Summary
B‐cell depletion induced by anti‐cluster of differentiation 20 (CD20) monoclonal antibody (mAb) therapy of patients with lymphoma is expected to impair humoral responses to severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) vaccination, but effects on CD8 T‐cell responses are unknown. Here, we investigated humoral and CD8 T‐cell responses following two vaccinations in patients with lymphoma undergoing anti‐CD20‐mAb therapy as single agent or in combination with chemotherapy or other anti‐neoplastic agents during the last 9 months prior to inclusion, and in healthy age‐matched blood donors. Antibody measurements showed that seven of 110 patients had antibodies to the receptor‐binding domain of the SARS‐CoV‐2 Spike protein 3–6 weeks after the second dose of vaccination. Peripheral blood CD8 T‐cell responses against prevalent human leucocyte antigen (HLA) class I SARS‐CoV‐2 epitopes were determined by peptide‐HLA multimer analysis. Strong CD8 T‐cell responses were observed in samples from 20/29 patients (69%) and 12/16 (75%) controls, with similar median response magnitudes in the groups and some of the strongest responses observed in patients. We conclude that despite the absence of humoral immune responses in fully SARS‐CoV‐2‐vaccinated, anti‐CD20‐treated patients with lymphoma, their CD8 T‐cell responses reach similar frequencies and magnitudes as for controls. Patients with lymphoma on B‐cell depleting therapies are thus likely to benefit from current coronavirus disease 2019 (COVID‐19) vaccines, and development of vaccines aimed at eliciting T‐cell responses to non‐Spike epitopes might provide improved protection.
Keywords: anti‐CD20 antibody, CD8 T‐cell response, coronavirus disease 2019 (COVID‐19) vaccination, humoral response, lymphoma, severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) epitopes
INTRODUCTION
Patients with cancer receiving immunosuppressive treatment are among the groups that are most susceptible to complications from coronavirus disease 2019 (COVID‐19). Patients with haematological malignancies may be at higher risk of COVID‐19 with a fatal course. 1 , 2 Worldwide efforts have been initiated to protect against COVID‐19 by vaccination programmes. High levels of protection have been achieved with approved vaccines among individuals without comorbidities. Less is known about the efficacy of these vaccines in subgroups treated with immunosuppressants.
A particular patient group that may be less likely to benefit from vaccination are those treated with monoclonal antibodies (mAbs) against CD20 (anti‐CD20 mAb, e.g. rituximab). Such antibodies are a standard part of anti‐neoplastic therapies in haematological malignancies like non‐Hodgkin lymphoma and chronic lymphocytic leukaemia, and are also used in treatment of various autoimmune disorders. 3 , 4 Prolonged B‐cell depletion is rapidly induced by anti‐CD20 mAbs and recovery of normal B‐cell counts will usually take 9–12 months after completed therapy. 5 Recently, anti‐CD20‐mAb therapy was shown to reduce levels of antibodies induced by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) vaccination in patients with autoimmune disease, such as multiple sclerosis (MS) and rheumatoid arthritis (RA). 6 , 7 , 8 , 9 , 10 In a previous study conducted during the H1N1 ‘swine‐flu’ pandemic, we demonstrated that none of the rituximab‐treated patients developed protective serological immunity after H1N1 influenza vaccination. 11 Available documentation indicates that SARS‐CoV‐2 vaccination of anti‐CD20‐mAb‐treated patients with lymphoma/leukaemia induces low antibody levels. 12 These levels seem lower when compared with those found in studies of patients with autoimmune diseases treated with rituximab, 6 , 13 , 14 , 15 although no direct comparison has been performed, to the best of our knowledge. There might be several reasons for potentially lower antibody levels in patients with lymphoma. First, the dose of anti‐CD20 mAb is higher than those administered in immune‐mediated disorders (e.g. rituximab, 375 mg/m2 every 1–4 weeks, given six to eight times as compared to dosing given every 6–12 months). Therefore, B‐cell depletion is completer and more prolonged. Second, immunotherapy is often combined with intensive chemotherapy regimens expected to enhance immunosuppression. Third, an inherently impaired immune system in patients with haematological malignancies might contribute to an inferior vaccine response. However, a recent report showed no difference in antibody response between treatment‐naïve patients with B‐cell lymphoma and healthy controls. In contrast, none of the patients who had received anti‐CD20 therapy within the last 6 months developed blocking antibodies, while the majority of patients that were off such treatment for >1 year were able to generate a serological response. 16
Studies investigating cytokine responses among peripheral blood mononuclear cells (PBMC) to peptide pools covering the Spike protein have indicated that overall T‐cell responses are little affected in patients with lymphoma receiving anti‐CD20 mAb therapy. 17 , 18 , 19 A similar observation was made in CD20‐treated patients with MS and RA. 6 , 13 , 15 , 20 , 21 , 22 , 23 However, low resolution T‐cell assays did not allow conclusions regarding the magnitudes and specificities of CD8 T‐cell responses. A recent study showed that CD8, but not CD4, T‐cell numbers correlated positively with survival upon COVID‐19 infection in situations of insufficient humoral immunity in patients with haematological malignancies. 24 Moreover, CD8 T‐cell immunity was shown to be critical for viral control in convalescent rhesus macaques with suboptimal antibody levels. 25 It is therefore of importance to specifically detect SARS‐CoV‐2 vaccine‐induced CD8 T‐cell responses in patients with impaired humoral immunity.
Here, we perform the first study investigating epitope‐specific CD8 T‐cell responses in COVID‐19 vaccinated patients with lymphoma undergoing treatment with anti‐CD20 mAbs as a single agent or combined with other anti‐neoplastic treatments. The use of peptide pools (overlapping 15 mers) in previous studies requires processing by antigen‐presenting cells for cross‐presentation to CD8 cells on HLA class I molecules. 26 , 27 Memory CD8 T‐cell responses in samples with reduced B‐cell counts might thus be underestimated. To avoid such a bias, we therefore studied responses to exact HLA class I SARS‐CoV‐2 epitopes that we recently validated by mass spectrometry and/or endogenous presentation. 28 We show that whereas almost all patients lack humoral responses, vaccination induces strong, epitope‐specific CD8 T‐cell responses, likely to contribute to protection against infection.
METHODS
Patient characteristics and vaccinations against SARS‐CoV‐2
Adult patients with CD20‐positive B‐cell lymphoma/leukaemia treated with anti‐CD20‐mAb therapy were recruited from hospitals all over Norway (for patients’ characteristics see Table 1). Patients received two vaccine doses (BioNTech/Pfizer, Moderna, Astra/Zeneca) separated by 4–8 weeks. Blood samples were drawn before the first vaccine and then 3–6 weeks after the second. Sera from 110 patients were analysed for immunoglobulin G (IgG) antibodies against SARS‐CoV‐2. A total of 38 individuals were recruited for collection of PBMC, for analysis of T‐cell responses. Published data from healthy individuals and a cohort of patients treated with anti‐CD20 antibodies for MS were used as reference. 29 The project was approved by the Regional Research Ethics Committee (REK#229747) according to the Declaration of Helsinki. Eligible participants provided informed consent.
TABLE 1.
Characteristics of complete patient cohort
| Patient characteristics | Value |
|---|---|
| Number of patients enrolled | 135 |
| Excluded from analysis, n (%) | 17 (13) |
| Not fully vaccinated due to COVID‐19 infection, n (%) | 3 (2) |
| Not fully vaccinated or did not meet for blood draw, n (%) | 12 (9) |
| Withdrawn consent, n (%) | 1 (1) |
| Not lymphoma, n (%) | 1 (1) |
| Included patients, n (%) | 118 (87) |
| Age, years, median (range) | 71 (22–89) |
| Diagnosis, n (%) | |
| DLBCL | 31 (26) |
| FL | 35 (30) |
| tDLBCL | 5 (4) |
| HL | 2 (2) |
| MZL | 7 (6) |
| MCL | 30 (25) |
| CLL/SLL | 3 (3) |
| Waldenström | 2 (2) |
| Burkitt | 3 (3) |
| Treatment status for lymphoma, n (%) | |
| Ongoing | 82 (69) |
| Completed | 36 (31) |
| Days from end of treatment to 1. Vaccine (n = 36), median (range) | 133 (2–222) |
| Days from end of treatment to 2. Vaccine (n = 36), median (range) | 166 (20–261) |
| Treatment regimen, n (%) | |
| R/O‐Chemo | 72 (61) |
| R‐mono | 12 (10) |
| R‐maintenance | 21 (18) |
| R‐bortezomib | 1 (1) |
| R‐ibrutinib | 5 (4) |
| R‐lenalidomide | 1 (1) |
| R‐lenalidomide/venetoclax | 4 (3) |
| R‐venetoclax | 2 (2) |
| Vaccine manufacturer, n (%) | |
| BioNTech/Pfizer | 103 (87) |
| Moderna | 14 (12) |
| Astra/Zeneca | 1 (1) |
Abbreviations: Burkitt, Burkitt lymphoma; CLL, chronic lymphocytic leukaemia; (t)DLBCL, (transformed) diffuse large B‐cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; MZL, marginal zone lymphoma; MCL, mantel cell lymphoma; O, obinutuzumab; R, rituximab; SLL, small lymphocytic lymphoma.
Analysis of humoral responses against SARS‐CoV‐2
For the measurements of SARS‐CoV‐2 receptor‐binding domain (RBD) and full‐length Spike IgG antibodies in sera of patients and controls, a bead‐based multiplexing assay was used. Briefly, purified His‐tagged SARS‐CoV‐2 RBD and full‐length Spike proteins (production see Methods S1) were biotinylated at a biotin to protein ratio of 1:1 and bound to neutravidin‐conjugated polymer microspheres with fluorescent barcodes. 30 , 31 Beads with different proteins and barcodes were mixed in assay buffer (phosphate‐buffered saline [PBS] with Tween‐20, bovine serum albumin, Neutravidin, D‐biotin and NaN3) and kept at 4°C until use. Bead‐multiplexes were added to serum diluted 1:100 in assay buffer. After a 1‐h incubation with constant agitation, the beads were washed with PBS‐Tween 20 (1%), labelled with R‐phycoerythrin (PE)‐goat‐anti‐human IgG‐Fc (Jackson Immunoresearch; 1:600) and analysed with an Attune Next flow cytometer (Thermo). Flow cytometry data were analysed with WinList 10.0 and median fluorescence intensity (MFI) values exported to Excel. Results are reported as: arbitrary units (au) = (MFIviral protein beads)/(MFIno protein beads). A double cut‐off of 5 au for RBD and Spike was used to classify positives.
Analysis of T‐cell responses against SARS‐CoV‐2
Subject details
Healthy control donors (HD): buffy‐coats from 20 age‐matched individuals, collected 3–7 weeks after the second vaccine dose (T1), were obtained from Oslo Blood Bank. The PBMC were isolated using a standard Ficoll isolation protocol.
Patient samples: blood from 38 patients before vaccination (T0) and after the second dose (T1) was collected in cell preparation tubes with sodium citrate (BD Vacutainer® CPT) and processed following the manufacturer’s instructions.
The PBMC were frozen in 60% fetal bovine serum (FBS)/30% RPMI/10% dimethyl sulphoxide (DMSO) and stored in liquid nitrogen.
HLA typing
DNA was isolated from PBMC using the DNeasy Blood and Tissue Kit (Qiagen). HLA typing was performed by next‐generation sequencing (NGS) using NGSgo®‐AmpX v2 HLAGeneSuite (GenDX) for sample library preparations and ran on a Miseq sequencer (Illumina), following the manufacturer’s instructions.
Synthetic peptides
Six SARS‐CoV‐2‐specific Spike peptides presented by HLA‐A*01:01, HLA‐A*02:01, or HLA‐A*03:01 previously identified by our group as immunogenic in COVID‐19 convalescent individuals were included in this study (Table 2). 28 Peptides were synthesised at Genscript (purity >70%) and dissolved in DMSO.
TABLE 2.
Peptides included in study
| Peptide name | Sequence | HLA allele | Immuno‐prevalence, % | Fluorochrome 1 a | Fluorochrome 2 a |
|---|---|---|---|---|---|
| S269‐277 | YLQPRTFLL | A*02:01 | ≥45 | SA‐APC (Invitrogen) | SA‐PE (Invitrogen) |
| S378‐387 | KCYGVSPTKL | A*03:01 | SA‐BV421 (Biolegend) | SA‐PE (Invitrogen) | |
| S89‐97 | GVYFASTEK | A*03:01 | SA‐APC (Invitrogen) | SA‐APC‐R700 (BD) | |
| S865‐874 | LTDEMIAQYT | A*01:01 | SA‐APC‐R700 (BD) | SA‐PE (Invitrogen) | |
| S1000‐1008 | RLQSLQTYV | A*02:01 | 13 | SA‐APC (Invitrogen) | SA‐PE‐CF594 (BD) |
| S367‐378 | VLYNSASFSTFK | A*03:01 | 11 | SA‐APC‐R700 (BD) | SA‐PE‐Cy5 (BD) |
Abbreviations: APC, allophycocyanin; BV421, brilliant violet 421; Cy5, cyanine 5; PE, R‐phycoerythrin; SA, streptavidin.
staining with dual fluorochrome combination.
Analysis of CD8 T‐cell responses in HD and patients with lymphoma by combinatorial peptide‐HLA (pHLA)‐multimer staining
The pHLA‐multimers carrying the peptides of interest were generated as previously described, 28 , 32 , 33 and for multimerization streptavidin‐tagged fluorochromes were added to the pMonomer solution (Table 2).
The PBMC of the HD and anti‐CD20‐mAb‐treated patients with lymphoma expressing at least one of the following prevalent HLA alleles were analysed: HLA‐A*02:01, HLA‐A*01:01 and HLA‐A*03:01. For the HD, T1 samples from 16 of 20 individuals were analysed based on matching HLA type, while 29 of 38 patients were included based on availability of sufficient cell numbers and matching HLA type (Figure 1). For one patient the pre‐vaccination sample (T0) was missing. On Day 0, PBMC were thawed, washed, and loaded for 2 h at 37°C with a peptide mix containing the six pre‐selected SARS‐CoV‐2‐specific Spike peptides (1 × 106 cells/ml in Iscove’s modified Dulbecco’s medium [IMDM]; each peptide at 100 ng/mL). Excess peptide was washed away, and 7.5 × 105 cells/well were plated out in IMDM medium (ThermoFisher Scientific) containing 1% penicillin/streptomycin and 5% normal human serum (Trina Bioreactives AG). On Day 3, half‐medium exchange was performed, and 10 IU/ml interleukin 2 (Peprotech) added. On Day 5, the medium was completely replenished. On Day 7, cells from each individual were pooled and washed. Then, 3–5 × 106 PBMC were stained with pHLA‐multimers carrying six distinctive peptides as dual fluorochrome combination in PBS. After a 10‐min incubation at room temperature, fluorescein isothiocyanate (FITC)‐anti‐human CD8a; BV785‐anti‐human CD19; BV785‐anti‐human CD56; BV785‐anti‐human CD14; BV785‐anti‐human CD4 (all Biolegend) diluted in LIVE/DEAD™ fixable near‐IR stain (LD‐NIR; Life Technologies) were added to the sample for 30 min at 4°C. After extensive washing, the samples were acquired on a BD Symphony A5. Data were analysed in FlowJo version 10.8.0 (for gating strategy, see Figure S1). For one patient, the T0 sample had to be excluded from the analysis, as the inclusion criteria of at least 2000 live CD8 cells in the acquisition was not met. An individual was classified as a ‘responder’ to a peptide if the pHLA‐multimer population had: (i) at least five clearly double‐positive pHLA‐multimer events, (ii) constituted ≥0.005% of the live CD8, (iii) formed a tight cluster, and (iv) the population was not detected in CD8‐negative cells.
FIGURE 1.
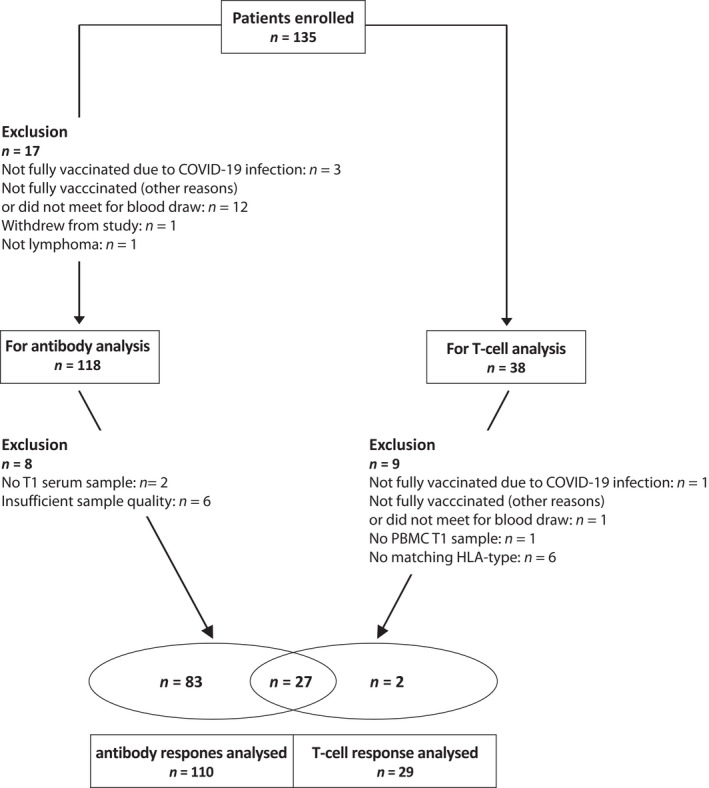
Study flow diagram. Adult patients with CD20‐positive B‐cell lymphoma/leukaemia were recruited at Oslo University Hospital, Akershus University Hospital, Haukeland University Hospital, Stavanger University Hospital, St Olavs University Hospital, and University Hospital of Northern Norway. Participants were undergoing treatment with anti‐CD20 antibody alone or in combination with chemotherapy or other neoplastic agents or had finished such therapies <9 months prior to inclusion. Serum samples (110 patients) and peripheral blood mononuclear cells (PBMC; 29) were analysed for B‐ and T‐cell responses before and after the second dose of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) vaccination
Details about used antibodies and pHLA‐multimers see Methods S1.
Statistics
Statistical analysis was performed in R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria). Unless otherwise specified, we used Fisher’s exact test for contingency tables and Wilcoxon rank‐sum test for non‐parametric continuous variables. A two‐sided p < 0.05 was considered statistically significant. Binomial 95% confidence intervals (CIs) were calculated for the response frequency to each T‐cell epitope. To estimate T‐cell response rates in a standardised Caucasian population given the measured response rates for each epitope in patients and HD in our study, we applied our recently developed algorithm. 28 For a brief description, see Methods S1.
RESULTS
Patient characteristics
A total of 135 patients with B‐cell lymphoma were included in this study of which 17 were excluded for various reasons, leaving 118 patients for serum antibody analysis (Table 1, Figure 1). The most common subtypes were diffuse large B‐cell lymphoma, follicular lymphoma, and mantle cell lymphoma. The median (range) age was 71 (22–89) years with a male/female ratio of 1:2. The majority of patients (n = 82) was under treatment, whereas 36 patients had completed therapy. For the latter group, the median time from last cycle of therapy and first vaccine dose was 133 days. Vaccines received by the participants were manufactured by BioNTech/Pfizer (n = 103), Moderna (n = 14) or Astra/Zeneca (n = 1). All patients received two doses of vaccine.
Only a minor subset of patients on anti‐CD20‐mAb therapy produce antibodies to the RBD of the SARS‐CoV‐2 Spike protein after COVID‐19 vaccination
We analysed sera from 110 patients within 2 months after the second dose of COVID‐19 vaccine. IgG antibodies to RBD and Spike were detected in seven patients, but four of these had very low levels compared to those observed in HD (n = 150, Figure 2A,B). The antibody response in patients with lymphoma was weaker than that observed in patients treated with anti‐CD20 mAbs for MS (Figure 2A,C). A subset of patients had antibodies that bound the full‐length Spike, but not RBD. We are currently investigating the possibility that this reflects cross‐reactive memory responses to seasonal coronaviruses. We did not find any correlation between time from last anti‐CD20‐mAb treatment and antibody responses (Figure 2D). However, two of the strongest antibody responses were observed in patients more than 6 months since last treatment, whereas all patients under active treatment showed no or very low antibody levels, indicating a positive correlation between the probability of mounting humoral immune response and the time since anti‐CD20‐mAb treatment.
FIGURE 2.
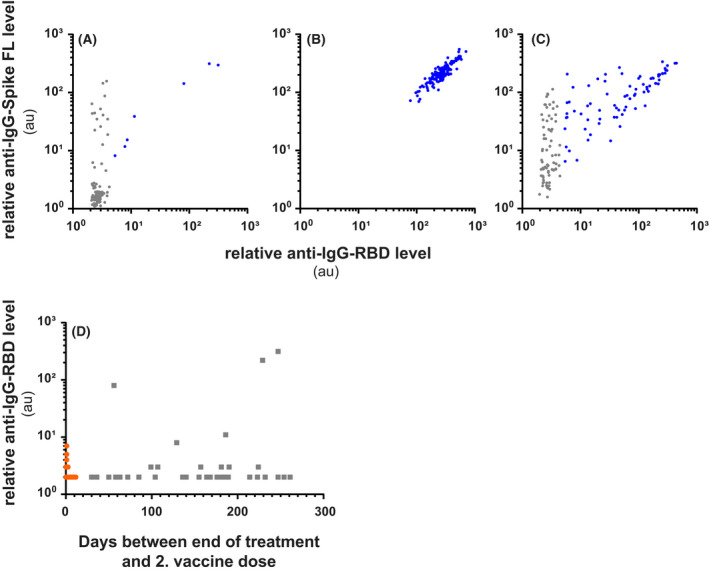
Most rituximab‐treated patients with lymphoma lack antibody responses to Spike or receptor‐binding domain (RBD) after vaccination. (A–D) Relative levels of immunoglobulin G (IgG) antibodies after vaccination to full‐length (FL) Spike from severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) (y‐axis) and the RBD (x‐axis) in patients treated with anti‐CD20 monoclonal antibodies (mAbs) for B‐cell lymphoma (A) or multiple sclerosis (C), compared to healthy controls (B). Relative antibody levels are reported as arbitrary units (au) = (MFIviral protein beads)/(MFIno protein beads). Each dot represents one individual. Blue dots indicate sera with antibody levels above the double cut‐off for RBD and FL Spike (au ≥5). (D) Anti‐RBD in patients according to days after last dose of treatment. Orange dots indicate patients on treatment, while grey squares indicate patients where treatment is terminated. MFI, median fluorescence intensity
Strong vaccine‐induced CD8 T‐cell responses in anti‐CD20‐mAb‐treated patients
The PBMCs were collected for a subset of patients to measure antigen‐specific CD8 T‐cell responses to vaccination. In total, 29 anti‐CD20‐mAb‐treated patients with lymphoma and 16 age‐matched HD with at least one of the following prevalent alleles: HLA‐A*02:01, HLA‐A*01:01 and HLA‐A*03:01 were analysed (Table 3, Figure 1). We used combinatorial pHLA‐multimer staining to determine patient T‐cell responses before and after vaccination towards exact SARS‐CoV‐2 Spike‐derived epitopes that we recently identified as immunogenic and prevalent in a large cohort of otherwise healthy COVID‐19 convalescents. 28 In total, we assessed the response to six prevalent Spike epitopes, restricted by the prevalent HLA‐A*02:01, HLA‐A*01:01 and HLA‐A*03:01 alleles, expressed by 50%, 31% and 27% of the Caucasian population respectively (Figure 3A). Robust T‐cell responses were observed towards five of the six epitopes in the anti‐CD20‐mAb‐treated patient cohort after vaccination (T1), which were absent in the pre‐vaccination samples (T0), demonstrating specificity (Figure 3B, Table 4). The median values for response magnitudes to single epitopes were similar in patients and HD after vaccination. For 20 of 29 (69%) patients we detected a response to at least one epitope, similar to the response rates observed in HD (12/16 [75%]; Figure 3C, Table S1). To account for the different HLA‐allele distributions among patients and HD, we calculated the expected response rates in a standardised Caucasian population given the observed immuno‐prevalence of each epitope in each of the cohorts. The standardised population response rates were similar, with largely overlapping CIs: 54.2% (95% CI: 40.6%–59.5%) for patients and 57.9% (95% CI: 42.6%–61.8%) for HD. Within the patient cohort, neither the treatment regimen (rituximab‐Chemo vs. rituximab‐Other, Fisher’s exact test p = 0.7), the vaccine manufacturer (Moderna vs. BioNTech/Pfizer, Fisher’s exact test p = 1), nor the number of HLA alleles matching the tested epitopes (one vs. two, Fisher’s exact test p = 0.7) affected the T‐cell response rate for a given epitope. However, we observed a trend towards lower response rates for patients on anti‐CD20‐mAb therapy as compared with patients that no longer received such treatment (56% vs. 91%, Fisher’s exact test p = 0.1, Figure 3C). Taken together, patients treated with anti‐CD20 mAbs who lack antibody responses, mount strong vaccine‐induced CD8 T‐cell responses to immuno‐prevalent Spike epitopes comparable with those seen in vaccinated HD (Figure 3D).
TABLE 3.
Characteristics of patient and healthy donor (HD) cohort used in T‐cell analysis
| RTX‐treated patients | HD | |
|---|---|---|
| Total, n | 29 | 16 |
| Gender, n | ||
| Male | 17 | 6 |
| Female | 12 | 10 |
| Age, years, median (range) | 66 (37–89) | 67 (62–74) |
| Diagnosis, n | ||
| DLBCL | 8 | ‐ |
| FL | 10 | ‐ |
| tDLBCL | 0 | ‐ |
| HL | 0 | ‐ |
| MZL | 1 | ‐ |
| MCL | 8 | ‐ |
| CLL/SLL | 0 | ‐ |
| Waldenström | 0 | ‐ |
| Burkitt | 2 | ‐ |
| Treatment regimen, n | ||
| R‐Chemo | 12 | ‐ |
| R‐Ibrutinib | 2 | ‐ |
| R‐Len/Ven | 2 | ‐ |
| R‐Mono | 3 | ‐ |
| R‐maintenance | 10 | ‐ |
| Vaccine manufacturer, n | ||
| BioNTech/Pfizer | 19 | 11 |
| Moderna | 9 | 5 |
| Astra/Zeneca | 1 | 0 |
| Days ‐ second vaccine and T1 sample, median (range) | 28 (19–40) | 30 (20–52) |
| HLA class I, n | ||
| A*01:01 | 9 | 4 |
| A*02:01 | 24 | 9 |
| A*03:01 | 5 | 5 |
Abbreviations: Burkitt, Burkitt lymphoma; CLL, chronic lymphocytic leukaemia; (t)DLBCL, (transformed) diffuse large B‐cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; Len, lenalidomide; MCL, mantel cell lymphoma; MZL, marginal zone lymphoma; R, rituximab; SLL, small lymphocytic lymphoma; Ven, venetoclax.
FIGURE 3.
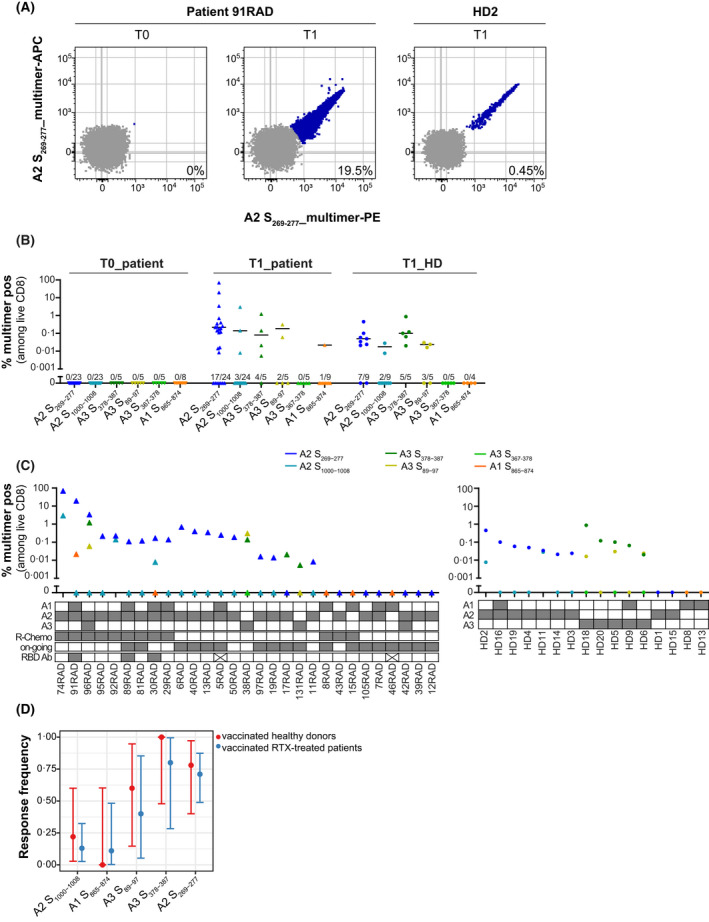
T‐cell responses in vaccinated healthy donors (HD) and patients. (A) Epitope S269‐277 induces responses of high magnitude in vaccinated individuals after in vitro stimulation. Flow plots show peptide‐HLA (pHLA)‐multimer staining for rituximab (RTX)‐treated patient 91RAD before (T0) and after (T1) vaccination (second strongest responder among RTX‐treated patients) and the HD2 after vaccination (T1; strongest responder among HD controls). (B) Magnitude of CD8 T‐cell responses to six SARS‐CoV‐2 Spike‐specific peptides in RTX‐treated patients and HD. T‐cell responses were determined by pHLA‐multimer staining in HLA‐typed individuals (individual data points with median of responders). ● HD (T1: 3–7 weeks after vaccination; n = 16); Δ RTX‐treated patients (T0: before vaccination, n = 27; T1: 3–6 weeks after vaccination, n = 29). For each peptide the number of responses identified among the number of individuals tested is displayed above the x‐axis. (C) Response distribution among patients after vaccination (T1; left) and HD (T1; right). In the table below the graph, HLA‐type is displayed for each individual. For patients, the treatment regimen (R‐Chemo or not), treatment status (on‐going or completed), and receptor‐binding domain (RBD)‐immunoglobulin G (IgG)‐antibody response (x means no antibody data available) is displayed. (D) Immuno‐prevalence data with exact binomial 95% confidence intervals for every studied epitope in the HD (red) and patient (blue) cohort (Fisher’s exact test p > 0.5). A1, A2 and A3 refer to HLA‐A*01:01, HLA‐A*02:01 and HLA‐A*03:01 respectively
TABLE 4.
T‐cell responses to six spike epitopes in vaccinated healthy donors (HD) and rituximab‐treated patients with lymphoma 3–6 weeks after the second vaccine dose
| Peptide | Sequence | HLA allele | BA_rank a , % | Patient T1 | HD T1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Positive, n | Frequency, % | Median response (range) | n | Positive, n | Frequency, % | Median response (range) | ||||
| S269‐277 | YLQPRTFLL | A*02:01 | 0.023 | 24 | 17 | 71 | 0.22 (0.01–69.9) | 9 | 7 | 78 | 0.05 (0.02–0.45) |
| S378‐387 | KCYGVSPTKL | A*03:01 | 7.898 | 5 | 4 | 80 | 0.08 (0.01–1.22) | 5 | 5 | 100 | 0.10 (0.02–0.87) |
| S89‐97 | GVYFASTEK | A*03:01 | 0.054 | 5 | 2 | 40 | 0.19 (0.06–0.31) | 5 | 3 | 60 | 0.02 (0.02–0.03) |
| S1000‐1008 | RLQSLQTYV | A*02:01 | 0.161 | 24 | 3 | 13 | 0.14 (0.01–3.0) | 9 | 2 | 22 | 0.02 (0.01–0.03) |
| S865‐874 | LTDEMIAQYT | A*01:01 | 0.122 | 9 | 1 | 11 | 0.022 | 4 | 0 | 0 | 0 |
| S367‐378 | VLYNSASFSTFK | A*03:01 | 0.011 | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
BA_Rank: NetMHCpan4.1.
DISCUSSION
Here, we present the first study to characterise COVID‐19 vaccine‐induced epitope‐specific CD8 T‐cell responses in anti‐CD20‐mAb‐treated patients with lymphoma. We show that there is an almost complete absence of humoral immunity after vaccination in B‐cell lymphoma and that the immunosuppression is more severe than that observed in patients with MS treated with anti‐CD20. This provided a unique setting to study T‐cell responses. Our data show that patients with lymphoma mounted robust CD8 T‐cell responses against defined HLA class I‐restricted epitopes from the Spike protein of SARS‐CoV‐2, at similar frequencies and magnitudes as age‐matched HD. Therefore, patients with lymphoma undergoing anti‐CD20‐mAb therapy will likely benefit from current COVID‐19 vaccines despite absent humoral responses.
Patients with lymphoma have an increased risk of severe COVID‐19 with fatal outcome. 34 In fact, haematological malignancy represents an independent risk factor for COVID‐19 mortality. 24 It is therefore of great importance to investigate if this patient group benefits from current vaccines. Our study showed an almost complete lack of seroconversion after two vaccinations in anti‐CD20‐mAb‐treated patients with lymphoma <6 months after therapy. This is in agreement with other reports. 35 , 36 Even 6–8 months after termination of treatment, the large majority of patients did not have a serological response (Figure 2D). These response rates are clearly inferior to those published for vaccinated patients treated with anti‐CD20 mAbs for non‐malignant diseases. 6 , 7 , 8 , 9 , 13 , 14 , 15 Our results shown in Figure 2 for MS are in line with those published by others, and reports on patients with immune‐mediated glomerulonephritis and systemic rheumatological diseases using rituximab. 6 , 7 , 8 , 9
An important question is the degree to which cellular vaccine responses occur in a setting of a failed humoral response. The importance of CD8 T‐cell immunity for protection against SARS‐CoV‐2 infection was demonstrated in convalescent rhesus macaques with suboptimal antibody levels. 25 There is also evidence that CD8 T cells contribute to survival in patients with COVID‐19 and haematological cancer. 37 When clinical outcomes to SARS‐CoV‐2 infection were correlated with immune profiles in patients with cancer, those with depleted T cells had the highest mortality, regardless of B‐cell numbers. Moreover, patients with haematological cancer who survived had higher CD8 T‐cell numbers, whereas CD4 T‐cell counts were not associated with survival. 24 Strikingly, anti‐CD20‐mAb therapy was not associated with higher fatality, disease severity or viral load when compared to chemotherapy or observation, although humoral immunity was deficient. 24 However, the degree and type of vaccine‐induced T‐cell immune response required for viral control is still not known. Current SARS‐CoV‐2 vaccines induce CD4 T‐cell responses 38 , 39 , 40 , 41 that might improve weak antibody responses and also support CD8 T‐cell responses.
T‐cell responses depend on (i) the percentage of responders to an epitope presented by a given HLA allele among the individuals expressing this allele (immuno‐prevalence), and (ii) the frequency distribution of this HLA allele in a population. We recently mapped epitope‐specific CD8 T‐cell responses in otherwise healthy convalescents in a large HLA‐typed Norwegian Caucasian cohort. 28 We identified 29 epitopes restricted by four of the most prevalent HLA alleles in Caucasians. The epitopes were validated as endogenously presented and eluted from HLA for sequencing by mass spectrometry. Previous studies have shown that measurements of responses to peptide pools (usually overlapping 15–18 mers), and using assays such as intracellular cytokine staining or enzyme‐linked immunospot (ELISPOT), might underestimate the magnitude of responses seen to single epitopes partly due to high background activation. 42 , 43 Moreover, the need for processing of long epitopes (in peptide pools) to fit into the peptide‐binding groove of HLA class I molecules (most often 9–10 mers) in vitro in samples lacking normal, functional B cells, might underestimate the CD8 T‐cell responses. We therefore used combinatorial pHLA‐multimer staining to determine patient T‐cell responses before and after vaccination towards exact SARS‐CoV‐2 Spike‐derived epitopes. In our convalescent study, we found that nine of 29 epitopes were highly prevalent, derived from ORF3a (four), nucleocapsid (three) and Spike (two). 28 Here, we found that the two Spike epitopes were recognised by ~70% of patients with lymphoma (and HD) expressing the restricting HLA allele following vaccination. Overall, prevalence and magnitude of responses to all Spike epitopes were similar among patients and HD. Strikingly, some of the strongest CD8 responses were, however, seen in the patient cohort.
Taken together, the data presented here indicate that SARS‐CoV‐2 vaccine‐induced epitope‐specific CD8 T‐cell responses in anti‐CD20‐mAb‐treated patients with lymphoma are independent of normal, functioning B cells, as they are very similar to those induced in age‐matched HD. Current European and North American vaccines induce immunity to Spike only. Our study indicates that a third dose of standard SARS‐CoV‐2 vaccination could provide improved protection against infection for patients with lymphoma undergoing anti‐CD20‐mAb therapy by boosting the CD8 T‐cell response to Spike. However, mapping of epitope prevalence in COVID‐19 convalescents demonstrated that seven of nine epitopes recognised by ≥70% are derived from non‐Spike proteins. 28 Importantly, all nine epitopes are conserved in the SARS‐CoV‐2 variant ‘omicron’. For the manufacturing process it would be very challenging, if at all possible, to use the complete genomic sequence of SARS‐CoV‐2 due to its length. Moreover, it might be challenging to reach an effective dose of mRNA, as the amount encoding immunogenic epitopes would be very small relative to the amount encoding non‐immunogenic mRNA. We therefore propose that T‐cell‐targeting vaccines including epitopes as string of beads encoded by mRNA from multiple SARS‐CoV‐2 proteins could be designed in a similar way as previously done by Sahin et al. 44 to induce anti‐cancer T‐cell responses. Such an approach for COVID‐19 vaccination would be expected to induce broader CD8 T‐cell responses in patients with lymphoma with deficient humoral immunity, possibly providing better protection against infection. For global coverage of such vaccines, additional epitopes restricted by HLA alleles frequently expressed in populations other than the one investigated here would have to be identified. Taken together, our results showing robust CD8 T‐cell responses upon SARS‐CoV‐2 vaccination in patients with lymphoma on B‐cell‐depleting therapies represent important knowledge for both healthcare workers and patients and are most likely valid for other common vaccines as well.
CONFLICT OF INTEREST
A patent application was filed by the institutional technology transfer office Inven2 covering SARS‐CoV‐2 epitopes (Inventors: Johanna Olweus, Fridtjof Lund‐Johansen, Saskia Meyer, Isaac Blaas, Even Holth Rustad). All other co‐authors confirm no competing interest.
AUTHOR CONTRIBUTIONS
Conceptualisation: Saskia Meyer, Isaac Blaas, Johanna Olweus, Arne Kolstad. Methodology: Saskia Meyer, Isaac Blaas, Fridtjof Lund‐Johansen, Johanna Olweus, Arne Kolstad. Resources: Saskia Meyer, Marina Delic‐Sarac, Lise Nissen‐Meyer, Arne Kolstad, Jon Riise, Marton König, Gro Nygaard. Investigation: Saskia Meyer, Isaac Blaas, Trung T. Tran, Ke‐Zheng Dai, John Torgils Vaage, Fridtjof Lund‐Johansen, Johanna Olweus, Malu Lian Hestdalen, Ellen Brodin, Even Holth Rustad, Fredrik Sund, Karin F. Wader, Anne T. Bjornevik, Peter A. Meyer, Arne Kolstad, Jon Riise, Adity Chopra. Formal analysis: Saskia Meyer, Even Holth Rustad, Jon Riise, Arne Kolstad. Visualisation: Saskia Meyer, Even Holth Rustad, Jon Riise, Arne Kolstad. Funding acquisition: Fridtjof Lund‐Johansen, Johanna Olweus, Arne Kolstad, Sigbjørn Smeland. Supervision: Johanna Olweus, Arne Kolstad. Writing – original draft: Saskia Meyer, Even Holth Rustad, Fridtjof Lund‐Johansen, Johanna Olweus, Arne Kolstad, Jon Riise. Writing – review and editing: Johanna Olweus, Saskia Meyer, Even Holth Rustad, Arne Kolstad, Jon Riise, Sigbjørn Smeland.
Supporting information
Appendix S1
Riise J, Meyer S, Blaas I, Chopra A, Tran TT, Delic‐Sarac M, et al. Rituximab‐treated patients with lymphoma develop strong CD8 T‐cell responses following COVID‐19 vaccination. Br J Haematol. 2022;197:697–708. 10.1111/bjh.18149
Jon Riise, Saskia Meyer and Isaac Blaas contributed equally.
Johanna Olweus and Arne Kolstad contributed equally.
Contributor Information
Fridtjof Lund‐Johansen, Email: fridtjol@gmail.com.
Johanna Olweus, Email: johanna.olweus@medisin.uio.no.
Arne Kolstad, Email: arnek2@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available within the article and its supplementary information files. Raw data are available from the corresponding authors upon reasonable request.
REFERENCES
- 1. Desai A, Gupta R, Advani S, Ouellette L, Kuderer NM, Lyman GH, et al. Mortality in hospitalized patients with cancer and coronavirus disease 2019: a systematic review and meta‐analysis of cohort studies. Cancer. 2021;127:1459–68. [DOI] [PubMed] [Google Scholar]
- 2. Liu H, Yang D, Chen X, Sun Z, Zou Y, Chen C, et al. The effect of anticancer treatment on cancer patients with COVID‐19: a systematic review and meta‐analysis. Cancer Med. 2021;10:1043–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salles G, Barrett M, Foà R, Maurer J, O'Brien S, Valente N, et al. Rituximab in B‐cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34:2232–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaegi C, Wuest B, Schreiner J, Steiner UC, Vultaggio A, Matucci A, et al. Systematic review of safety and efficacy of rituximab in treating immune‐mediated disorders. Front Immunol. 2019;10:1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McLaughlin P, Grillo‐Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti‐CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four‐dose treatment program. J Clin Oncol. 1998;16:2825–33. [DOI] [PubMed] [Google Scholar]
- 6. Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, et al. Cellular and humoral immune responses following SARS‐CoV‐2 mRNA vaccination in patients with multiple sclerosis on anti‐CD20 therapy. Nat Med. 2021;27:1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brill L, Rechtman A, Zveik O, Haham N, Oiknine‐Djian E, Wolf DG, et al. Humoral and T‐cell response to SARS‐CoV‐2 vaccination in patients with multiple sclerosis treated with Ocrelizumab. JAMA Neurol. 2021;78:1510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sormani MP, Inglese M, Schiavetti I, Carmisciano L, Laroni A, Lapucci C, et al. Effect of SARS‐CoV‐2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72:103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tallantyre EC, Vickaryous N, Anderson V, Asardag AN, Baker D, Bestwick J, et al. COVID‐19 vaccine response in people with multiple sclerosis. Ann Neurol. 2022;91:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Achiron A, Mandel M, Dreyer‐Alster S, Harari G, Magalashvili D, Sonis P, et al. Humoral immune response to COVID‐19 mRNA vaccine in patients with multiple sclerosis treated with high‐efficacy disease‐modifying therapies. Ther Adv Neurol Disord. 2021;14:17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yri OE, Torfoss D, Hungnes O, Tierens A, Waalen K, Nordoy T, et al. Rituximab blocks protective serologic response to influenza a (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118:6769–71. [DOI] [PubMed] [Google Scholar]
- 12. Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with B‐cell non‐Hodgkin lymphoma. Blood Adv. 2021;5:3053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asplund Högelin K, Ruffin N, Pin E, Månberg A, Hober S, Gafvelin G, et al. Development of humoral and cellular immunological memory against SARS‐CoV‐2 despite B cell depleting treatment in multiple sclerosis. iScience. 2021;24:103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frey S, Connolly CM, Chiang TP, Teles M, Alejo JL, Boyarsky BJ, et al. Antibody kinetics in patients with rheumatic diseases after SARS‐CoV‐2 mRNA vaccination. Lancet Rheumatol. 2021;3:e753–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mrak D, Tobudic S, Koblischke M, Graninger M, Radner H, Sieghart D, et al. SARS‐CoV‐2 vaccination in rituximab‐treated patients: B cells promote humoral immune responses in the presence of T‐cell‐mediated immunity. Ann Rheum Dis. 2021;80:1345–50. [DOI] [PubMed] [Google Scholar]
- 16. Shree T, Shankar V, Lohmeyer JJ, Czerwinski DK, Schroers‐Martin JG, Rodriguez GM, et al. CD20‐targeted therapy ablates De novo antibody response to vaccination but spares pre‐established immunity. Blood Cancer Discov. 2022;3:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marasco V, Carniti C, Guidetti A, Farina L, Magni M, Miceli R, et al. T‐cell immune response after mRNA SARS‐CoV‐2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol. 2021;196:548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Candon S, Lemee V, Leveque E, Etancelin P, Paquin C, Carette M, et al. Dissociated humoral and cellular immune responses after a three‐dose schema of BNT162b2 vaccine in patients receiving anti‐CD20 monoclonal antibody maintenance treatment for B‐cell lymphomas. Haematologica. 2021;107:755–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liebers N, Speer C, Benning L, Bruch PM, Kraemer I, Meissner J, et al. Humoral and cellular responses after COVID‐19 vaccination in anti‐CD20‐treated lymphoma patients. Blood. 2022;139:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moor MB, Suter‐Riniker F, Horn MP, Aeberli D, Amsler J, Möller B, et al. Humoral and cellular responses to mRNA vaccines against SARS‐CoV‐2 in patients with a history of CD20 B‐cell‐depleting therapy (RituxiVac): an investigator‐initiated, single‐centre, open‐label study. Lancet Rheumatol. 2021;3:e789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Felten R, Gallais F, Schleiss C, Chatelus E, Javier RM, Pijnenburg L, et al. Cellular and humoral immunity after the third dose of SARS‐CoV‐2 vaccine in patients treated with rituximab. Lancet Rheumatol. 2021;4:e13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonelli MM, Mrak D, Perkmann T, Haslacher H, Aletaha D. SARS‐CoV‐2 vaccination in rituximab‐treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis. 2021;80:1355–6. [DOI] [PubMed] [Google Scholar]
- 23. Simon D, Tascilar K, Schmidt K, Manger B, Weckwerth L, Sokolova M, et al. Humoral and cellular immune responses to SARS‐CoV‐2 infection and vaccination in autoimmune disease patients with B cell depletion. Arthritis Rheumatol. 2022;74:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bange EM, Han NA, Wileyto P, Kim JY, Gouma S, Robinson J, et al. CD8(+) T cells contribute to survival in patients with COVID‐19 and hematologic cancer. Nat Med. 2021;27:1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS‐CoV‐2 in rhesus macaques. Nature. 2021;590:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zwaveling S, Ferreira Mota SC, Nouta J, Johnson M, Lipford GB, Offringa R, et al. Established human papillomavirus type 16‐expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169:350–8. [DOI] [PubMed] [Google Scholar]
- 27. Faure F, Mantegazza A, Sadaka C, Sedlik C, Jotereau F, Amigorena S. Long‐lasting cross‐presentation of tumor antigen in human DC. Eur J Immunol. 2009;39:380–90. [DOI] [PubMed] [Google Scholar]
- 28. Meyer S, Blaas I, Bollineni RC, Delic‐Sarac M, Tran TT, Knetter C, et al. Public T‐cell epitopes shared among SARS‐CoV‐2 variants are presented on prevalent HLA class I alleles. bioRxiv. 2021; 2021.10.13.463911. [Google Scholar]
- 29. König M, Lorentzen ÅR, Torgauten HM, Tran TT, Schikora‐Rustad S, Vaage EB, et al. Humoral immunity to SARS‐CoV‐2 mRNA vaccination in multiple sclerosis: the relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J Neurol Neurosurg Psychiatry. 2021;jnnp‐2021‐327612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu W, Slastad H, de la Rosa CD, Frey T, Tjonnfjord G, Boretti E, et al. Antibody array analysis with label‐based detection and resolution of protein size. Mol Cell Proteomics. 2009;8:245–57. [DOI] [PubMed] [Google Scholar]
- 31. Sikorski K, Mehta A, Inngjerdingen M, Thakor F, Kling S, Kalina T, et al. A high‐throughput pipeline for validation of antibodies. Nat Methods. 2018;15:909–12. [DOI] [PubMed] [Google Scholar]
- 32. Toebes M, Coccoris M, Bins A, Rodenko B, Gomez R, Nieuwkoop NJ, et al. Design and use of conditional MHC class I ligands. Nat Med. 2006;12:246–51. [DOI] [PubMed] [Google Scholar]
- 33. Toebes M, Rodenko B, Ovaa H, Schumacher TN. Generation of peptide MHC class I monomers and multimers through ligand exchange. Curr Protoc Immunol. 2009;87:18.16.1–20. [DOI] [PubMed] [Google Scholar]
- 34. Zakeri K, Yu Y, Lee N. An imbalance in competing mortality favouring Debio 1143. Lancet Oncol. 2020;21:e502. [DOI] [PubMed] [Google Scholar]
- 35. Cattaneo C, Cancelli V, Imberti L, Dobbs K, Sottini A, Pagani C, et al. Production and persistence of specific antibodies in COVID‐19 patients with hematologic malignancies: role of rituximab. Blood Cancer J. 2021;11:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vijenthira A, Gong I, Betschel SD, Cheung M, Hicks LK. Vaccine response following anti‐CD20 therapy: a systematic review and meta‐analysis of 905 patients. Blood Adv. 2021;5:2624–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen‐specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID‐19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–9. [DOI] [PubMed] [Google Scholar]
- 39. Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, et al. Rapid induction of antigen‐specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS‐CoV‐2 mRNA vaccination. Immunity. 2021;54:2133–42 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Angyal A, Longet S, Moore SC, Payne RP, Harding A, Tipton T, et al. T‐cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS‐CoV‐2‐naive UK health‐care workers: a multicentre prospective cohort study. Lancet Microbe. 2022;3:e21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woldemeskel BA, Garliss CC, Blankson JN. SARS‐CoV‐2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS‐CoV‐2 variants and HCoV‐NL63. J Clin Invest. 2021;131:e149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oberhardt V, Luxenburger H, Kemming J, Schulien I, Ciminski K, Giese S, et al. Rapid and stable mobilization of CD8(+) T cells by SARS‐CoV‐2 mRNA vaccine. Nature. 2021;597:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 vaccine induces neutralizing antibodies and poly‐specific T cells in humans. Nature. 2021;595:572–7. [DOI] [PubMed] [Google Scholar]
- 44. Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, et al. Personalized RNA mutanome vaccines mobilize poly‐specific therapeutic immunity against cancer. Nature. 2017;547:222–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available within the article and its supplementary information files. Raw data are available from the corresponding authors upon reasonable request.


