Abstract
COVID-19, the syndrome caused by the novel coronavirus SARS-CoV-2, has spread throughout the world, causing the death of at least three million people. For the over 81 million who have recovered, however, the long-term effects are only beginning to manifest. We performed a bilateral lung transplant on a 31-year-old male patient for chronic hypoxic respiratory failure, severe pulmonary hypertension and radiographically identified pulmonary fibrosis five months after an acute COVID-19 infection. The explant demonstrated moderate pulmonary vascular remodeling with intimal thickening and medial hypertrophy throughout, consistent with pulmonary hypertension. The parenchyma demonstrated an organizing lung injury in the proliferative phase, with severe fibrosis, histiocytic proliferation, type II pneumocyte hyperplasia, and alveolar loss consistent with known COVID-19 pneumonia complications.
This report highlights a novel histologic finding in severe, chronic COVID-19. Although the findings in acute COVID-19 pneumonia have been well-examined at autopsy, the chronic course of this complex disease is not yet understood. The case presented herein suggests that COVID-induced pulmonary hypertension may become more common as more patients survive severe SARS-CoV-2-related pneumonia. Pulmonologists and pulmonary pathologists should be aware of this possible association and look for the clinical, radiographic, and histologic criteria in the appropriate clinical setting.
Keywords: COVID-19, pneumonia, pulmonary hypertension, lung transplant
Introduction
Vaccination for SARS-CoV-2 has significantly reduced the risk of severe disease from acute infection.1 Despite the thorough histologic examinations performed on decedents’ tissues performed through the world,2–5 there is a paucity of histological data on those who do not develop severe disease, likely driven by a combination of variable post-infection sequelae and unclear benefit of histopathologic characterization for these patients. This is particularly relevant because of so-called “breakthrough” cases despite adequate vaccine protection, as well as the severe worldwide dearth of vaccine.
Here, a patient developed severe COVID-19 pneumonia, eventually requiring transplantation.
Case Report
The patient is a 31-year-old male resident of the rural Midwestern United States with a past medical history significant only for Grade 3 obesity who was diagnosed with COVID-19 by two distinct RT-PCR assays under emergency use authorization. He had not received COVID-19 vaccination and initially felt well enough to perform physically rigorous work. Over the next few days, he worsened, requiring hospital admission for metabolic encephalopathy and hypoxic respiratory failure for which he received dexamethasone, remdesivir, and convalescent plasma. His chest CT demonstrated typical findings of acute COVID-19 pneumonia. His condition deteriorated such that he required mechanical ventilation and prone positioning for hypoxia. His course was complicated by acute kidney injury, septic shock, deep venous thrombosis and pneumothorax requiring a thoracostomy tube. After several weeks of mechanical ventilation, weaning proved unlikely and a tracheostomy was placed. Although he recovered from the acute phase of multi-organ dysfunction, he remained ventilator- dependent with persistent, severe hypoxia; for this he underwent lung transplant evaluation. A COVID screen on a tracheal aspirate (Cobas e680, Roche Diagnostics, Mannheim, Germany) was negative during evaluation. Repeat CT imaging at that time revealed fibrotic changes and ground glass throughout both lungs. Right heart catheterization performed for transplant evaluation showed severe pulmonary arterial hypertension of 72/31 mm Hg which reduced to 47/16 with nitric oxide. The patient was initiated on inhaled trepostinil for the purposes of improved V/Q matching in hopes of enabling physical therapy. At the time of evaluation, the patient's BMI precluded lung transplant. In the two months following evaluation, the patient's exertional capacity improved and BMI declined. At the time of transplant, the patient was able to walk 728 feet in 6 min while mechanically ventilated on 100% oxygen. Notably, a rapid nasopharyngeal COVID screen (Xpert Xpress, Cepheid, Sunnyvale, CA) performed without symptoms four days prior to transplant demonstrated a positive screen with a cycle threshold value (Ct) of 43 cycles; a new sample taken the following day was negative.
The patient received a deceased donor bilateral lung transplant 140 days after his initial diagnosis with COVID-19 pneumonia. Intraoperative findings include no loss of tissue planes and no adhesions. The explanted lungs were subjected to formalin fixation prior to sectioning. Transbronchial fixation failed due to poor lung filling, and formalin injection was necessary. The parenchyma in all lobes was mottled, congested, consolidated, and mahogany to brown in color. No area of normal-appearing lung was identified. The right upper lobe was also remarkable for a 0.5 × 0.5 × 0.5 cm well-encapsulated, sub-pleural, red-brown heterogeneous lesion.
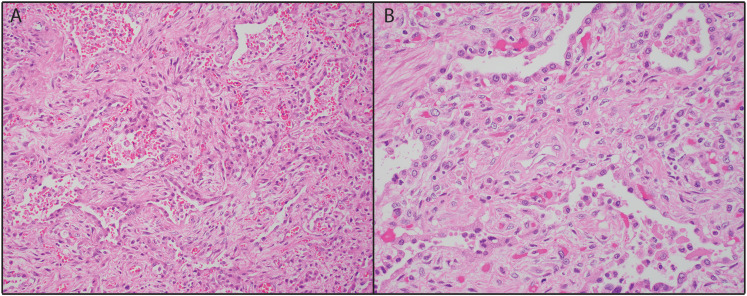
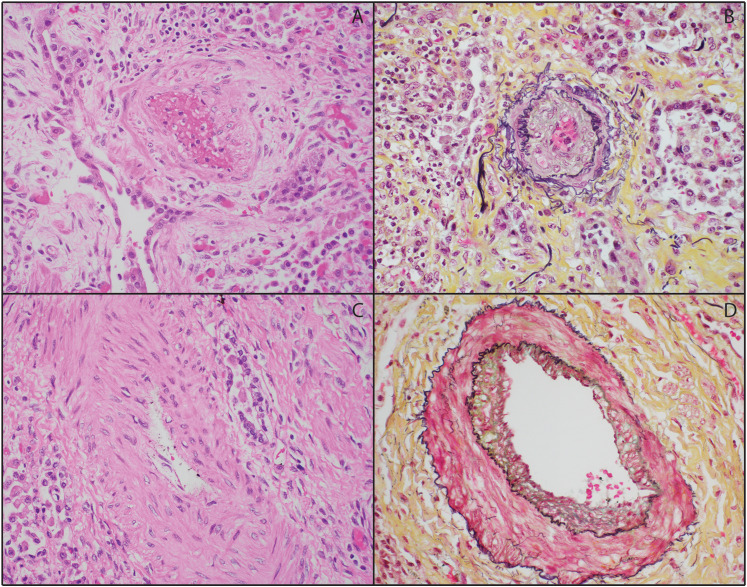
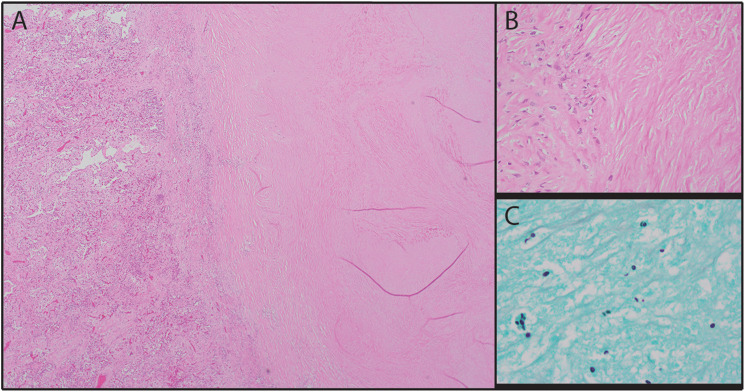
Histologic examination of the lung parenchyma revealed organizing diffuse alveolar damage in the proliferative phase with prominent fibrosis, type II pneumocyte hyperplasia, and near-complete loss of alveolar air spaces (Figure 1). The remaining intra-alveolar spaces were filled with chronic inflammatory cells and scattered histiocytes. There was no evidence of viral cytopathic effect, bacteria, acute inflammation, or hyaline membranes. Parenchymal sections were also remarkable for mild to moderate arteriolar intimal thickening and medial hypertrophy patchily distributed throughout both lungs (Figure 2). Plexiform lesions were not identified. The right upper lobe lesion was composed of a necrotizing granuloma with scattered yeast forms compatible with Histoplasma (Figure 3), but further microbiological workup was not performed.
Figure 1.
Representative lung explant. A: The lung parenchyma demonstrates the organizing phase of diffuse alveolar damage (DAD), with prominent fibrosis (H&E, × 200). B: Type II pneumocyte hyperplasia and collapsed alveoli with interspersed histiocytes are easily identified (H&E, × 400).
Figure 2.
Representative vascular changes in lung explant. A-B: Scattered arterioles demonstrated moderate intimal thickening (A: H&E, B: Movat pentachrome; × 200). C-D: Scattered arteries had moderate medial hyperplasia (C: H&E, B: Movat pentachrome; × 200).
Figure 3.
Subpleural necrotizing granuloma. A-B: The lesion consisted of a necrotic core surrounded by epithelioid histiocytes (A: H&E, × 40; B: H&E, × 400). C: The necrotic core contained numerous yeast forms with narrow-based budding consistent with Histoplasma (Grocott methenamine silver, × 1000).
The patient was successfully transplanted, and fully weaned from mechanical ventilation on post-operative day 5. He is currently doing well and progressing through pulmonary rehabilitation similarly to non-COVID transplant recipients.
Discussion
The novel coronavirus SARS-CoV-2, the causative agent in COVID-19, irrevocably altered human interactions from its discovery in Wuhan, China in late 2019 and its subsequent distribution throughout the globe. The pandemic has infected over 224 million people worldwide and is tied to over 4.6 million deaths at the time of writing.6 Though numerous primary care and autopsy reports have highlighted the systemic physiological changes focused on the lungs caused by acute and fatal COVID-19 cases,2–5 as the pandemic continues, the long-term histologic sequelae in patients become more apparent. “Long COVID,” a group of physical, physiological, and psychological symptoms that follow SARS-CoV-2 infection for an extended period,7 is a related concept, but this patient's rapid and prolonged deterioration does not fit with the use of this term.
We report here a patient with chronic respiratory failure secondary to COVID-19 who received a bilateral lung transplant for post-COVID respiratory failure. He developed marked pulmonary hypertension in the months leading up to his transplantation. His explant demonstrated pulmonary hypertensive changes in addition to end-stage fibrosis and chronic-phase diffuse alveolar damage / organizing pneumonia. The obliterative changes that are identified in this explant are consistent with what we and others have previously described at autopsy in fatal acute and subacute COVID-19 pneumonia.3–5 We also report probable histoplasmosis, a likely incidental finding that is common in our geographic area. Of note, a recent report of COVID pneumonia requiring lung transplantation demonstrated a granuloma containing Candida.8 This patient did not receive any treatment for his Histoplasma but receives prophylaxis post-transplant due to his now immunocompromised status.
In two case series describing lung transplantation for COVID-19 pneumonia, pulmonary hypertension was a common finding.9,10 Other groups as well have found a possible association between pulmonary hypertension and COVID,11 including on autopsy specimens.12 To our knowledge, this case report is unique in demonstrating pictorially the histologic changes of pulmonary hypertension in a patient on maximal pre-transplant prostaglandin therapy, as well as the longest reported ventilatory requirement (128 days). Further work is needed to describe the prevalence of pulmonary hypertension and the associated pathologic changes in patients who develop chronic symptoms after COVID-19. Given the large number of people who have been infected with the virus, this could have enormous clinical relevance to patients and the medical community.
Interestingly, the patient's first positive COVID screen was three months prior to his negative test during transplant evaluation. However, a nasopharyngeal rapid test performed shortly prior to transplant had a Ct value of 43 cycles, a detectable but low level of virus. Although we cannot necessarily exclude reinfection, a high Ct value in ultrasensitive assays is thought to represent remaining viral nucleic acid but not transmissible virus and can persist for months after primary infection.13 His repeat test the next day was negative.
In contrast to other cases of COVID-related lung transplant in the literature,9,10 in which the majority of the patients demonstrated severe pulmonary adhesions, the patient reported here did not have any adhesions. The significance of this finding is unclear.
As this is a single case, there are some limitations. We cannot know what the patient's baseline pulmonary function was prior to infection because he had never been tested. He was obese, but had never presented for any significant medical workup, including pulmonary or cardiac, prior to his hospitalization. We also cannot generalize our findings to all infected individuals without more specimens.
Conclusion
This case report contributes to the growing body of evidence supporting the development of pulmonary hypertension in patients with chronic COVID-19 pneumonia. As the post-COVID population will only increase, pulmonologists and pulmonary pathologists should be aware of the possibility of COVID-induced pulmonary arteriolar remodeling and consider clinical, radiologic, and perhaps histologic correlation when the situation arises.
Footnotes
Author Contributions: Conception and design of study: BJS, SJR, JMR. Medical management and surgical performance: HS, DB, AS. Pathologic performance: JMR, SJR, BJS. Drafting of manuscript: JMR. All authors edited and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Swanson serves on the scientific advisory board of Cogen Biosciences and owns stock in the company.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical Approval: At our institution, single case studies with participant approval do not require IRB oversight.
Informed Consent: The patient gave informed consent for this study.
Trial Registration: N/A
ORCID iD: Joseph M. Rohr https://orcid.org/0000-0002-9710-6471
References
- 1.Moline H, Whitaker M, Deng L, et al. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥65 Years — COVID-NET, 13 States, February–April 2021. Vol. 70. 2021:1088-1093. Morbidity and Mortality Weekly Report. August 13, 2021. [DOI] [PMC free article] [PubMed]
- 2.Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Aug. 11 2020;370(m3026). doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 3.Alshomrani A, Rohr J, Carda M, et al. A Sticky Issue: Thrombotic Phenomena Amidst Autopsy Findings in Fatal Cases of COVID-19. Video and abstract, United States and Canadian Academy of Pathology Annual Meeting (virtual meeting), March 2021.
- 4.Elsoukkary SS, Mostyka M, Dillard A, et al. Autopsy findings in 32 patients with COVID-19: a single-institution experience. Pathobiology. 2021;88(1):56-68. doi: 10.1159/000511325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satturwar S, Fowkes M, Farver C, et al. Postmortem findings associated With SARS-CoV-2: systematic review and meta-analysis. Am J Surg Pathol. May 1 2021;45(5):587-603. doi: 10.1097/PAS.0000000000001650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. May 2020;20(5):533-534. doi: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. Apr 2021;27(4):626-631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aesif SW, Bribriesco AC, Yadav R, et al. Pulmonary pathology of COVID-19 following 8 weeks to 4 months of severe disease: a report of three cases, including One With bilateral lung transplantation. Am J Clin Pathol. Mar 15 2021;155(4):506-514. doi: 10.1093/ajcp/aqaa264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharat A, Querrey M, Markov NS, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. Dec 16 2020;12(574). doi: 10.1126/scitranslmed.abe4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharat A, Machuca TN, Querrey M, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. Mar 31 2021;9(5):487–497. doi: 10.1016/S2213-2600(21)00077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JD, Burger CD, Delossantos GB, et al. A survey-based estimate of COVID-19 incidence and outcomes among patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension and impact on the process of care. Ann Am Thorac Soc. Dec 2020;17(12):1576-1582. doi: 10.1513/AnnalsATS.202005-521OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki YJ, Nikolaienko SI, Shults NV, Gychka SG. COVID-19 patients may become predisposed to pulmonary arterial hypertension. Med Hypotheses. Feb 2021;147:110483. doi: 10.1016/j.mehy.2021.110483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plebani M. Persistent viral RNA shedding in COVID-19: caution, not fear. EBioMedicine. Feb 2021;64:103234. doi: 10.1016/j.ebiom.2021.103234 [DOI] [PMC free article] [PubMed] [Google Scholar]