29) | 9 (0.25) | 13 (0.34) | 10 (0.27) | |
| Elective resection surgery | 3033 | 2990 | 3102 | 3263 | 3171 | |
| Diverting stoma preoperatively | 135 (4.4) | 124 (4.2) | 149 (4.8) | 142 (4.4) | 193 (6.1) | 0.0020 |
| Preoperative chemotherapy | 92 (3.0) | 96 (3.2) | 86 (2.8) | 113 (3.5) | 161 (5.1) | 0.0016 |
| Laparoscopic surgery | 1168 (39) | 1279 (43) | 1499 (48) | 1771 (54) | 1845 (58) | 0.0017 |
| Number of days from diagnosis to surgery d median (IQR) | 32 (22–43) | 32 (22–46) | 33 (24–44) | 33 (24–44) | 29 (22–41) | <0.0001 |
| Days of hospital stay c median (IQR) | 6 (4–10) | 6 (4–9) | 6 (4–9) | 5 (3–8) | 5 (3–8) | 0.17 |
| 30‐day outcomes | ||||||
| Surgical complication | 409 (13) | 382 (13) | 427 (14) | 407 (12) | 364 (11) | 0.23 |
| Treated in intensive care | 152 (5.0) | 141 (4.7) | 134 (4.3) | 108 (3.3) | 97 (3.1) | 0.57 |
| Reoperation | 229 (7.6) | 223 (7.5) | 245 (7.9) | 234 (7.2) | 201 (6.3) | 0.20 |
| Readmission | 256 (8.4) | 203 (6.8) | 254 (8.2) | 297 (9.1) | 264 (8.3) | 0.29 |
| Death | 41 (1.4) | 50 (1.7) | 40 (1.3) | 44 (1.4) | 34 (1.1) | 0.36 |
Abbreviation: N.A, not applicable.
All stomas created, including stoma as the only procedure and permanent and diverting stoma at resection surgery.
Information on acute or elective surgery missing in two patients in 2016, one patient in 2017 and two patients in 2020.
All resection surgery (acute and elective).
Restricted to patients diagnosed the same year.
DISCUSSION
Our study shows that despite a reduction in performed surgery during the first wave of COVID‐19 in Sweden in April–May 2020 this reduction was compensated for in June to December, and thus colon cancer treatment over the entire year was largely similar to 2019. We observed a change in treatment with more preoperative diverting ostomies and more preoperative oncological treatment, and an increase in the proportion of laparoscopic surgery.
There are, to date, no nationwide studies on colon cancer incidence for the entire year 2020. A study from England reported that 3500 fewer people had been diagnosed and treated for colorectal cancer than would have been expected from April to October 2020 [9]. In the Paris region in France, a 31% decrease in referrals for colon cancer was observed from March to May relative to the average of the previous 2 years, and an 8% decrease in June to October [21]. Similarly, a tertiary care hospital in Spain has reported an almost halved incidence of colorectal cancer diagnosis during the first period of the pandemic [11], and surveys from Italy a 62% reduction in cases from 9 March to 17 May 2020 [6]. New Zealand, which actively pursued elimination of COVID‐19 had a 40% decline in cancer registrations during a national shutdown in March–April 2020 but were back to preshutdown levels over the following months [22]. In Sweden there were 27% fewer colon cancer patients diagnosed during April–May 2020 compared to the year before, but this rapidly caught up during the rest of the year.
We did not observe any significant changes in surgical treatment of colon cancer in Sweden in year 2020, apart from an increase in the proportion of patients receiving a diverting stoma prior to resection surgery (6.1% compared to 4.4% the year before). More ostomies instead of anastomoses compared to the prepandemic period has also been reported from CovidSurg Collaborative, probably due to a wish to reduce complications [23]. In Sweden in 2020, proportionally more patients were diagnosed with locally advanced tumours, which also could explain the increase in use of preoperative diverting stoma.
The proportion of patients who were treated with laparoscopic surgery increased from 54% to 58%, contrary to England [9] and Korea [10], who reported a decline in procedures performed with the minimally invasive technique during the COVID‐19 pandemic. In the international survey from covidsurg Collaborative, the proportion of laparoscopic surgeries were comparable to the Swedish numbers (57.2%) [23]. The increase in laparascopic surgery may be due to a general trend and not connected to COVID‐19, although without the pandemic the increase might have been even greater.
Concerns have been raised that diagnostic delay due to the COVID‐19 pandemic may decrease resectability in colorectal cancer, but we found no evidence of this occurring in Sweden. In the present study, more patients were diagnosed in stage cT4 in 2020 compared with 2019, but this trend was also seen during the preceding years and can be explained by a smaller proportion of unstaged patients. It is possible that the relative increase in proportion of cT4 tumours was due to a reduction of cases with less advanced tumours, and therefore, fewer symptoms. If the increase in cT4 tumours is, in fact, a sign of stage migration due to diagnostic delay will become evident during the following years.
Our study showed no difference in complication rate, ICU care, or 30‐day mortality. This is in line with reports from Spain, where surgery was delayed but complications and mortality remained unchanged [24]. An international cohort study representing 40 countries, showed a higher 30‐day mortality rate during the COVID‐19 pandemic, but fewer anastomotic leakages. The mortality in case of anastomotic leakage was higher during the pandemic compared to the prepandemic period [23].
During the first wave of the COVID‐19 pandemic, Sweden experienced a 9% reduction in overall cancer cases and a general reduction in patients seeking care for other conditions than COVID‐19 [16]. The Swedish National Board of Health and Welfare published guidelines with the aim to guide physicians in prioritizing. All patients were to have an assessment of their condition. After the assessment, care could be delayed, but not for conditions that were assumed to have a large impact on life span or quality of life, that is, cancer care was prioritized [25]. This may explain why the quality of colon cancer treatment seems to have been maintained. An Australian review including data from 20 countries reported a 30% reduction in health care seeking during the pandemic [26]. For colon cancer patients in Sweden, the disruptions seem to have been mild. There was mainly a delay in diagnosis and treatment during the first wave of the pandemic due to the screening call off, and patient delay because of fear of COVID‐19, but there was a quick return to normal activity and an increase in the number of diagnosed cases and surgeries during the end of the year. Time from diagnosis to surgery was shorter in 2020 than in 2019, possibly as a consequence of the prioritization of cancer care over other diseases.
The strength of the study is the use of prospectively collected register data with high validity and near complete coverage. Limitations include lack of information of patient characteristics that are not included in the register, for example, lack of data on the COVID‐19 infection. Possible misclassification bias due to registration errors should be randomly distributed and not affect the results in any major way.
CONCLUSION
On a nationwide level, we did not observe major adverse effects of the COVID‐19 pandemic on colon cancer treatment and short time outcomes in Sweden when comparing year 2020 to preceding years, indicating that quality of care for these patients was maintained, but the long‐term consequences are not yet known. There were small increases in the use of preoperative chemotherapy and ostomies, which may have future implications for quality of life in these patients.
CONFLICT OF INTERESTS
All authors have nothing to declare.
AUTHOR CONTRIBUTIONS
Guarantor of article: Åsa H Everhov. Specific author contributions: Conception and design: All authors. Acquisition of data: Åsa H Everhov, Karolina Eklöv. Statistical analysis: Åsa H Everhov, Interpretation of data: All authors. Drafting the manuscript: Karolina Eklöv, Åsa H Everhov. Critical revision for intellectual content. All authors. Final approval: All authors. Accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
ETHICS STATEMENT
The study was approved by the Regional Ethics committee in Stockholm (Dnr 2020–04169). Since this was a strictly register‐based study, individual informed consent was not required.
Eklöv K, Nygren J, Bringman S, Löfgren J, Sjövall A, Nordenvall C, Colon cancer treatment in Sweden during the COVID‐19 pandemic: A nationwide register‐based study. Colorectal Dis. 2022;00:1–8. 10.1111/codi.16129
Funding information
This project was supported by grants from Bengt Ihre Research Fellowship. None of the funding organizations had any role in the design and conduct of the study; in the collection, management, and analysis of the data; or in the preparation, review, and approval of the manuscript.
DATA AVAILABILITY STATEMENT
KE and ÅHE had full access to data.
REFERENCES
- 1. Maringe C, Spicer J, Morris M, Purushotham A, Nolte E & Sullivan R et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21(8):1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sud A, Torr B, Jones ME, Broggio J, Scott S, Loveday C, et al. Effect of delays in the 2‐week‐wait cancer referral pathway during the COVID‐19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mason SE, Scott AJ, Markar SR, Clarke JM, Martin G, Winter Beatty J, et al. Insights from a global snapshot of the change in elective colorectal practice due to the COVID‐19 pandemic. PLoS One 2020;15(10):e0240397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cavaliere D, Parini D, Marano L, Cipriani F, Di Marzo F, Macrì A, et al. Surgical management of oncologic patient during and after the COVID‐19 outbreak: practical recommendations from the Italian society of surgical oncology. Updates Surg. 2020;73(1):321–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ren X, Chen B, Hong Y, Liu W, Jiang Q, Yang J, et al. The challenges in colorectal cancer management during COVID‐19 epidemic. Ann Transl Med 2020;8(7):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Vincentiis L, Carr RA, Mariani MP, Ferrara G. Cancer diagnostic rates during the 2020 'lockdown', due to COVID‐19 pandemic, compared with the 2018‐2019: an audit study from cellular pathology. J Clin Pathol. 2020;74:187–9. [DOI] [PubMed] [Google Scholar]
- 7. Nunoo‐Mensah JW, Rizk M, Caushaj PF, Giordano P, Fortunato R, Dulskas A, et al. COVID‐19 and the global impact on colorectal practice and surgery. Clin Colorectal Cancer 2020;19(3):178‐90. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang Z, Yan J, Jin T, Huang X, Zeng G, Adashek ML, et al. The challenges of urgent radical sigmoid colorectal cancer resection in a COVID‐19 patient: a case report. Int J Surg Case Rep. 2020;71:147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris EJA, Goldacre R, Spata E, Mafham M, Finan PJ & Shelton J et al. Impact of the COVID‐19 pandemic on the detection and management of colorectal cancer in England: a population‐based study. Lancet Gastroenterol Hepatol. 2021;6(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi JY, Park IA‐O, Lee HG, Cho EA‐O, Kim YA‐O, Kim CA‐O, et al. Impact of the COVID‐19 Pandemic on Surgical Treatment Patterns for Colorectal Cancer in a Tertiary Medical Facility in Korea. Cancers (Basel). 2021;13(9):2221. 10.3390/cancers13092221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suárez J, Mata E, Guerra A, Jiménez G, Montes M, Arias F, et al. Impact of the COVID‐19 pandemic during Spain's state of emergency on the diagnosis of colorectal cancer. J Surg Oncol. 2020;123:32–6. [DOI] [PubMed] [Google Scholar]
- 12. Socialstyrelsen . Analys av första och andra covid‐19‐vågen – produktion, köer och väntetider i vården. [cited 2021 May 01]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/artikelkatalog/ovrigt/2021‐5‐7371.pdf
- 13. Samverkan RCi . Uppskjuten cancervård ‐Jämförelse av antalet nyregistrerade cancerfall under covid‐19‐pandemin 2020 och motsvarande period 2019. [cited 2021 May 01]. Available from: https://cancercentrum.se/globalassets/covid‐19/rapport_uppskjuten_cancervard_covid19‐varen2020_vers1.0.pdf
- 14. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies conducted using observational routinely‐collected health data (RECORD) statement. PLoS Med 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anell A. The public–private pendulum — patient choice and equity in Sweden. N Engl J Med. 2015;372(1):1–4. [DOI] [PubMed] [Google Scholar]
- 16. RCC . Report from Swedish cancer center 2021. [cited 2021 Aug 16]. Available from: https://cancercentrum.se/globalassets/covid‐19/uppskjuten_cancervard_covid19_delrapport3.pdf
- 17. RCC . Swedish nationel guidelines for CRC temporarily during covid 19 pandemic 2020 [cited 2021 May 01]. Available from: https://cancercentrum.se/globalassets/covid‐19/kolorektalcancer_avvikelse_covid19_10november20.pdf
- 18. Pesola F, Eloranta S, Martling A, Saraste D, Smedby KE. Family history of colorectal cancer and survival: a Swedish population‐based study. J Intern Med. 2020;287(6):723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moberger P, Sköldberg F, Birgisson H. Evaluation of the Swedish colorectal cancer registry: an overview of completeness, timeliness, comparability and validity. Acta Oncol. 2018;57(12):1611–21. [DOI] [PubMed] [Google Scholar]
- 20. Bolliger M, Kroehnert JA, Molineus F, Kandioler D, Schindl M, Riss P. Experiences with the standardized classification of surgical complications (Clavien‐Dindo) in general surgery patients. Eur Surg. 2018;50(6):256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Priou S., Lamé G, Chatellier G, Tournigand C, Kempf E. Effect of the COVID‐19 pandemic on colorectal cancer care in France. Lancet Gastroenterol Hepatol. 2021;6(5):342–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gurney JK, Millar E, Dunn A, Pirie R, Mako M & Manderson J et al. The impact of the COVID‐19 pandemic on cancer diagnosis and service access in New Zealand‐a country pursuing COVID‐19 elimination. Lancet Reg Health. 2021;10:100127. 10.1016/j.lanwpc.2021.100127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhangu A, Li E, Fisher A, Manku B. Outcomes from elective colorectal cancer surgery during the SARS‐CoV‐2 pandemic. Colorectal Dis. 2020. Epub ahead of print. 10.1111/codi.15431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tejedor P, Simó V, Arredondo J, López‐Rojo I, Baixauli J, Jiménez LM, et al. The impact of SARS‐CoV‐2 infection on the surgical management of colorectal cancer: lessons learned from a multicenter study in Spain. Rev Esp Enferm Dig 2020;113:85–91. [DOI] [PubMed] [Google Scholar]
- 25.Nationella principer för prioritering av rutinsjukvård under covid‐19‐pandemin 2020. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint‐dokument/dokument‐webb/ovrigt/nationella‐principer‐for‐prioritering‐av‐rutinsjukvard‐covid19.pdf
- 26. Moynihan R, Sanders S, Michaleff ZA, Scott AM, Clark J, To EJ, et al. Impact of COVID‐19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open 2021;11(3):e045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
KE and ÅHE had full access to data.


