Summary
Although eyelid reconstruction by transplanting an autologous free tarsoconjunctival graft (FTG) is a well-established technique, few studies have examined the postoperative course of FTG transplantation for East Asian eyelids, including those of Japanese patients. Therefore, this study investigated complication and reoperation rates after FTG transplantation in the reconstruction of East Asian (Japanese) eyelids.
This study included 42 eyelids wherein posterior lobe reconstruction after resection of a malignant tumour of the eyelid was performed by FTG transplantation between 2007 and 2019 at Niigata University Medical and Dental Hospital. We investigated complications and need for revision surgery during the patients’ postoperative courses. The relationship between postoperative complications, tumour diameter, and eyelid defect width was statistically examined.
Of 42 cases reconstructed with FTG, the upper eyelid was reconstructed in 23. Postoperative complications were observed in 12 cases (52%): entropion in eight and corneal epithelial disorder in four. Revision surgery was required in three of those cases (13%). There were 19 cases of lower eyelid reconstruction. Postoperative complications were observed in seven cases (32%): ectropion in three and corneal epithelial disorder in two and one lower eyelid ptosis. Two of these cases (11%) required revision surgery. There was no statistically significant difference in tumour diameter between cases with and without postoperative complications. There was also no significant association between the width of the eyelid defect and the presence/absence of complications. Entropion and ectropion were more likely to occur in the upper and lower eyelids, respectively.
For Japanese eyelids, complication rates after FTG transplantation were approximately 50% and 30% for the upper and lower eyelids, respectively. The revision surgery rate was approximately 10% for both upper and lower eyelids. As these revision surgery rates are low, FTG transplantation may be an option for the reconstruction of Japanese eyelids.
Keywords: Tarsoconjunctival graft, Eyelid tumour, Surgery, Japanese
Introduction
Malignant eyelid tumours represented by basal cell carcinoma and sebaceous carcinoma are diseases that frequently occur in the elderly.1 Treatment is mainly surgical resection and radiation therapy, but radiation therapy is often selected for patients who refuse surgery or have no indication for radical surgery.1 The basis of treatment for malignant eyelid tumours is complete resection of the lesion and reconstruction of the eyelid.1 For eyelid reconstruction, it is important to select a reconstructive method that does not reduce postoperative quality of life and vision. This is one of the most challenging treatments in oculoplastic surgery.2
Reconstruction of the full thickness of the eyelid requires anterior and posterior lamella reconstruction.3 Particularly, reconstruction of the posterior lamella, which has direct contact with the ocular surface, is important for maintaining postoperative quality of life and vision.4 Use of an autologous tarsoconjunctival graft is the best option for reconstructing the posterior lamella.5 Several methods of posterior lamella reconstruction that use an autologous tarsoconjunctival graft have been reported previously. The Hughes flap used to reconstruct the lower eyelid and the Cutler-Beard method for reconstructing the upper eyelid are standard techniques for reconstructing the posterior and anterior lamella of the eyelid with autologous tissue.6,7 Recently, some modifications of these techniques have been reported.8, 9, 10 Additionally, the switch flap is a useful method in that it uses autologous tissue for posterior lamella reconstruction, similar to the Hughes or Cutler-Beard techniques.11,12 However, all aforementioned surgical procedures are two-stage surgeries. During the weeks between the first and second surgeries, the surgical eye remains covered with a transplant graft. Two-stage surgery requires temporary reductions in the patient's quality of vision and life. Furthermore, malignant eyelid tumours are more common in elderly patients,13 and thus covering one eye can lead to accidents such as falls. If tumour resection and eyelid reconstruction can be performed in a one-stage surgery, the reductions described above can be avoided.
Reconstruction of the eyelid by transplanting an autologous free tarsoconjunctival graft (FTG) is another well-established approach; with this method, it is possible to resect the tumour and reconstruct the eyelid in a single surgery.14 One-stage surgery does not require covering one eye and seems to be a useful method. However, to the best of our knowledge, only a limited number of reports have been published on the comprehensive postoperative results after one-stage eyelid reconstruction by autologous FTG transplantation.15, 16, 17 In particular, there are few studies on the postoperative course of FTG transplantation for East Asian eyelids, including patients from Japan. Therefore, this study aimed to investigate the postoperative course (the complications and revision surgery rates) of cases in which FTG transplantation was used for eyelid reconstruction after resection of a malignant eyelid tumour in East Asians (Japanese). In addition, we compared the postoperative courses of our Japanese patients with those from previous reports on Caucasian populations.
Patients and methods
Ethics statements
This study was approved by the Ethics Committee of Niigata University School of Medicine (approval number: 2019–0435). This single-centre retrospective study follows the STROBE guidelines. As the study was based on data from medical records, an opt-out method was used to obtain patients’ consent. The study adheres to the ethical principles outlined in the Declaration of Helsinki as amended in 2013, and the research was compliant with the Health Insurance Portability and Accountability Act of 1996.
Study population
This study included cases in which a malignant tumour of the eyelid was resected with full thickness and the posterior lamella of the eyelid was reconstructed by FTG transplantation at Niigata University Medical and Dental Hospital from January 2007 to December 2019. Exclusion criteria were cases with a postoperative follow-up period of less than 12 months and those with a positive margin in the pathological diagnosis. The participants of this study were only Japanese.
Surgical procedure
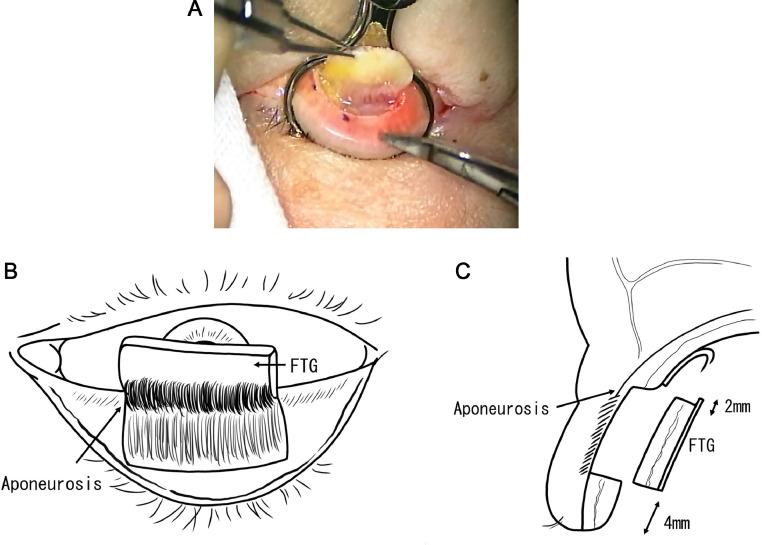
The lesion of the eyelid was resected in full thickness with a safety margin of 2–5 mm (basal cell carcinoma: 2–3 mm, sebaceous carcinoma: 4–5 mm, squamous cell carcinoma and Merkel cell carcinoma: 5 mm). Subsequently, the posterior lamella was reconstructed with FTG harvested from the ipsilateral upper eyelid or the contralateral upper eyelid. FTG was harvested by full-layer incision of the palpebral conjunctiva and tarsus at 4 mm from the upper eyelid margin, dissecting the aponeurosis, then cutting the conjunctiva at 2 mm from the upper edge of the tarsus (Fig. 1). The harvested FTG was suture-fixed to the cut ends of the tarsus and aponeurosis of the defect area (Fig. 2). In the case of the lower eyelid, FTG was fixed to the cut ends of the tarsus and lower eyelid retractors. If there was no tarsus stump left, it was fixed to the medial or lateral canthal tendon. If the tendon was not long enough to reconstruct the posterior lamella, the orbital periosteum was incised to create a flap to fix the FTG (orbital periosteal flap).
Fig. 1.
FTG harvesting. a. The upper eyelid tarsus and palpebral conjunctiva are incised at full-layers, and the aponeurosis is detached (surgeon's view). b. Schema of the FTG harvesting (surgeon's view). c. Schema of sagittal section: the graft is harvested 4 mm away from the upper eyelid margin. The conjunctiva is cut at 2 mm from the upper edge of the tarsus. FTG: free tarsoconjunctival graft.
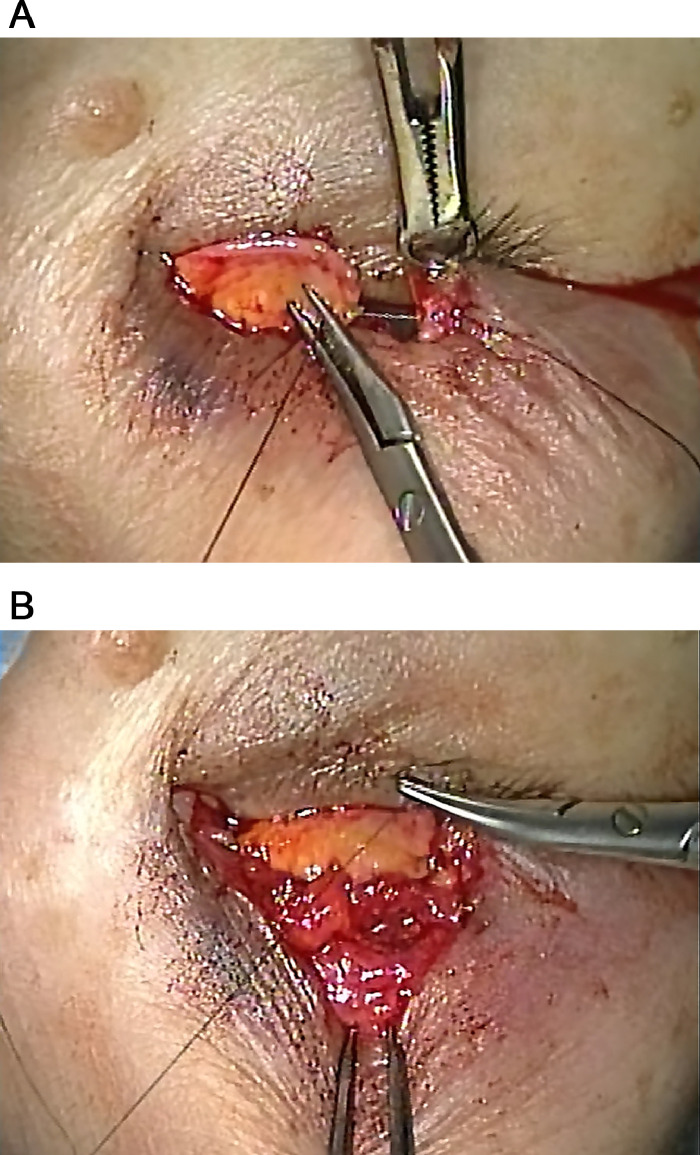
Fig. 2.
Reconstruction of the posterior lamella with FTG (upper eyelid, surgeon's view) Suture fixation of the graft to the cut end of the tarsus (Fig. 2-a) and aponeurosis (Fig. 2-b). FTG: free tarsoconjunctival graft.
Anterior lamella reconstruction was performed using the redundant skin and orbicularis oculi muscle to cover the defect. The redundant skin was trimmed as needed. If the redundant skin was not sufficient to cover the defect, a local myocutaneous flap was created to reconstruct the anterior lamella. Local myocutaneous flaps such as the V-Y advancement flap (Fig. 3) and transposition flap (Fig. 4) were used properly depending on the case. In all cases, tumour resection and eyelid reconstruction were completed with one-stage surgery.
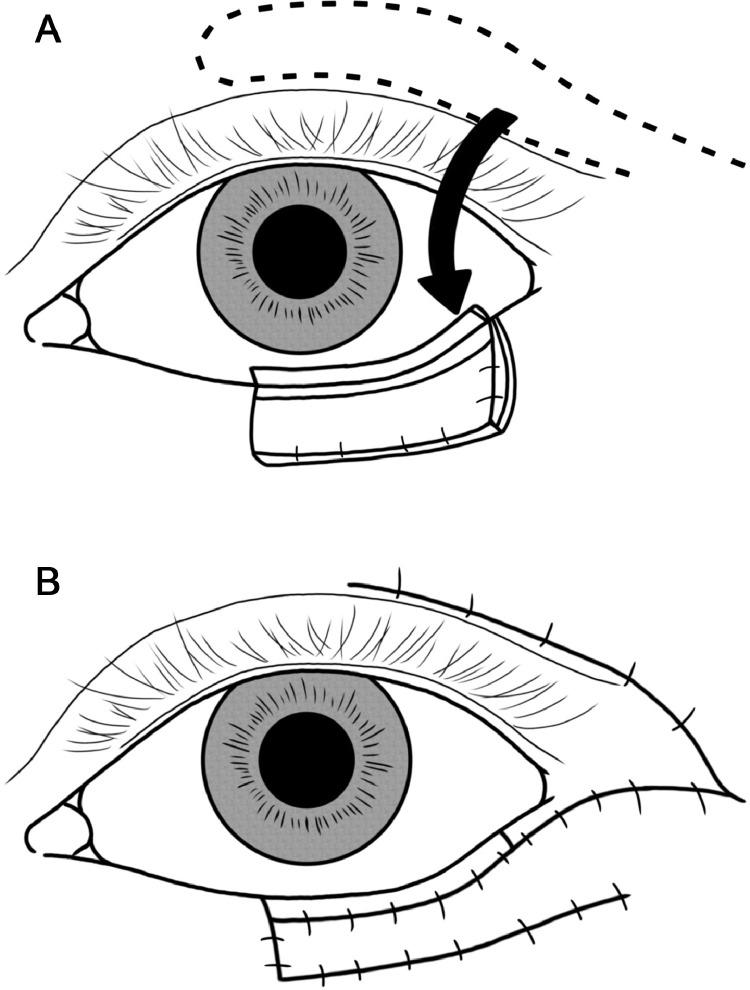
Fig. 3.
Schema of V-Y advancement flap (upper eyelid). a. Create a V-shaped myocutaneous flap from the defect site toward the lateral side.b. Advance the myocutaneous flap horizontally and suture it in a Y-shape.
Fig. 4.
Schema of transposition flap (lower eyelid). a. Create a flap with the anterior lamella of the upper eyelid with a pedicle on the lateral side of the lateral canthal angle.b. Suture the area covered by the flap and the area where the flap was created, respectively. Normal skin is partially trimmed as needed.
Data collection and statistical analysis
All patient data were collected from the electronic medical record system at our hospital. Age, sex, the postoperative follow-up period (month), preoperative tumour diameter (mm), tumour pathological diagnosis, width of the eyelid defect in the lateral diameter after tumour resection (%), and posterior and anterior lamella reconstruction procedures were obtained from patients’ medical records and noted. We also investigated the presence or absence of postoperative complications and the need for revision surgery. Postoperative complications included corneal epithelial disorders, entropion, lower eyelid ptosis, and ectropion. Even if there was no subjective eye symptom of the patient, we considered that there was a complication if it occurred objectively. Furthermore, the relationship between postoperative complications, the tumour diameter, and width of the eyelid defect was statistically examined using Student's t-tests, Fisher's exact tests, and Welch's tests. All statistical analyses were performed using SPSS statistical software version 23.0 (IBM Corp., Armonk, NY, USA), and statistical significance was defined as p < 0.05.
Results
Between January 2007 and December 2019, 49 eyelids in 49 patients underwent reconstruction of the posterior lobe with FTG after resection of the malignant tumour of the eyelid. Of these, five patients with an observation period of less than 12 months, one patient with a positive margin on histological diagnosis, and one Caucasian patient were excluded.
The mean (±standard deviation) age of the 42 included patients who underwent FTG transplantation for posterior lamella reconstruction was 73.5 (±11.8) years. There were 16 males and 26 females. The mean postoperative follow-up period was 49.5 (±33.2) months (Table 1).
Table 1.
Clinical characteristics of patients who underwent eyelid reconstruction with a free tarsoconjunctival graft.
| Characteristic | |
|---|---|
| Total number of patients, n | 42 |
| Age (years) | 73.5 (±11.8, 38–93) |
| Men/women, n | 16/26 |
| Postoperative follow-up period (months) | 49.5 (±33.2, 12–141) |
| Upper/lower eyelid, n | 23/19 |
| Type of eyelid tumour, n (%) | |
| Sebaceous carcinoma | 26 (61.9%) |
| Basal cell carcinoma | 10 (23.8%) |
| Squamous cell carcinoma | 4 (9.5%) |
| Merkel cell carcinoma | 2 (4.8%) |
Data are presented as the mean (±standard deviation, minimum–maximum) unless otherwise noted.
Of 42 cases in which FTG transplantation was used for posterior lamella reconstruction, 23 cases involved reconstruction of the upper eyelid. The mean lesion size was 9.8 (±3.1) mm. The width of the eyelid defect was 75% or more of the lateral width (large defect) in eight cases, 50–75% or less (moderate defect) in 12 cases, and less than 50% (small defect) in three cases. FTGs used for posterior lamella reconstruction were harvested from the contralateral upper eyelid in all cases. There were 17 cases of an advancement flap from the superior skin and six cases of a V-Y advancement flap from the temporal side in anterior lamella reconstruction. Postoperative complications were observed in 12/23 cases (52%): entropion in eight cases and corneal epithelial disorder in four. Three of those cases (13%) required revision surgery (Table 2).
Table 2.
Overview of the 23 cases of reconstructed upper eyelids.
| Case | Diagnosis | Tumour diameter (mm) | Operative method | Defect width | Postoperative complication | Revision surgery |
|---|---|---|---|---|---|---|
| 1 | SC | 12 | Contralateral FTG + orbital periosteal flap + redundant skin flap | Large | (-) | (-) |
| 2 | SC | 10 | Contralateral FTG + redundant skin flap | Large | (-) | (-) |
| 2 | SC | 10 | Contralateral FTG + redundant skin flap | Large | Entropion | (-) |
| 4 | SC | 12 | Contralateral FTG + redundant skin flap | Large | Corneal epithelial disorder | (-) |
| 5 | SC | 10 | Contralateral FTG + V-Y flap | Large | Corneal epithelial disorder | (-) |
| 6 | SC | 15 | Contralateral FTG + V-Y flap | Large | Corneal epithelial disorder | (-) |
| 7 | SC | 10 | Contralateral FTG + redundant skin flap | Moderate | (-) | (-) |
| 8 | SC | 10 | Contralateral FTG + redundant skin flap | Moderate | (-) | (-) |
| 9 | SC | 8 | Contralateral FTG + V-Y flap | Moderate | Entropion | (-) |
| 10 | SC | 6 | Contralateral FTG + redundant skin flap | Moderate | Entropion | (+) |
| 11 | SC | 6 | Contralateral FTG + redundant skin flap | Moderate | Entropion | (+) |
| 12 | SC | 8 | Contralateral FTG + V-Y flap | Moderate | Entropion | (+) |
| 13 | SC | 8 | Contralateral FTG + redundant skin flap | Moderate | Entropion | (-) |
| 14 | SC | 8 | Contralateral FTG + redundant skin flap | Moderate | Entropion | (-) |
| 15 | SC | 7 | Contralateral FTG + V-Y flap | Moderate | Entropion | (-) |
| 16 | SC | 8 | Contralateral FTG + redundant skin flap | Moderate | (-) | (-) |
| 17 | SC | 8 | Contralateral FTG + redundant skin flap | Small | (-) | (-) |
| 18 | SC | 5 | Contralateral FTG + redundant skin flap | Small | (-) | (-) |
| 19 | BCC | 10 | Contralateral FTG + redundant skin flap | Moderate | (-) | (-) |
| 20 | BCC | 10 | Contralateral FTG + redundant skin flap | Small | (-) | (-) |
| 21 | MCC | 18 | Contralateral FTG + redundant skin flap | Large | (-) | (-) |
| 22 | MCC | 14 | Contralateral FTG + redundant skin flap | Large | Corneal epithelial disorder | (-) |
| 23 | SCC | 12 | Contralateral FTG + V-Y flap | Moderate | (-) | (-) |
SC: sebaceous carcinoma, FTG: free tarsoconjunctival graft, large: width of the eyelid defect was over 75% of the lateral width, BCC: basal cell carcinoma, moderate: width of the eyelid defect was 50–75% of the lateral width, MCC: Merkel cell carcinoma, small: width of the eyelid defect was less than 50% of the lateral width, SCC: squamous cell carcinoma, V-Y flap: V-Y advancement flap, -: no, +: yes.
In 19 cases, the lower eyelids were reconstructed with FTG transplantation. The mean lesion size was 9.8 (±3.7) mm. The width of the eyelid defect was a large defect in one case; all other cases had moderate defects. FTGs were harvested from the ipsilateral upper eyelid in 18 cases and from the contralateral upper eyelid in two cases. In anterior lamella reconstruction, there were 11 cases of a transposition flap from the upper eyelid, three cases of an advancement flap from the inferior skin, and two cases of a V-Y advancement flap from the lateral side. The other three cases were reconstructed with a combination of multiple flaps. Postoperative complications were observed in 6/19 cases (32%): ectropion in three cases, corneal epithelial disorder in two cases, and lower eyelid ptosis in one case. Two of those cases (11%) required revision surgery (Table 3).
Table 3.
Overview of the 19 cases of reconstructed lower eyelids.
| Case | Diagnosis | Tumour diameter (mm) | Operative method | Defect width | Postoperative complication | Revision surgery |
|---|---|---|---|---|---|---|
| 24 | SC | 20 | Ipsilateral FTG + V-Y flap | Large | Ectropion | (-) |
| 25 | SC | 10 | Ipsilateral FTG + advancement flap with lateral Z-plasty | Moderate | (-) | (-) |
| 26 | SC | 6 | Ipsilateral FTG + redundant skin flap | Moderate | (-) | (-) |
| 27 | SC | 13 | Ipsilateral FTG + transposition flap | Moderate | (-) | (-) |
| 28 | SC | 8 | Ipsilateral FTG + transposition flap | Moderate | (-) | (-) |
| 29 | SC | 8 | Ipsilateral FTG + transposition flap | Moderate | (-) | (-) |
| 30 | SC | 7 | Ipsilateral FTG + transposition flap | Moderate | (-) | (-) |
| 31 | SC | 5 | Ipsilateral FTG + transposition flap | Moderate | Corneal epithelial disorder | (+) |
| 32 | BCC | 5 | Ipsilateral FTG + transposition flap | Moderate | Corneal epithelial disorder | (-) |
| 33 | BCC | 14 | Ipsilateral FTG + transposition flap | Moderate | Lower lid ptosis | (+) |
| 34 | BCC | 7 | Ipsilateral FTG + transposition flap | Moderate | (-) | (-) |
| 35 | BCC | 10 | Ipsilateral FTG + transposition flap | Moderate | (-) | (-) |
| 36 | BCC | 10 | Ipsilateral FTG + redundant skin flap | Moderate | (-) | (-) |
| 37 | BCC | 7 | Ipsilateral FTG + V-Y flap | Moderate | Ectropion | (-) |
| 38 | BCC | 10 | Ipsilateral FTG + V-Y flap + transposition flap | Moderate | (-) | (-) |
| 39 | BCC | 12 | Contralateral FTG + vertical & horizontal V-Y flap, bilobed flap | Moderate | Ectropion | (-) |
| 40 | SCC | 13 | Ipsilateral FTG + transposition flap | Moderate | (-) | (-) |
| 41 | SCC | 12 | Ipsilateral FTG + transposition flap | Moderate | (-) | (-) |
| 42 | SCC | 10 | Contralateral FTG + redundant skin flap | Moderate | (-) | (-) |
SC: sebaceous carcinoma, FTG: free tarsoconjunctival graft, large: width of the eyelid defect was over 75% of the lateral width, BCC: basal cell carcinoma, moderate: width of the eyelid defect was 50–75% of the lateral width, SCC: squamous cell carcinoma, V-Y flap: V-Y advancement flap, -: no, +: yes.
We compared tumour diameters between the groups with and without complications after resection and reconstruction surgery, but no statistically significant difference was found. We also examined the relationship between the width of the eyelid defect and the presence or absence of complications, but without significant difference (Table 4). Further, we compared the types of complications that occurred in the upper and lower eyelids and found that entropion was more likely to occur in the upper eyelid, whereas ectropion was more likely to occur in the lower eyelid (Table 5, p = 0.003, Fisher's exact test).
Table 4.
Comparison of cases with and without complications.
| Postoperative complications |
|||
|---|---|---|---|
| (+) | (-) | p-value | |
| Upper lid, n | 12 | 11 | |
| Tumour size (mm) | 9.3 (±3.0) | 10.3 (±3.2) | 0.48* |
| Width of the upper lid defect, n | |||
| Large | 5 | 3 | |
| Moderate | 7 | 5 | |
| Small | 0 | 3 | 0.23⁎⁎ |
| ower lid, n | 6 | 13 | |
| Tumour size (mm) | 10.5 (±6.0) | 9.5 (±2.3) | 0.72† |
| Width of the lower lid defect, n | |||
| Large | 1 | 0 | |
| Moderate | 5 | 13 | 0.32⁎⁎ |
Data are presented as the mean (±standard deviation) unless otherwise noted.
Calculated using Student's t-test.
Calculated using Fisher's exact test.
Calculated using Welch's test.
Large: width of the eyelid defect was over 75% of the lateral width, moderate: width of the eyelid defect was 50–75% of the lateral width, small: width of the eyelid defect was less than 50% of the lateral width, -: no, +: yes.
Table 5.
Comparison of complications between upper and lower eyelids.
| Cases with complications |
||
|---|---|---|
| Upper eyelids (n = 12) | Lower eyelids (n = 6) | |
| Entropion | 8 | 0 |
| Corneal epithelial disorder | 4 | 2 |
| Ectropion | 0 | 3 |
| Lower eyelid ptosis | 0 | 1 |
Discussion
This study examined 42 upper and lower eyelids reconstructed with FTG. Of the 42 eyelids, 18 eyelids (43%) had postoperative complications, and five eyelids (12%) required revision surgery. Hawes and Jamell15 reported that of 44 upper and lower eyelids reconstructed with FTG transplantation, 37 (84%) had postoperative complications, and five (11%) required revision surgery. The authors stated that the complication rate was high, but that most of the complications were mild. In addition, there are several cases where complications have improved spontaneously.15 Because some cases had a short postoperative observation period, it appears that there were cases where complications improved over time after the research.15 Owing to this background, it is difficult to make a simple comparison with our study, but the complication rate and reoperation rate after FTG transplantation in East Asians (Japanese) were not bad. The tarsal plate of the Japanese is smaller than that of Caucasians,18 but there were no cases in our study in which the eyelid reconstruction with FTGs was difficult. Ethnic differences in tarsal plates are thought to have little effect on this surgical procedure.
Of 42 eyelids reconstructed by FTG transplantation, 19 included the lower eyelids. Postoperative complications occurred in 32% of cases, and revision surgery was needed in 11%. In recent postoperative studies in which the lower eyelid was reconstructed with the Hughes flap, the complication rate was 15.5–27.3%, and the revision surgery rate was 0–11%, although there were variations amongst the reports.5,8,19,20 Compared with the previous reports using the Hughes flap, our study using FTG transplantation showed a slightly higher postoperative complication rate for the lower eyelid. However, Hawes et al.5 directly compared the Hughes flap and FTG transplantation and stated that FTG transplantation had fewer complications and required revision surgery less frequently. It is difficult to conclude which procedure is better, but FTG transplantation seems to be as good as using the Hughes flap in lower eyelid reconstruction. In particular, FTG transplantation appears to have a great advantage in that excision and reconstruction can be completed by one-stage surgery.
Of 42 eyelids reconstructed by FTG transplantation, 23 involved the upper eyelids. Postoperative complications occurred in 52% of cases, and revision surgery was needed in 13%. It is difficult to interpret the results of the present study because no previous report has examined the outcome of using FTG transplantation for upper eyelid reconstruction in detail; therefore, it is impossible to compare our study with previous reports. Kopecky et al.21 reported that postoperative revision surgery was required in three of 13 patients (23%) who underwent the Cutler-Beard method. A comparison of these findings with our results revealed that FTG transplantation may have a lower postoperative revision surgery rate than the Cutler-Beard method, which is a two-stage surgery. In our study, 8/23 cases (35%) of the upper eyelids had major defects with a defect width of 75% or more of the lateral width. Of these, 5/8 cases (63%) had complications, but no cases required postoperative revision surgery. Furthermore, recent studies reported that a large defect of the upper eyelid was reconstructed by one-stage surgery using FTG transplantation.22,23 Postoperative complications were observed in 40–60% of these reports, and Patrinely et al.24 noted that reoperation was performed in about 35% of these cases. Our study results showed that postoperative complications occurred in 5 of the 8 patients with large upper eyelid defects, as noted above. The complication rate was slightly higher than previously reported. However, none of the 8 patients required reoperation after surgery. Large upper eyelid defects are often difficult to reconstruct and become challenging cases, but such a case has coursed without reoperation. In Japanese patients, reconstruction of large upper eyelid defects by FTG is prone to minor complications, but the results suggest that it may be possible to course without the need for revision. Moreover, the review of eyelid reconstruction by Hada25 cited FTG transplantation as one of the methods for reconstructing large defects. Our study in East Asians likewise showed that FTG transplantation could be successfully used to reconstruct large upper eyelid defects.
In the current study, the most common complication of the upper eyelid was entropion, whereas the most common complication of the lower eyelid was ectropion. It was speculated that the upper eyelid entropion or lower eyelid ectropion was caused by the downward pulling of the anterior lamella owing to gravity. In addition, the length of the posterior lobe was insufficient, the anterior lobe was retracted, and the penetration branch of the aponeurosis did not function in the reconstructed eyelid. To solve these problems, it is necessary to review and revise the procedure in the future.
It is important to note that this study did not examine which cases developed complications and what should be done to prevent the complications. Future studies should also consider how best to further reduce complications associated with using FTG transplantation.
In conclusion, the complication rate after posterior lamella reconstruction by FTG transplantation in East Asians (Japanese) was approximately 50% in the upper eyelid and approximately 30% in the lower eyelid. The reoperation rate was about 10% for both upper and lower eyelids. The complication and reoperation rates were not high compared to those reported previously in Caucasians. Posterior lamella reconstruction by FTG transplantation is also useful for East Asian eyelids. One-stage surgery is possible even for large defects, and it may be the first choice for posterior lamella reconstruction of medium or larger defects. However, further research is needed on preventive measures for surgical complications.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This study was approved by the Ethics Committee of Niigata University School of Medicine (approval number: 2019-0435).
Declaration of Competing Interest
None.
Acknowledgments
The authors are deeply grateful to illustrator Momoko Ishiguro for the illustrations and to Editage (www.editage.jp) for English-language editing.
Footnotes
Meeting presentation: A portion of the study results was presented at the 32nd annual meeting of the Japan Society of Eye Lid & Eye Socket Surgery on February 20, 2021, in Kyoto, Japan (via website).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jpra.2022.04.003.
Appendix. Supplementary materials
References
- 1.Cook B.E., Bartley G.B. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology. 2001;108:2088–2098. doi: 10.1016/s0161-6420(01)00796-5. quiz 2099. [DOI] [PubMed] [Google Scholar]
- 2.Alghoul M., Pacella S.J., McClellan W.T., Codner M.A. Eyelid reconstruction. Plast Reconstr Surg. 2013;132:288e–302e. doi: 10.1097/PRS.0b013e3182958e6b. [DOI] [PubMed] [Google Scholar]
- 3.Rafii A.A., Enepekides D.J. Upper and lower eyelid reconstruction: the year in review. Curr Opin Otolaryngol Head Neck Surg. 2006;14:227–233. doi: 10.1097/01.moo.0000233592.76552.d2. [DOI] [PubMed] [Google Scholar]
- 4.Fin A., De Biasio F.D., Lanzetta P., Mura S., Tarantini A., Parodi P.C. Posterior lamellar reconstruction: a comprehensive review of the literature. Orbit. 2019;38:51–66. doi: 10.1080/01676830.2018.1474236. [DOI] [PubMed] [Google Scholar]
- 5.Hawes M.J., Grove A.S., Hink E.M. Comparison of free tarsoconjunctival grafts and Hughes tarsoconjunctival grafts for lower eyelid reconstruction. Ophthalmic Plast Reconstr Surg. 2011;27:219–223. doi: 10.1097/IOP.0b013e318217e194. [DOI] [PubMed] [Google Scholar]
- 6.Hughes W.L. A new method for rebuilding a lower lid. Arch Ophthalmol. 1937;17:1008–1017. [Google Scholar]
- 7.Cutler N.L., Beard C. A method for partial and total upper lid reconstruction. Am J Ophthalmol. 1955;39:1–7. doi: 10.1016/0002-9394(55)92646-5. [DOI] [PubMed] [Google Scholar]
- 8.Hishmi A.M., Koch K.R., Matthaei M., Bölke E., Cursiefen C., Heindl L.M. Modified Hughes procedure for reconstruction of large full-thickness lower eyelid defects following tumor resection. Eur J Med Res. 2016;21:27. doi: 10.1186/s40001-016-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sa H.S., Woo K.I., Kim Y.D. Reverse modified Hughes procedure for uppereyelid reconstruction. Ophthalmic Plast Reconstr Surg. 2010;26:155–160. doi: 10.1097/IOP.0b013e3181b8e5fd. [DOI] [PubMed] [Google Scholar]
- 10.Bengoa-González Á., Laslău B.M., Martín-Clavijo A., Mencía-Gutiérrez E., Lago-Llinás M.D. Reconstruction of upper eyelid defects secondary to malignant tumors with a newly modified Cutler-Beard technique with tarsoconjunctival graft. J Ophthalmol. 2019;2019:1–7. doi: 10.1155/2019/6838415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnamurthy A., Vaidhyanathan A. ‘Switch flap’ for full thickness upper eyelid reconstruction. J Cutan Aesthet Surg. 2011;4:148–150. doi: 10.4103/0974-2077.85045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y.C., Dai H.Y., Xing X., Lv C., Zhu J., Xue C.Y. Pedicled lower lid-sharing flap for full-thickness reconstruction of the upper eyelid. Eye (Lond) 2014;28:1292–1296. doi: 10.1038/eye.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quigley C., Deady S., Hughes E., McElnea E., Zgaga L., Chetty S. National incidence of eyelid cancer in Ireland (2005-2015) Eye (Lond) 2019;33:1534–1539. doi: 10.1038/s41433-019-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leone C.R., Jr, Hand S.I., Jr. Reconstruction of the medial eyelid. Am J Ophthalmol. 1979;87:797–801. doi: 10.1016/0002-9394(79)90357-x. [DOI] [PubMed] [Google Scholar]
- 15.Hawes M.J., Jamell G.A. Complications of tarsoconjunctival grafts. Ophthal Plast Reconstr Surg. 1996;12:45–50. doi: 10.1097/00002341-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson C.M., Brown B.Z. The use of tarsus as a free autogenous graft in eyelid surgery. Ophthal Plast Reconstr Surg. 1985;1:43–50. doi: 10.1097/00002341-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Hawes M.J. Free autogenous grafts in eyelid tarsoconjunctival reconstruction. Ophthal Surg. 1987;18:37–41. [PubMed] [Google Scholar]
- 18.Goold L.A., Casson R.J., Selva D., Kakizaki H. Tarsal height. Ophthalmology. 2009;116 doi: 10.1016/j.ophtha.2009.05.035. 1831-31.e2. [DOI] [PubMed] [Google Scholar]
- 19.Mashima A., Goto H., Kimura K., Shibata M. Outcome of the modified Hughes procedure for eyelid sebaceous carcinoma. Nippon Ganka Gakkai Zasshi. 2017;121:125–129. [PubMed] [Google Scholar]
- 20.Ekin M.A., Ugurlu S.K. Impact of the type of anterior lamellar reconstruction on the success of modified Hughes procedure. Arq Bras Oftalmol. 2020;83:11–18. doi: 10.5935/0004-2749.20200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopecky A., Koch K.R., Bucher F., Cursiefen C., Heindl L.M. Results of Cutler-Beard procedure for reconstruction of extensive full thickness upper eyelid defects following tumor resection. Ophthalmologe. 2016;113:309–313. doi: 10.1007/s00347-015-0146-z. [DOI] [PubMed] [Google Scholar]
- 22.Toft P.B. Reconstruction of large upper eyelid defects with a free tarsal plate graft and a myocutaneous pedicle flap plus a free skin graft. Orbit. 2016;35:1–5. doi: 10.3109/01676830.2015.1078372. [DOI] [PubMed] [Google Scholar]
- 23.Yazici B., Oztuker C., Efe A.C. Reconstruction of large upper eyelid defects with bilobed flap and tarsoconjunctival graft. Ophthal Plast Reconstr Surg. 2019;36:372–374. doi: 10.1097/IOP.0000000000001557. [DOI] [PubMed] [Google Scholar]
- 24.Patrinely J.R., O'Neal K.D., Kersten R.C., Soparkar C.N. Total upper eyelid reconstruction with mucosalized tarsal graft and overlying bipedicle flap. Arch Ophthalmol. 1999;117:1655–1661. doi: 10.1001/archopht.117.12.1655. [DOI] [PubMed] [Google Scholar]
- 25.Hada M. Eyelid reconstruction techniques: an overview. DJO. 2018;28:51–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.