Dear Editor,
A 43‐year‐old man was seen at the dermatology clinic with 3‐month history of skin rash. One day after receiving his first dose of Moderna (mRNA‐1273) COVID‐19 vaccine, a painful rash developed over the cheeks that eventually spread to the scalp, back and chest over 10 days. He also complained of difficulty in squatting, lifting arms and swallowing, as well as weight loss of 5‐kilograms. He had no past medical history or pre‐existing skin conditions, and is not on any long‐term medications.
Physical examination revealed symmetrical, non‐scaly, fiery red plaques with telangiectasia in a centrofacial distribution sparing nasolabial folds, and erythematous plaques on posterior scalp, upper chest, and back (Figure 1A, B). He also had faint linear erythematous rash over the metacarpophalangeal joints of both hands, and macular erythematous rash over the lateral aspects of both thighs. Weakness in neck flexion and proximal upper and lower limbs was elicited.
FIGURE 1.
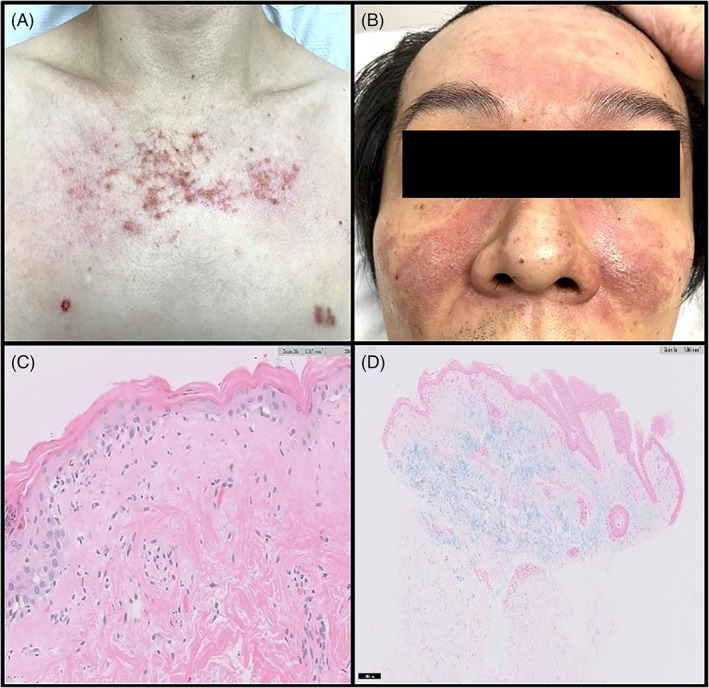
(A) and (B) Erythematous rash with telangiectasis on chest and both medial cheeks sparing nasolabial folds. (C) Histology from the punch biopsy of the chest lesion shows epidermal atrophy, vacuolar interphase changes with sparse inflammatory infiltrate. (D) Mucin, highlighted by special stain Alcian blue, is noted in the superficial dermis
Laboratory investigations showed raised muscle enzymes with serum aldolase levels of 6.8 U/L and alanine transaminase levels of 70 U/L. Erythrocyte sedimentation rate was elevated at 56 mm/hr. Full blood count, urea and electrolyte levels, and thyroid‐stimulating hormone levels were unremarkable. Anti‐nuclear antibody test was positive with a titer of 1:160 (nucleolar staining pattern), but anti‐double‐stranded DNA antibody levels and serum complements (C3 and C4) were within normal range. Anti‐extractable nuclear antigens antibody tests (Smith, ribonucleoprotein, Ro, La, Scl‐70, Jo‐1) were negative. An extended myositis antibody panel did not detect the presence of myositis‐specific or myositis‐associated antibodies. Needle electromyography revealed spontaneous insertional activity with fibrillation potentials, positive sharp waves, and small polyphasic motor unit potentials in the proximal upper and lower limbs, suggestive of a myopathic disorder. Skin punch biopsy of a chest lesion was performed. Histopathological examination revealed epidermal atrophy, attenuation of the rete ridges and vacuolar interface changes, associated with sparse dermal inflammatory infiltrate composed of lymphocytes and no eosinophils (Figure 1C). Dermal mucin was increased as highlighted by Alcian Blue stain (Figure 1D). However, no immune deposits were noted on direct immunofluorescence. Chest radiograph had no features of interstitial lung disease. The patient declined muscle biopsy and further evaluation for malignancy.
The patient was initially given topical corticosteroid and a tapering course of oral prednisolone. The rashes and systemic symptoms improved after the treatment but worsened each time prednisolone was tapered below 20 mg per day. Intravenous immunoglobulin infusion and hydroxychloroquine was subsequently added, resulting in resolution of symptoms and normalization of his serum muscle enzymes. At last follow‐up, his prednisolone dose has been tapered to 10 mg per day.
A spectrum of cutaneous reactions has been reported with both Moderna and Pfizer mRNA COVID‐19 vaccines, 1 with most skin biopsies showing a range of spongiotic and interface changes. 2 , 3 , 4 Whilst there have been reported cases of exacerbation of autoimmune disorders such as subacute cutaneous lupus erythematosus flare triggered by COVID‐19 vaccine, 5 , 6 we describe, in this report, a novel case of dermatomyositis as an adverse reaction to mRNA COVID‐19 vaccine, with no prior history of connective tissue disorder. The patient did not receive the second dose in view of the adverse event after the first dose. Whilst it is possible that the dermatomyositis is coincidental to administration of the vaccine, the balance of probability given the temporal relationship and the severity of the symptoms following the first dose makes it difficult to recommend completing the vaccination regime.
Infections and vaccines can occasionally cause new‐onset or flare of autoimmune‐mediated diseases. 7 , 8 COVID‐19 infections were found to increase autoantibodies targeting a wide variety of tissues. 9 As the mRNA vaccines provide genetic information for the synthesis of SARS‐CoV‐2 spike protein, we postulate that a similar, albeit narrower range of autoantibodies may be induced.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
AUTHORS CONTRIBUTION
All authors were involved in data collection for manuscript preparation, review of literature, manuscript preparation, and review.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. McMahon D, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85:46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larson V, Seidenberg R, Avrom C, Brinster N, Meehan S, Kim R. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID‐19 vaccination. J Cut Pathol. 2021;49:34‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McMahon D, Kovarik C, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID‐19 vaccine reactions including V‐REPP: a registry‐based study. J Am Acad Dermatol. 2021;S0190‐9622(21):2442‐2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magro C, Crowson N, Franks L, et al. The histologic and molecular correlates of COVID‐19 vaccine‐induced changes in the skin. Clin Dermatol. 2021;39:966‐984. doi: 10.1016/j.clindermatol.2021.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joseph AK, Chong BF. Subacute cutaneous lupus erythematosus flare triggered by COVID‐19 vaccine. Dermatol Ther. 2021;34:e15114. doi: 10.1111/dth.15114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kreuter A, Licciardi‐Fernandez MJ, Burmann SN, et al. Induction and exacerbation of subacute cutaneous lupus erythematosus following mRNA‐based or adenoviral vector‐based SARS‐CoV‐2 vaccination. Clin Exp Dermatol. 2021;22:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guimarães LE, Baker B, Perricone C, Shoenfeld Y. Vaccines, adjuvants and autoimmunity. Pharmacol Res. 2015;100:190‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watad A, De Marco G, Mahajna H, et al. Immune‐mediated disease flares or new‐onset disease in 27 subjects following mRNA/DNA SARS‐CoV‐2 vaccination. Vaccines (Basel). 2021;9(5):435. doi: 10.3390/vaccines9050435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang E, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID‐19. Nature. 2021;595:283‐288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


