Abstract
Background
We investigated the ocular surface disturbances in COVID‐19 patients discharged from the hospital.
Methods
One hundred and seventy‐nine eyes of 109 healthy participants and 456 eyes of 228 post‐COVID‐19 patients received comprehensive eye examinations; the latter were interviewed with questionnaires on ocular symptoms before and after COVID‐19 diagnosis. Associations of ocular surface manifestations with virological and ophthalmic parameters were evaluated by multivariable mixed linear or logistic regression models.
Results
Mean interval between COVID‐19 diagnosis and ophthalmic evaluation was 52.23 ± 16.12 days. The severity of meibomian gland dysfunction (MGD) based on clinical staging was higher in post‐COVID‐19 than healthy eyes (1.14 ± 0.67 vs. 0.92 ± 0.68, p = 0.002) and so was ocular surface staining score (0.60 ± 0.69 vs. 0.49 ± 0.68, p = 0.044). Patients requiring supplementary oxygen during hospitalisation had shorter tear break‐up time (β −1.63, 95% CI ‐2.61 to −0.65). Cycle threshold (Ct) value from upper respiratory samples (inversely correlated with viral load) at diagnosis had an OR = 0.91 (95% CI 0.84–0.98) with new ocular surface symptoms 4 weeks after diagnosis. The presence of ocular surface symptoms 1 week prior to COVID‐19 diagnosis showed an OR of 20.89 (95% CI 6.35–68.66) of persistent or new ocular symptoms 4 weeks afterward.
Conclusions
MGD and ocular surface staining are more common and severe in post‐COVID‐19 patients. Patients with higher viral loads have greater risks of ocular surface symptoms. Patients requiring supplementary oxygen are more likely to show tear film instability. Ocular surface evaluation should be considered 1–3 months following hospital discharge for any COVID‐19 patient.
Keywords: meibomian gland dysfunction, ocular surface, COVID‐19, tear break up time
1. INTRODUCTION
By the end of October 2021, COVID‐19 caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) had resulted in more than 230 million confirmed cases and over 4.6 million deaths worldwide. Angiotensin‐converting enzyme, the receptor for SARS‐CoV‐2 and transmembrane serine protease 2, a cell surface‐associated membrane protein that facilitates viral entry, are highly expressed along the respiratory tract. 1 These viral entry factors were present on the cornea and conjunctival epithelia from human cell lines and postmortem tissues. 2 , 3 The ocular surface mucosa has been a possible route of respiratory infection and transmission. Virus may enter the ocular surface via droplets and pass through the nasolacrimal duct down into the respiratory tract. 3 , 4 A 63% concordance was found between conjunctival and nasopharyngeal reverse transcription polymerase chain reactions (RT‐PCR) when swabbed within 2 days of one another. 5 Ocular manifestations have been reported with a prevalence ranging from 0.8% to 31.6%. 6 , 7 However, the detection of SARS‐CoV‐2 from tear and conjunctival sampling using RT‐PCR showed an inconsistent relationship with the ocular surface manifestations in different reports. 8 , 9 The heterogeneity may be related to the timing and method of sampling, detection and a change from acute viral conjunctivitis to post‐infectious ocular surface disturbances.
‘Long‐COVID’ describes the persistent or new symptoms 4 weeks or more after acute COVID‐19 in a subset of survivors. The multisystem manifestations after the acute infection have been the subject of investigation while the potential long‐term sequelae remain unknown. 10 The WHO Clinical Case Definition Working Group on Post‐COVID‐19 Condition defined ‘long‐ COVID’ symptoms as those that arise within 3 months from the disease diagnosis. 11 Understanding on the ocular surface consequences of post‐COVID‐19 is also limited. In a meta‐analysis of 16 studies involving 2347 acute COVID‐19 patients, a variety of ocular complications was detected: 11.64% of COVID‐19 patients had ocular surface manifestations including 31.2% ocular pain, 19.2% discharge, 10.8% redness and 7.7% follicular conjunctivitis. 12 A recent cohort study on patients examined at a mean of 36.1 days after hospital discharge reported a greater ocular surface disease index (OSDI) score, lower Schirmer's test results and shorter tear break‐up times (TBUT) as compared to the control group. 13 In Hong Kong, all patients with COVID‐19 are admitted to publicly funded hospitals under the Hospital Authority (HA) for management. The purpose of this study is to evaluate the ocular surface manifestations of post‐COVID‐19 patients after hospital discharge as compared to healthy controls and identify the associated risk factors.
2. METHODS
2.1. Study subjects
Consecutive COVID‐19 patients aged 18 and above admitted to two regional HA hospitals (the Prince of Wales Hospital or the United Christian Hospital) in Hong Kong between February 2020 and December 2020 were invited for ophthalmic evaluation on their first follow‐up visits after discharge from the hospital. The length of stay was defined as the period from hospital admission to discharge. Patients discharged before 6 July 2020, were required to have 2 negative nasopharyngeal swabs SARS‐CoV‐2 RT‐PCR tests taken at least 24 h apart before hospital discharge. Patients had to receive RT‐PCR tests every 2–3 days until the nasopharyngeal swab results were negative. The discharge criteria were modified on 6 July 2020, including any detectable SARS‐CoV‐2 anti‐nucleoprotein immunoglobulin G (IgG) antibodies in the serum regardless of a positive respiratory specimen RT‐PCR result. Discharged patients were evaluated 4–12 weeks after COVID‐19 diagnosis. Healthy controls had no prior history of COVID‐19 who attended our eye clinics for general eye checkups or cataract surgery. We excluded eyes with the previous history of refractive surgery and ocular trauma, ophthalmic surgery within the past year, contact lens wear within the past 3 months, pre‐existing ocular surface conditions (e.g., dry eyes, allergic conjunctivitis, pterygium and cicatricial conjunctivitis) or eyes on topical medications (e.g., topical lubricants or glaucoma eye drops). SARS‐CoV‐2 infection was confirmed by two consecutive RT‐PCR tests targeting different regions of the RdRp gene performed by the local Public Health Laboratory Service. SARS‐CoV‐2 viral load was represented by cycle threshold (Ct) values from RT‐PCR of the respiratory specimen collected on admission. The RT‐PCR test amplifies the viral RNA from the sample until a detectable concentration exceeds the threshold value: the Ct value is equal to the number of cycles necessary for this. In other words, the lower the Ct value of a sample, the higher the viral load. 14
2.2. Ophthalmic examinations
All participants underwent comprehensive eye examination, including corrected distance visual acuity (CDVA), slit‐lamp examination, applanation tonometry and dilated fundus biomicroscopy by two ophthalmologists masked to the course of COVID‐19 and laboratory results. Fluorescein impregnated strips were wetted with a drop of non‐preserved saline solution and were instilled into the inferior fornix. The patient was asked to blink three or four times and was examined using a slit lamp with a cobalt blue light for 30 s. The duration from the last complete blink to the first break in the precorneal fluorescein solution was defined as TBUT. TBUT was measured three times consecutively after the instillation of a drop of fluorescein and the mean value was recorded. Corneal staining was then scored semi‐quantitatively using the van Bijsterveld score for the entire cornea: 0: none; 1: sparsely scattered; 2: densely scattered; 3: confluent spots. 15 Clinical severity of meibomian gland dysfunction (MGD) was graded as proposed by The International Workshop on Meibomian Gland Dysfunction from Stages 1 to 4, 16 or 0 if MGD was absent. In Stage 1, there are MGD signs with a meibum quality score of 2 to <4, meibum expressibility score of 1 and without staining. In Stage 2, there are scattered lid margin features with meibum quality score of 4 to <8, meibum expressibility score of 1, in Stage 3, there is plugging of the gland orifices and vascularity at lid margin with a meibum quality score of 8 to <13, meibum expressibility score of 2; in Stage 4 there are meibomian gland dropout or gland displacement with a meibum quality score of more than 13, meibum expressibility score of 3. 16 Conjunctival follicles and papillae were scored on a 4‐point scale as proposed by Fukushima et al (for follicles: Grade 3 = 20 or more follicles; Grade 2 = 10–19 follicles; Grade 1 = 1–9 follicles; Grade 0 = none, for papillae: Grade 3 = papillae size: 0.6 mm or more; Grade 2 = papillae size: 0.3–0.5 mm; Grade 1 = papillae size: 0.1–0.2 mm; Grade 0 = none). 17 , 18 Patients with severe follicular and papillary changes had conjunctival swabs taken for RT‐PCR at the time of ophthalmic examination.
2.3. Questionnaires
Based on subjective assessment, post‐COVID‐19 patients completed a self‐administered questionnaire at their ophthalmic evaluation after hospital discharge enquiring about the presence of the ocular symptoms including eye redness, swelling, itch, pain, burning sensation, grittiness, photophobia, tearing, discharge or blurring that developed 1 week before their diagnosis to 4 weeks after their diagnosis (Appendix A). Demographics, laboratory results and medical history were retrieved from the electronic medical records from the Hong Kong Hospital Authority Clinical Management System. The study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committees and was conducted following the Declaration of Helsinki; all patients provided written consent.
2.4. Statistical analysis
Statistical analyses were performed using Stata (version 16.0; Stata Corporation, TX). Data were presented as mean with standard deviation (SD) or number with percentage as appropriate. Variables between the convalescent and control groups were compared using a mixed linear or mixed ordinal logistic regression model adjusted for the age and sex with random intercept (random effects) at the subject level. Any ocular surface symptom associated with demographic, virological and clinical parameters were evaluated using a logistic regression model at the patient level. The associations of TBUT, grading of ocular surface staining, MGD staging, papillae and follicles with demographic and clinical parameters were analysed with a mixed linear or mixed ordinal logistic regression model with random intercept (random effects) at the subject level to adjust for the correlation between fellow eyes.
3. RESULTS
3.1. Clinical features
Three hundred and four post‐COVID‐19 patients were enrolled, 76 patients were excluded from the analysis because they were on ocular lubricants (n = 16), anti‐glaucoma eyedrops (n = 5), contact lens within the past 3 months (n = 14), had prior history of allergic conjunctivitis (n = 5), refractive surgery (n = 25), ocular trauma (n = 4), underwent ocular surgery or laser within the past year (n = 4) or found to have pterygium or corneal scar (n = 3). After exclusion, a total of 228 (49% females, mean age 47.25 ± 17.01 years) post‐COVID‐19 patients and 109 (64% females, mean age 51.96 ± 24.36 years) healthy individuals were included (Table 1). There were no significant differences in age (p = 0.086) between the groups but more females (p = 0.009) in the control group. Among the convalescent COVID‐19 patients, 120 patients (52.63%) wore spectacles, 49 patients (21.49%) had hypertension, 30 (13.16%) had diabetes and 7 (3.07%) had autoimmune disorders. The length of hospital stay was 16.67 ± 11.12 days (range: 1–56 days), respiratory symptoms were present in 179 (78.5%), 30 (13.2%) required supplementary oxygen and 12 (5.3%) were admitted to intensive care unit (ICU) during the acute phase. The mean duration from diagnosis to the ophthalmic evaluation was 52.23 ± 16.12 days (range: 29–120 days), corresponding to a mean time from hospital discharge to an ophthalmic examination of 35.56 ± 15.43 days.
TABLE 1.
Demographics and clinical features of the study cohort
| Variables | Post‐COVID‐19 | Healthy | p |
|---|---|---|---|
| Patient/eye, n | 228/456 | 109/179 | |
| Age (year) | 47.26 ± 17.01 | 51.96 ± 24.36 | 0.086 |
| Female, n (%) | 112 (49.12%) | 70 (64.22%) | 0.009 |
| Duration of hospital admission (days) | 16.67 ± 11.16 | ||
| Time from discharge to assessment (days) | 35.57 ± 15.43 | ||
| Time from diagnosis to assessment (days) | 52.23 ± 16.12 | ||
| ICU admission, n (%) | 12 (5.26%) | ||
| Supplementary oxygen, n (%) | 30 (13.16%) | ||
| Respiratory symptoms, n (%) | 179 (78.51%) | ||
| Ct value | 22.54 ± 5.73 | ||
| CDVA (logMAR) | −0.01 ± 0.18 | 0.00 ± 0.13 | 0.286* |
| IOP (mmHg) | 17.11 ± 3.41 | 16.71 ± 3.53 | 0.194* |
| TBUT (s) | 4.48 ± 2.33 | 5.09 ± 2.65 | 0.070* |
| Ocular surface staining score 15 | 0.60 ± 0.69 | 0.49 ± 0.68 | 0.044* |
| MGD stage 16 | 1.14 ± 0.67 | 0.92 ± 0.68 | 0.002* |
| Papillae grading 17 , 18 | 0.57 ± 0.64 | 0.49 ± 0.56 | 0.284* |
| Follicles grading 17 , 18 | 0.44 ± 0.67 | 0.34 ± 0.58 | 0.029* |
Abbreviations: CDVA, corrected distance visual acuity; Ct, cycle threshold; ICU, intensive care unit; IOP, intraocular pressure; MGD, meibomian gland dysfunction; SD, standard deviation; TBUT, tear breakup time.
Adjusted for age and sex.
3.2. Ophthalmic features
Ophthalmic examination revealed a mean CDVA (logMAR) of −0.01 ± 0.18 and mean intraocular pressure (IOP) of 17.11 ± 3.41 mmHg for the 456 eyes of the 228 patients, comparable to the CDVA (0.00 ± 0.13) from 179 eyes of 109 healthy controls and their IOP (16.71 ± 3.53 mmHg) (p ≥ 0.194). Cotton wool spots were present in six eyes of four patients and retinal microhemorrhages in one eye of a patient. None of the healthy individuals had cotton wool spots or retinal haemorrhages on fundal exam (Table 1). MGD was present in 387 eyes (84.9%), conjunctival papillae in 222 eyes (48.7%) and follicles in 154 eyes (33.8%) of the patients. The prevalence of MGD was significantly higher in the eyes of patients than those of the healthy eyes (n = 130, 72.6%, p = 0.006), while the presence of papillary (n = 82, 45.8%, p = 0.624) or follicular changes (n = 50, 27.9%, p = 0.176) was not. The mean grades of MGD were higher (p = 0.002) for the convalescent (1.14 ± 0.67) than healthy eyes (0.92 ± 0.68), but the TBUT was similar (p = 0.070) between the two groups (4.84 ± 2.33 vs. 5.09 ± 2.65, respectively). Likewise, in the convalescent and healthy eyes, the mean grades of follicles were higher (p = 0.029) in the former (0.44 ± 0.67) than the latter (0.34 ± 0.58), while the papillae grading were comparable between the two groups: 0.57 ± 0.64 and 0.49 ± 0.56, respectively (p = 0.284). The presence of any positive ocular surface staining was greater in the convalescent than healthy eyes (223 eyes (48.9%) vs. 69 eyes (38.6%), respectively, p = 0.038). Notably, the corneal staining score was also higher in the former (0.60 ± 0.69 vs. 0.49 ± 0.68, p = 0.044). Table 1 summarised the comparison between the two groups. The RT‐PCR for SARS‐CoV‐2 was negative for eyes with severe conjunctival papillae and/or follicles. No patient or healthy participants had conjunctival hyperemia or chemosis.
Based on self‐reporting through questionnaires, 32 (14.0%) convalescent patients had at least one new‐onset ocular symptom 1 week prior to diagnosis of COVID‐19, while 49 (21.5%) had those symptoms 4 weeks following diagnosis (Figure 1). The most commonly reported symptom was itchy eyes (8.3%) before and blurred vision (9.2%) after the COVID‐19 diagnosis. After multivariate adjustment, those patients with higher Ct values (indicative of lower viral load) at diagnosis showed an OR 0.91 (95% CI 0.84–0.98) for ocular surface symptoms within 4 weeks following diagnosis. Any new‐onset ocular surface symptom 1 week prior to diagnosis showed an OR of 20.89 (95% CI 6.35–68.66) for persistent or other new ocular symptoms. Demographics, spectacle‐wearing and other clinical characteristics covariates were not significant in the model (Table 2).
FIGURE 1.
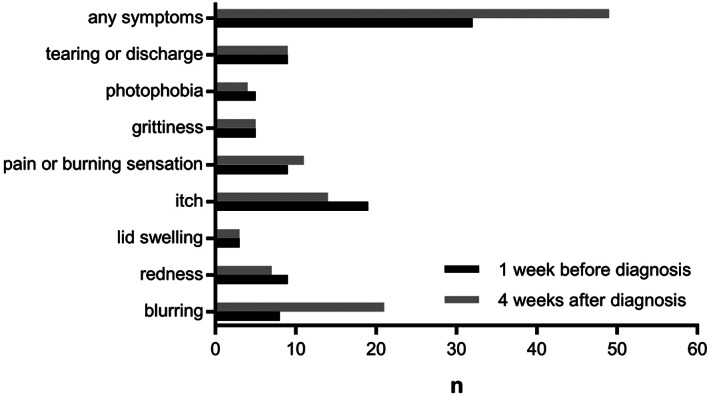
Number of patients with self‐reported symptoms 1 week before and 4 weeks after COVID‐19 diagnosis
TABLE 2.
Multivariable logistic regression model evaluating the association between any ocular symptoms 4 weeks after diagnosis with demographics and clinical characteristics in post‐COVID‐19 patients
| OR (95% CI) | p | |
|---|---|---|
| Age | 1.00 (0.97–1.03) | 0.955 |
| Sex (female) | 0.75 (0.32–1.76) | 0.514 |
| Spectacle wear | 1.14 (0.48–2.7) | 0.761 |
| Hypertension | 1.16 (0.39–3.42) | 0.791 |
| Diabetes | 1.18 (0.32–4.34) | 0.807 |
| Autoimmune disease | 1.22 (0.15–10.13) | 0.854 |
| ICU admission | 1.26 (0.23–6.96) | 0.788 |
| Supplementary oxygen | 2.80 (0.81–9.69) | 0.105 |
| Respiratory symptoms | 1.12 (0.35–3.55) | 0.847 |
| Ct value | 0.91 (0.84–0.98) | 0.020* |
| Any ocular symptoms 1 week before diagnosis | 20.89 (6.35–68.66) | <0.001* |
Abbreviations: CI, confidence interval; Ct, Cycle threshold; ICU, intensive care unit; OR, odds ratio.
Statistically significant.
Among the convalescent COVID‐19 patients, after multivariable adjustments for age, sex, underlying hypertension, diabetes, autoimmune disease and time after hospital discharge, patients requiring supplementary oxygen were associated with a lower TBUT (β −1.63, 95% CI ‐2.61 to −0.65). None of the other demographics and clinical characteristics covariates including spectacles wear, ICU admission, presence of respiratory symptoms, Ct value or presence of ocular symptoms showed significant association with the ocular surface signs (Table 3).
TABLE 3.
Mixed linear model or mixed ordinal logistic regression model evaluating the association of ocular surface signs with demographics and clinical characteristics in post‐COVID‐19 patients
| Tear breakup time | Corneal staining score | Meibomian gland dysfunction grading | Papillae grading | Follicles grading | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Glasses wear | −0.05 (−0.63–0.53) | 0.875 | 1.73 (0.7–4.29) | 0.239 | 0.74 (0.27–2.01) | 0.553 | 1.39 (0.43–4.5) | 0.584 | 2.80 (0.63–12.37) | 0.175 |
| Autoimmune disease | −1.24 (−2.89–0.41) | 0.141 | 1.36 (0.11–17.4) | 0.812 | 0.30 (0.02–4.85) | 0.394 | 0.47 (0.02–12.55) | 0.653 | 0.24 (0–15.53) | 0.500 |
| ICU admission | 1.28 (−0.17–2.72) | 0.083 | 1.00 (0.11–8.84) | 0.998 | 1.46 (0.12–17.68) | 0.765 | 1.86 (0.10–33.43) | 0.674 | 2.93 (0.07–119.29) | 0.569 |
| Supplementary oxygen | −1.63 (−2.61 ‐ ‐0.65) | 0.001* | 0.78 (0.17–3.56) | 0.753 | 0.38 (0.07–2.13) | 0.274 | 0.16 (0.02–1.19) | 0.073 | 1.78 (0.13–24.59) | 0.667 |
| Respiratory Symptoms | −0.53 (−1.28–0.22) | 0.168 | 1.23 (0.38–3.99) | 0.726 | 1.02 (0.28–3.73) | 0.979 | 1.15 (0.25–5.26) | 0.853 | 0.58 (0.09–3.85) | 0.572 |
| Ct value | 0.01 (−0.04–0.07) | 0.606 | 1.02 (0.94–1.11) | 0.633 | 0.95 (0.86–1.04) | 0.248 | 0.93 (0.83–1.04) | 0.192 | 0.89 (0.78–1.03) | 0.111 |
| Any ocular symptoms a | 0.20 (−0.45–0.86) | 0.544 | 1.96 (0.69–5.6) | 0.208 | 2.05 (0.65–6.45) | 0.221 | 1.44 (0.37–5.6) | 0.597 | 0.8 (0.15–4.28) | 0.798 |
Note: Covariates including age, sex, hypertension, diabetes, autoimmune disease, time after hospital discharge were adjusted for in these models.
Abbreviations: CI, confidence interval; OR, odds ratio.
Any ocular surface symptoms 1 week before or 4 weeks after COVID‐19 diagnosis.
Statistically significant.
4. DISCUSSION
As the number of COVID‐19 survivors continues to grow, it is paramount to understand the spectrum of multi‐systemic manifestations from acute to long COVID state. Long‐COVID is now recognised as a multi‐organ disease with increasing reports on persistent and new consequences including fatigue, dyspnea, chest pain, cognitive disturbances and arthralgia after acute COVID‐19. Cellular damage, inflammatory cytokine production from robust innate immune response and a pro‐coagulant state induced by SARS‐CoV‐2 may contribute to these sequelae. 19
Guideline for managing long‐COVID has been jointly developed by the National Institute for Health and Care Excellence (NICE), the Scottish Intercollegiate Guidelines Network (SIGN) and the Royal College of General Practitioners (RCGP). In long‐COVID patients, a recent in vivo confocal microscopy study revealed loss of small corneal nerve fibre and increased dendritic cell density at a mean of 3.7 months after diagnosis compared to healthy eyes. 20 Optical coherence tomography angiography showed a reduction of vessel density in the retinal superficial and deep capillary plexuses in COVID‐19 patients at a mean of 4.1 months after recovery compared to controls. 21 In post‐COVID‐19 patients assessed at a mean of 60 days, 87.4% reported the persistence of at least one systemic symptom. 22
To our knowledge, this present study is the largest cohort assessing the ocular surface consequences of adult patients after recovery from COVID‐19. At a mean evaluation of 52.23 days after diagnosis, we found a higher prevalence and mean grade of MGD in post‐COVID‐19 eyes than healthy eyes. Eyes with any ocular surface staining and the mean ocular staining score were also greater in the post‐COVID‐19 patients. There was no difference in TBUT between patients and controls. This observation is similar to the results of the Dry Eye Assessment and Management (DREAM) study, which reported no association between meibomian gland features and the severity of dry eye signs. 23 Using subjective recall during a telephone interview, Hong et al reported a significant increase of OSDI from 6.25 to 6.82 in 56 COVID‐19 patients before and after disease. Among them, 15 (27%) had worsening ocular symptoms and 6 (11%) had prodromal ocular symptoms prior to respiratory or systemic symptoms. 24 In a prospective cohort, 7 of the 50 patients (14%) reported ocular symptoms, but none was found to have any ocular surface sign upon slit lamp evaluation during hospitalisation. 25 During a subsequent follow‐up which included 27 patients (54%) examined at a median of 55 days (range: 38–205 days), no patients reported residual or new‐onset ocular symptoms. However, conjunctival follicles and papillae were found bilaterally in only one patient. 25 Unlike our study that excluded patients with self‐reported dry eye symptoms before COVID‐19 diagnosis, Costa et al reported signs and symptoms of dry eye disease in 11% of a cohort of 64 patients evaluated 82 days after the onset of COVID‐19's symptoms. Of note, 25% of these 64 patients had a history of dry eye symptoms prior to COVID‐19 diagnosis. 26
Patients often receive supplementary oxygen via nasal cannula mask or mechanical ventilation when admitted to ICU. The positive pressure from mechanical ventilation and tight endotracheal tube taping increases jugular venous pressure, which in turn decreases venous return causing fluid sequestration in the conjunctiva. 27 , 28 , 29 A high positive end‐expiratory pressure during mechanical ventilation further encourages sodium and water retention, worsening chemosis. 27 , 28 , 29 COVID‐19 patients may be nursed prone to improve the ventilation to perfusion ratio. This would cause facial and conjunctival edema due to venous pooling in the dependent area. Muscle relaxant and sedation used during ICU admission impair blink reflex and eyelid muscle tone, thus increasing the risk of ocular surface damage. Gas flow from oxygen delivery can cause evaporation of the tear film. Airflow from the supplementary oxygen delivery increases tear aqueous evaporation. Increased airflow of 1 m/s for 30 min showed a significant reduction in TBUT from 14.5 to 9.6 s. 30 High airflow of 1.5 m/s for only 5 min in a controlled environment chamber was shown to decrease the tear meniscus height and area measured with anterior segment optical coherence tomography. 31 In our multivariable regression model, supplementary oxygen was independently associated with a reduction of 1.6 s in TBUT after adjusting for other covariates including ICU admission, Ct level and the presence of respiratory or ocular symptoms (Table 3). A longitudinal follow‐up would be helpful to evaluate the long‐term effect on tear film following COVID‐19, particularly in those that had received supplementary oxygen. 27 , 28 , 29
Although we did not evaluate the non‐ophthalmic consequences of COVID‐19 in patients after hospital discharge, one of the largest cohort studies (n = 1733) with follow‐up duration at 6 months by Huang et al reported that patients requiring oxygen via high‐flow nasal cannula (HFNC), non‐invasive mechanical ventilation (NIV) or invasive mechanical ventilation had an increased risk of depression or anxiety (OR = 1.77). 32 The association between depression, anxiety and dry eyes is well documented, 33 and these findings by Huang et al corroborate with poor tear film stability in COVID‐19 patients requiring supplementary oxygen in the present study. The use of supplemental oxygen in any form clinically suggests a more severe course of COVID‐19 during the acute episode. 32 SARS‐CoV‐2 viral infection may alter the ocular surface microenvironment, including microbiome and can lead to ocular surface sequelae including papillary or follicular conjunctival changes, MGD and evaporative dry eye. 34 , 35 While conjunctival papillary and/or follicular changes may not carry long‐term consequences, ocular surface should be evaluated, with attention to the development or worsening of MGD.
The viral load of SARS‐CoV‐2 is inversely correlated to the Ct value. Our multivariable regression models adjusted for the covariate effect from morbidities such as severe pneumonia and ICU. Ct value is the only variable independently associated with any ocular symptoms 4 weeks within the diagnosis. An OR of 0.91 for Ct value indicates that higher viral load is associated with a greater likelihood of having ocular surface manifestations (Table 2). Chen et al evaluated the ocular symptoms of 534 patients with mild COVID‐19 via retrospective self‐reporting and found conjunctival congestion in 3.8%, increased conjunctival secretion in 10.6%, ocular pain in 5.7%, foreign body sensation in 19% and increased tearing in 13.3% of the patients. 36 Pre‐existing ophthalmic conditions were also reported, with coexisting dry eye in 31.2% of patients, history of conjunctivitis in 7.6%, keratitis in 4.2% and xerophthalmia in 8%. In their multivariable regression model, the frequency of hand‐eye contact was associated with conjunctival congestion. 36 Similar to our results, spectacle‐wearing was not associated with ocular surface changes.
Our study has several limitations. First, the ocular surface conditions before COVID‐19 were unavailable. Consequently, to minimise the impact of confounding ophthalmic conditions that may influence our findings, patients with history of refractive surgery, ocular trauma, ocular surgery, pre‐existing ocular surface disease (including known dry eye disease), use of any eye drops (including artificial lubricants) were excluded. Second, the patient‐reported symptoms were collected via a self‐administered questionnaire after discharge from the hospital, which was subjected to recall bias. Our questionnaire did not specifically focus on dry eyes as the ocular surface was hypothesised to be a potential transmission route. Thus future studies focusing on dry eyes should consider the use of standardised dry eyes related questionnaires (i.e., OSDI, SPEED or DEQ‐5). The retrospective nature of these questionnaires, administered after hospital discharge due to infectious control measures, are subject to recall bias. Furthermore, the ocular surface symptoms could be mixed and complicated and thus the questionnaire will be re‐designed accordingly in our future studies. Third, we did not assess the ocular surface symptoms and manifestations during the acute, hospitalised episode. It was not feasible due to the hospitals' infection control policy. Finally, we did not collect conjunctival swabs for SARS‐CoV‐2 for persistent viral shedding, tears for inflammatory cytokines and Schirmer's test from every patient at the time of ocular surface evaluation.
Ocular surface disturbances such as MGD and ocular surface staining were more common and severe in post‐COVID‐19 patients than healthy individuals. Patients with a higher viral load on presentation and those who required supplementary oxygen were at risk of developing new ocular surface symptoms and having reduced TBUT, respectively, during the convalescent period. Ocular surface evaluation should be considered for post‐COVID patients 1–3 months following hospital discharge.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
APPENDIX A. OCULAR SURFACE SYMPTOMS QUESTIONNAIRE
| New onset within 1 week before COVID‐19 diagnosis | New onset or persistent within 4 weeks after COVID‐19 diagnosis | |
|---|---|---|
| Tearing or discharge | ||
| Photophobia | ||
| Grittiness | ||
| Pain or burning sensation | ||
| Itch | ||
| Lid swelling | ||
| Redness | ||
| Blurring |
Note: Did you experience any of the following? (check if yes).
Wan KH, Lui GCY, Poon KCF, et al. Ocular surface disturbance in patients after acute COVID‐19. Clin Experiment Ophthalmol. 2022;50(4):398-406. doi: 10.1111/ceo.14066
Funding informationThis work was supported by Health and Medical Research Fund, Hong Kong SAR, project number COVID190106.
REFERENCES
- 1. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS‐CoV‐2 receptor ACE2 is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016, e19‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS‐CoV‐2 infection. Ocul Surf. 2020;18:537‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wan KH, Huang SS, Young AL, Lam DSC. Precautionary measures needed for ophthalmologists during pandemic of the coronavirus disease 2019 (COVID‐19). Acta Ophthalmol. 2020;98:221‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qing H, Li Z, Yang Z, et al. The possibility of COVID‐19 transmission from eye to nose. Acta Ophthalmol. 2020;98:e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azzolini C, Donati S, Premi E, et al. SARS‐CoV‐2 on ocular surfaces in a cohort of patients with COVID‐19 from the Lombardy region, Italy. JAMA Ophthalmol. 2021;139:956‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan W‐j, Ni Z‐y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID‐19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arora R, Goel R, Kumar S, et al. Evaluation of SARS‐CoV‐2 in tears of patients with moderate to severe COVID‐19. Ophthalmology. 2021;128:494‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou Y, Duan C, Zeng Y, et al. Ocular findings and proportion with conjunctival SARS‐COV‐2 in COVID‐19 patients. Ophthalmology. 2020;127:982‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post‐covid syndrome in individuals admitted to hospital with COVID‐19: retrospective cohort study. BMJ. 2021;372:n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post‐COVID‐19 condition by a Delphi consensus. Lancet Infect Dis. 2021. doi: 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aggarwal K, Agarwal A, Jaiswal N, et al. Ocular surface manifestations of coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. PLoS One. 2020;15:e0241661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gambini G, Savastano MC, Savastano A, et al. Ocular surface impairment after COVID‐19: a cohort study. Cornea. 2020;40:477‐483. [DOI] [PubMed] [Google Scholar]
- 14. Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS‐CoV‐2 in infected patients. Clin Infect Dis. 2020;71:793‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Bijsterveld OP. Diagnostic tests in the sicca syndrome. Arch Ophthalmol. 1969;82:10‐14. [DOI] [PubMed] [Google Scholar]
- 16. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on Meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922‐1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fukushima A, Ohashi Y, Ebihara N, et al. Therapeutic effects of 0.1% tacrolimus eye drops for refractory allergic ocular diseases with proliferative lesion or corneal involvement. Br J Ophthalmol. 2014;98:1023‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyazaki D, Fukushima A, Ohashi Y, et al. Steroid‐sparing effect of 0.1% tacrolimus eye drop for treatment of shield ulcer and corneal Epitheliopathy in refractory allergic ocular diseases. Ophthalmology. 2017;124:287‐294. [DOI] [PubMed] [Google Scholar]
- 19. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27:601‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bitirgen G, Korkmaz C, Zamani A, et al. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br J Ophthalmol. 2021. doi: 10.1136/bjophthalmol-2021-319450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cennamo G, Reibaldi M, Montorio D, D'Andrea L, Fallico M, Triassi M. Optical coherence tomography angiography features in post‐COVID‐19 pneumonia patients: a pilot study. Am J Ophthalmol. 2021;227:182‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324:603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daniel E, Pistilli M, Ying GS, et al. Association of meibomian gland morphology with symptoms and signs of dry eye disease in the dry eye assessment and management (DREAM) study. Ocul Surf. 2020;18:761‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hong N, Yu W, Xia J, Shen Y, Yap M, Han W. Evaluation of ocular symptoms and tropism of SARS‐CoV‐2 in patients confirmed with COVID‐19. Acta Ophthalmol. 2020;98:e649‐e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papazoglou A, Conen A, Haubitz S, et al. Ophthalmic screening in patients with coronavirus disease 2019: a prospective cohort study. J Clin Med. 2021;10:896. doi: 10.3390/jcm10050896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Costa ÍF, Bonifácio LP, Bellissimo‐Rodrigues F, et al. Ocular findings among patients surviving COVID‐19. Sci Rep. 2021;11:11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenberg JB, Eisen LA. Eye care in the intensive care unit: narrative review and meta‐analysis. Crit Care Med. 2008;36:3151‐3155. [DOI] [PubMed] [Google Scholar]
- 28. Grixti A, Sadri M, Edgar J, Datta AV. Common ocular surface disorders in patients in intensive care units. Ocul Surf. 2012;10:26‐42. [DOI] [PubMed] [Google Scholar]
- 29. Wan KH, Huang SS, Lam DSC. Conjunctival findings in patients with coronavirus disease 2019. JAMA Ophthalmol. 2021;139:254‐255. [DOI] [PubMed] [Google Scholar]
- 30. Wyon NM, Wyon DP. Measurement of acute response to draught in the eye. Acta Ophthalmol. 1987;65:385‐392. [DOI] [PubMed] [Google Scholar]
- 31. Koh S, Tung C, Kottaiyan R, Zavislan J, Yoon G, Aquavella J. Effect of airflow exposure on the tear meniscus. J Ophthalmol. 2012;2012:983182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan KH, Chen LJ, Young AL. Depression and anxiety in dry eye disease: a systematic review and meta‐analysis. Eye. 2016;30:1558‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang YJ. The respiratory microbiome and innate immunity in asthma. Curr Opin Pulm Med. 2015;21:27‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Banerjee R. Introduction to the thematic Minireview series: host‐microbiome metabolic interplay. J Biol Chem. 2017;292:8544‐8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen L, Deng C, Chen X, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID‐19 in Wuhan, China: a cross‐sectional study. Acta Ophthalmol. 2020;98:e951‐e959. [DOI] [PMC free article] [PubMed] [Google Scholar]


