Abstract
Peptide transporter 2 (PepT2) transports short peptides from the blood into bovine mammary epithelial cells (BMEC) to stimulate milk protein synthesis. Despite the fact that the effect of PepT2 is acknowledged in BMEC, little is known about its regulation. This study was completed to investigate the role of mammalian target of the rapamycin (mTOR) signaling in regulating the expression and function of PepT2 in BMEC. The regulation of PepT2 by mTOR in BMEC was studied in vitro using peptide transport assay, gene silencing, Western blot. The membrane expression of PepT2 and the uptake of β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid (β-Ala-Lys-AMCA), a model dipeptide, in BMEC were reduced by rapamycin (a mTOR inhibitor) and silencing of either mTOR complex 1 (mTORC1) or mTOR complex 2 (mTORC2), stimulated by DEP domain-containing mTOR-interacting protein (DEPTOR, endogenous inhibitor of mTORC1 and mTORC2) silencing. The trafficking of PepT2 to the membrane and the uptake of β-Ala-Lys-AMCA was promoted by neuronal precursor cell-expressed developmentally down-regulated 4 isoform 2 (Nedd4-2) silencing. The effects of knockdown of mTORC1, but not mTORC2, on cell membrane expression and transport activity of PepT2 was abolished by Nedd4-2 silencing. With immunofluorescence staining, PepT2 was identified to be interacting with Nedd4-2. The Nedd4-2 expression and the interaction between PepT2 and Nedd4-2 was increased through mTORC1 knockdown, indicating an increased ubiquitination of PepT2. The results revealed that mTORC1 can regulate the expression and function of PepT2 through Nedd4-2 in BMEC.
Keywords: mTORC1, mTORC2, Nedd4-2, PepT2, Peptide uptake, BMEC
1. Introduction
The supply of some free essential amino acids (EAA) in the blood is less than their output in milk proteins, and large amounts of peptide-bound amino acids (PBAA) can compensate for the shortage of EAA for milk protein synthesis (Bequette et al., 1999). PBAA represent an essential part of total amino acid flux across the portal-drained viscera and have been shown to contribute to milk protein synthesis (Mabjeesh et al., 2002). Our previous studies revealed that methionine (Met) containing peptides can be utilized to stimulate milk protein synthesis in bovine mammary epithelial cells (BMEC), and methionyl-methionine (Met–Met) has a higher efficiency in stimulating milk protein synthesis than free Met (Yang et al., 2015; Wang et al., 2018). Peptide transporter 2 (PepT2) acts an essential role in cellular uptake of di/tripeptides and peptidomimetic drugs in mammalian cells (Gilbert et al., 2008). Studies have shown that PepT2 is abundantly expressed in BMEC and the knockdown of PepT2 reduces the uptake of Met–Met and Met-Met-enhanced increase of αs1-casein (CN) expression in BMEC (Yang et al., 2015; Wang et al., 2018; Groneberg et al., 2002). As PepT2 plays a great role in small peptide absorption, understanding the regulation of PepT2 is of great significance.
The mammalian target of the rapamycin (mTOR) signaling pathway controls many cellular processes that produce or consume energy and nutrients (Laplante and Sabatini, 2012), including regulation of protein synthesis responding to the nutrient level and hormones in dairy cows (Huang and Fingar, 2014). Moreover, mTOR is currently established as a modulator of amino acid transporters (Burgos et al., 2010). mTOR is a key component of mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Loewith et al., 2002). The key difference between mTORC1 and mTORC2 is that mTORC1 comprises regulatory associated protein of mTOR (Raptor) whereas rapamycin-insensitive companion of mTOR (Rictor) is involved in mTORC2 (Peterson et al., 2009; Frias et al., 2006). Rosario et al. (2013) revealed mTORC1 and mTORC2 silencing significantly inhibits the trafficking and transport activity of system A and system L amino acid transporters in trophoblasts. However, the regulatory effect of mTOR signaling in the function of PepT2 in BMEC is still unclear.
Ubiquitination has been identified as a major regulatory mechanism of transporters (Acconcia et al., 2009). Ubiquitin-activating enzyme 3 (E3) is included in the last step of ubiquitination and transfers the ubiquitin to the target proteins (Acconcia et al., 2009; Wenzel et al., 2011). Neuronal precursor cell-expressed developmentally down-regulated 4 isoform 2 (Nedd4-2), participates in the ubiquitination of many nutrient transporters (Foot et al., 2017). Nedd4-2 regulates the plasma membrane expression of sodium-dependent neutral amino acid transporter 2 (SNAT2) and L-type amino acid transporter 1 (LAT1) and mediates the regulation of amino acid transporter by mTORC1 (Rosario et al., 2016).
The objective of this study was to define whether ubiquitination regulates the transport activity of PepT2 and find a link between the regulation of PepT2 by mTOR signaling and ubiquitination. In the study, we hypothesized that mTOR can modulate the expression and transport activity of PepT2 through Nedd4-2 in BMEC.
2. Materials and methods
2.1. Culture of BMEC
The procedures of BMEC culture were described previously (Wang et al., 2018). The mammary gland tissues were acquired from 3 midlactation dairy cows at a local abattoir. The use of animal tissues was approved by the Institutional Animal Use Committee of Zhejiang University. Briefly, the minced tissues collected from bovine mammary gland were digested in trypsin (0.25%, Amesco, Solon, OH, USA) at 37 °C for 30 min and subsequently in collagenase I (Amesco) and collagenase II (Amesco) for 4 h at 37 °C. The digested tissue homogenate was filtrated and centrifuged at 300 g or 5 min, the cells were cultivated in plastic dishes containing DMEM/F12 with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), transferrin (Sigma, St. Louis, MO, USA), insulin (Sigma), prolactin (5 mg/mL, Sigma), hydrocortisone (1 mg/mL, Sigma) and epithelial growth factor (10 ng/mL, Sigma). After the cells were starved for 12 h and treated with rapamycin (100 ng/mL, Sangon, Shanghai, China) for 12 h, they were then harvested with RIPA cell lysate buffer (Beyotime, Jiangsu, China) or used to study peptide uptake. BMEC used in the study were between passages 5 and 8.
2.2. The siRNA transient transfection
The siRNA was transfected into BMEC with lipofectamine RNAimax (Invitrogen, Carlsbad, CA, USA). In brief, the siRNA (60 pmol/L) and lipofectamine RNAimax were diluted, mixed and incubated for 5 min. BMEC were harvested at 24 h after transfection. The sequences of siRNA are presented in Appendix Table 1.
2.3. Quantitative reverse transcription real-time PCR (qRT-PCR)
Total RNA was extracted from BMEC using RNA isolation kit (Sangon). After confirming the absorbance at A260 nm/A280 nm (within 1.8 to 2.1) and the RNA integrity by electrophoresis, it was transcribed into cDNA. The cDNA was synthesized with a reverse transcription kit (Takara, Otsu, Shiga, Japan). The relative mRNA abundance of target genes was quantified by SYBR PrimeScript reagent kit (Takara) and an ABI 7500 (Applied Biosystems, Singapore). The designed primers were present in Appendix Table 2, and the amplification efficiency was between 90% and 110%. The gene expression was normalized to β-actin and calculated by the 2−ΔΔCt method (Schmittgen and Livak, 2008).
2.4. Surface biotinylation
BMEC were treated with 100 μmol/L Nacetyl-Leu-Leu-norleucinal for 2 h before harvest to avoid proteasomal degradation of ubiquitinated PepT2. BMEC were washed three times using PBS followed by incubating with sulfo–NHS–LC-biotin (1 mg/mL, Genescript, Nanjing, China) for 30 min on ice. Subsequently, the cells were quenched by PBS comprising 100 μmol/L glycine for 15 min at 4 °C, lysed with ice-cold RIPA and centrifuged at 16,000×g for 20 min. The supernatant was incubated with streptavidin-agarose beads for 2 h on ice to isolate the membrane proteins. The PepT2 at the cell membrane was detected by Western blot.
2.5. Western blot
Western blot was performed according to previously described procedures (Wang et al., 2018). Briefly, membrane proteins or cell lysates (20 μg) were separated on 10% polyacrylamide gels and then transferred to polyvinylidene fluoride microporous membranes. The PVDF membranes were blocked with blocking buffer (Sangon) and incubated by anti-β-actin (Boster, Wuhan, China, catalog number:BM0627), anti-Na+-K+-ATPase (Abcam, Cambridge, UK, catalog number:ab185065), anti-PepT2 (Abcam, catalog number: ab83771), anti-DEP domain-containing mTOR-interacting protein (DEPTOR, Abcam, catalog number: ab191841), anti-Rictor (1:1,000, Abcam, catalog number: ab105469), anti-Raptor (Abcam, catalog number: ab40768), p70S6K (Thr389, cell signaling technology, catalog number: 9234), protein Kinase B (Akt, Ser473, cell signaling technology, catalog number: 9272), Phospho-Akt antibody (Ser473, cell signaling technology, catalog number: 9271) and anti-Nedd4-2 (Abcam, catalog number: ab46521) at 4 °C overnight and then followed with horse radish peroxidase conjugated secondary antibody (Sangon) for 1 h. Finally, the bands were detected with chemiluminescence system (CLiNX Science Instrument, Shanghai, China). Membranes were analyzed by ImageJ software (version 1.50b, NIH, Bethesda, MD, USA). The relative protein levels of PepT2 and Nedd4-2 in cell membrane or cell lysate were normalized by the levels of Na+-K+-ATPase or β-actin, respectively.
2.6. Immunofluorescence staining
BMEC were cultured with 100 μmol/L Nacetyl-Leu-Leu-norleucinal for 2 h and then fixed with 4% paraformaldehyde and blocked by 3% serum albumin for half an hour. The cells were incubated with a PepT2 antibody (Abcam, 1:50) overnight at 4 °C. Subsequently, they were incubated with a FITC-conjugated secondary antibody (Sangon) for an hour in dark and counterstained with DAPI (2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride, Sangon) for 10 min. Finally, they were evaluated by confocal laser scanning microscope (Nikon, Tokyo, Japan).
2.7. Uptake of β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid (β-Ala-Lys-AMCA)
β-Ala-Lys-AMCA, a coumarin-tagged peptide probe, was used to detect the uptake of dipeptide in BMEC (Wang et al., 2018). After the cells were cultivated at 12-well plates for 1 d, they were preincubated with Krebs–Ringer modified buffer (KRB) for 0.5 h, followed by incubation with KRB containing 25 μmol/L β-Ala-Lys-AMCA for 30 min at 37 °C, the cells were washed 3 times with ice-cold KRB to stop the uptake of dipeptide and lysed with 1% triton-100. The uptake of β-Ala-Lys-AMCA in cells was detected by microplate reader (excitation at 350 nm, emission at 455 nm). Protein concentration was determined by BCA protein assay kit (Beyotime, Jiangsu, China). The uptake of β-Ala-Lys-AMCA in each well was standardized to the amount of protein.
2.8. Statistical analysis
Statistical analysis was examined with one-way ANOVA or unpaired Student's t-test by SAS software (SAS Institute, USA). Tukey's multiple range test was applied to detect the differences between means. Each experiment was repeated at least 3 times. Values are shown as the means and pooled SEM. P < 0.05 was considered as a significant difference.
3. Results
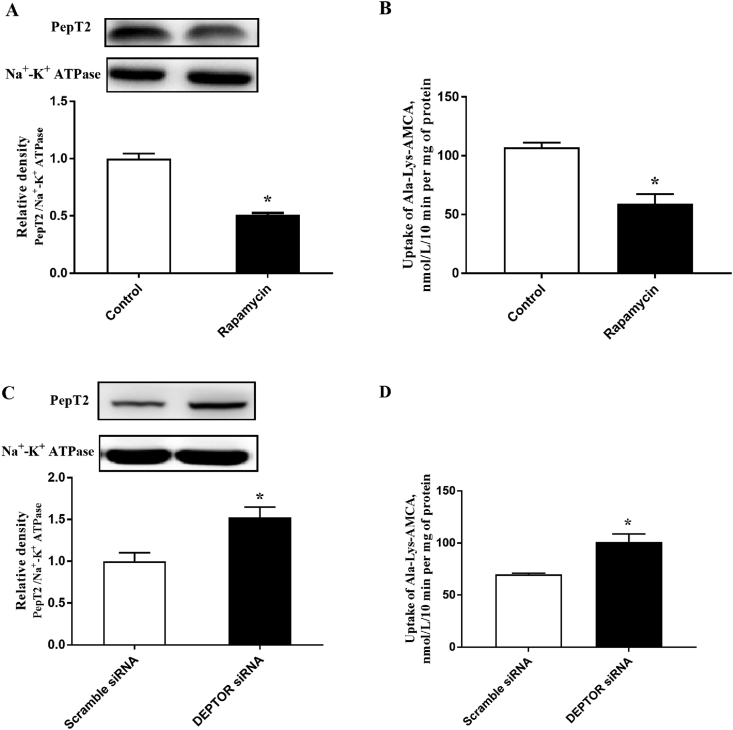
3.1. mTOR regulates the membrane expression and peptide transport activity of PepT2
Treatment of BMEC with rapamycin significantly decreased the PepT2 membrane expression (Fig. 1A) and reduced the uptake of β-Ala-Lys-AMCA (Fig. 1B), but the total cellular expression of PepT2 was not affected (Appendix Fig. 1). The interference efficiency of DEPTOR siRNA was illustrated in Appendix Fig. 2. The siRNA 3 decreased DEPTOR mRNA abundance by 70%. In contrast, knockdown of DEPTOR using siRNA significantly increased the PepT2 membrane expression (Fig. 1C) and stimulated the uptake of β-Ala-Lys-AMCA in BMEC (Fig. 1D).
Fig. 1.
The mTOR regulates the localization and activity of PepT2 in bovine mammary epithelial cells (BMEC). (A, C) The analysis of PepT2 in plasma membrane or whole lysate of BMEC treated with or without rapamycin (100 ng/mL) for 12 h (A) or DEPTOR siRNA was detected by Western blot (C). Upper panel: representative blot; bottom panel: quantitative representation. (B, D) The uptake of β-Ala-Lys-AMCA treated with or without rapamycin (B) or DEPTOR siRNA (D) was measured at 25 μmol/L, pH 6.5, and 37 °C for 30 min. Values are the means ± SD (n = 3). Means with a ∗ indicates significant difference (P < 0.05). mTOR = mammalian target of the rapamycin; PepT2 = peptide transporter 2; BMEC = bovine mammary epithelial cells; DEPTOR = DEP domain-containing mTOR-interacting protein; β-Ala-Lys-AMCA = β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid.
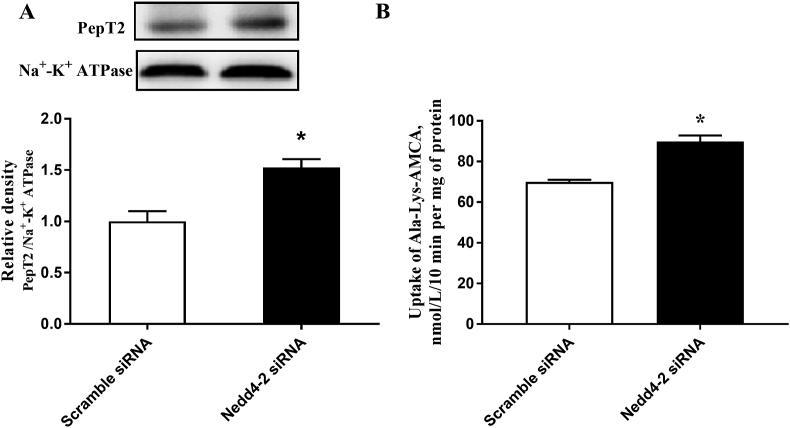
3.2. Nedd4-2 knockdown promotes membrane expression and cell peptide transport activity of PepT2
The transfection efficiency of Nedd4-2 siRNA was illustrated in Appendix Fig. 3. The siRNA 3 inhibited Nedd4-2 mRNA expression by about 70% in BMEC (Appendix Fig. 3). The membrane expression of PepT2 was enhanced when Nedd4-2 was knocked down using siRNA 3 (Fig. 2A). Consistently, the uptake of β-Ala-Lys-AMCA was also increased after Nedd4-2 is knocked down in BMEC (Fig. 2B).
Fig. 2.
Nedd4-2 knockdown promotes trafficking of PepT2 to the plasma membrane and peptide uptake activity in bovine mammary epithelial cells (BMEC). (A) The membrane expression of PepT2 in BMEC treated with or without Nedd4-2 siRNA. Upper panel: representative blot; bottom panel: quantitative representation. (B) The uptake of β-Ala-Lys-AMCA treated with or without Nedd4-2 siRNA was measured at 25 μmol/L, pH 6.5, and 37 °C for 30 min. Values are the means ± SD (n = 3). Means with a ∗ indicates P < 0.05. Nedd4-2 = neural precursor cell expressed developmentally downregulated 4 isoform 2; PepT2 = peptide transporter 2; BMEC = bovine mammary epithelial cells; β-Ala-Lys-AMCA = β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid .
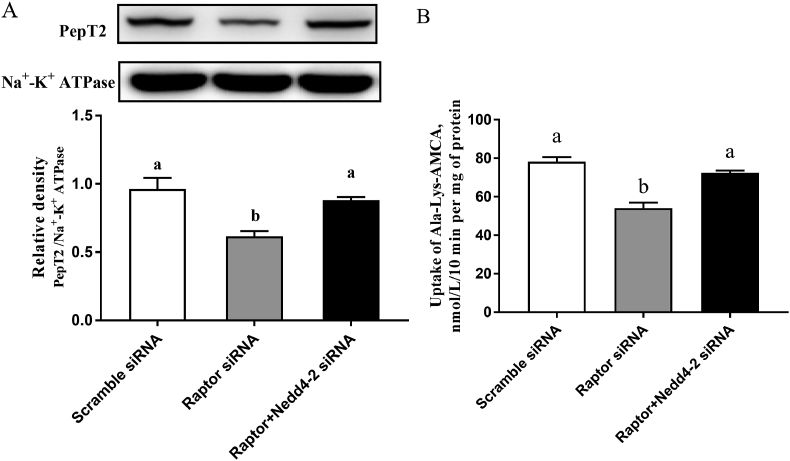
3.3. Nedd4-2 mediates the regulation of the membrane expression and peptide transport activity of PepT2 by mTORC1
The interference efficiency of Raptor siRNA was shown in Fig. S4. The siRNA 2 decreased Raptor mRNA abundance by almost 60% (Appendix Fig. 4). Compared with cells transfected with scramble siRNA, Raptor knockdown significantly decreased the PepT2 membrane expression (Fig. 3A) and the uptake of β-Ala-Lys-AMCA (Fig. 3B) in BMEC. However, the simultaneous knockdown of Raptor and Nedd4-2 canceled the inhibitory effect of mTORC1 knockdown on the membrane expression of PepT2 (Fig. 3A) as well as the uptake of β-Ala-Lys-AMCA in BMEC (Fig. 3B).
Fig. 3.
Nedd4-2 mediates the regulation of the plasma membrane expression and transport activity of PepT2 by mTORC1. (A) Western blot analysis of PepT2 in the plasma membrane of BMEC transfected with scramble, Raptor, or Raptor + Nedd4-2 siRNA. Upper panel: representative blot; bottom panel: quantitative representation. (B) The uptake of β-Ala-Lys-AMCA in BMEC transfected with scramble, Raptor, or Raptor + Nedd4-2 siRNA. Values are the means ± SD (n = 3). Means without a common letter differ (P < 0.05). Nedd4-2 = neural precursor cell expressed developmentally downregulated 4 isoform 2; PepT2 = peptide transporter 2; mTORC1 = mammalian target of the rapamycin complex 1; BMEC = bovine mammary epithelial cells; Raptor = regulatory associated protein of mTOR; β-Ala-Lys-AMCA = β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid.
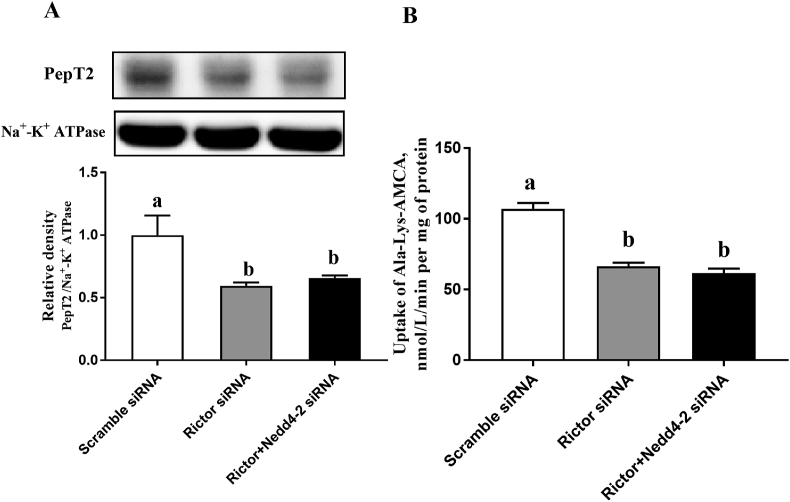
3.4. Nedd4-2 is not required in the regulation of the membrane expression and peptide transport activity of PepT2 by mTORC2
The interference efficiency of Rictor siRNA was described in Appendix Fig. 5. The three siRNAs downregulated Rictor mRNA abundance in BMEC (Appendix Fig. 5). Similar to Raptor knockdown, knockdown of Rictor by siRNA 3 also significantly reduced the plasma membrane expression of PepT2 (Fig. 4A) and the uptake of β-Ala-Lys-AMCA (Fig. 4B) in BMEC. However, Nedd4-2 knockdown did not affect the reduction in the plasma membrane expression of PepT2 (Fig. 4A) and the uptake of β-Ala-Lys-AMCA induced by mTORC2 inhibition (Fig. 4B) in BMEC.
Fig. 4.
Nedd4-2 was not required for mTORC2 regulation on the plasma membrane trafficking of PepT2 and transport activity of PepT2 in BMEC. (A) Representative western blots are shown for PepT2 in the membrane of scramble, Rictor or Rictor + Nedd4-2 silenced BMEC. Upper panel: representative blot; bottom panel: quantitative representation. (B) The uptake of β-Ala-Lys-AMCA transfected with scramble, Rictor or Rictor + Nedd4-2 siRNA. Values are the means ± SD (n = 3). Means without a common letter differ (P < 0.05). Nedd4-2 = neural precursor cell expressed developmentally downregulated 4 isoform 2; mTORC2 = mammalian target of the rapamycin complex 2; PepT2 = peptide transporter 2; BMEC = bovine mammary epithelial cells; Rictor = rapamycin-insensitive companion of mTOR; β-Ala-Lys-AMCA = β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid.
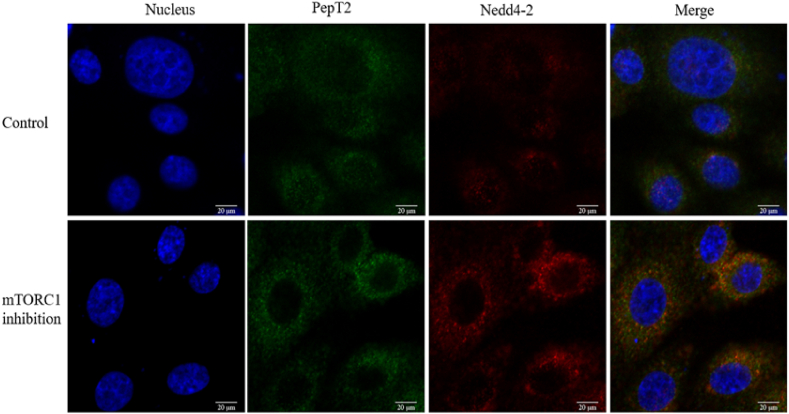
3.5. mTORC1 inhibition increases PepT2 ubiquitination
Raptor knockdown markedly enhanced the interaction between PepT2 and Nedd4-2, suggesting increased ubiquitination of PepT2 (Fig. 5).
Fig. 5.
The mTORC1 inhibition increases the interaction between Nedd4-2 and PepT2. Immunofluorescence were used to detect the interactions between PepT2 and Nedd4-2 following Raptor silencing, DAPI, DAPI nuclear staining. Scale bar = 20 μm. mTORC1 = mammalian target of the rapamycin complex 1; Nedd4-2 = neural precursor cell expressed developmentally downregulated 4 isoform 2; PepT2 = peptide transporter 2; DAPI = 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride.
4. Discussion
PepT2 acts pivotal role in cellular uptake of di/tripeptides and peptidomimetic drugs in BMEC (Zhao and Lu, 2015; Wang et al., 2019). Hence, it is of great significance to understand the regulation of PepT2 in physiological and pathological processes. The present study revealed that PepT2 is regulated by mTOR signaling, and Nedd4-2 mediates the regulation of PepT2 by mTORC1 in BMEC.
The mTOR signaling pathway regulates protein translation and modulates cell growth and metabolism (Saxton and Sabatini, 2017; Xie et al., 2022). There is evidence suggesting that nutrient transporters are the downstream targets of mTOR signaling in mammalian cells (Roos et al., 2007). Rosario et al. (2013) found that mTORC1 and mTORC2 silencing inhibited the membrane expression and transport activity of system A and system L amino acid transporters in human trophoblast cells. The protein level of PepT2 in cell lysates was not affected after rapamycin treatment, but rapamycin decreased the membrane expression and transport activity of PepT2 in BMEC in the present study. The subcellular localization of PepT2 determines its function in peptide uptake. Our observations indicated that mTOR signaling can direct the trafficking of PepT2 to the plasma membrane, which leads to the increased activity of peptide uptake. Indeed, knockdown of DEPTOR, an endogenous mTOR inhibitor, also enhanced the trafficking of PepT2 to the plasma membrane and promoted the uptake of β-Ala-Lys-AMCA, confirming the role of mTOR signaling in modulating the membrane expression of PepT2 and peptide transport activity in BMEC. As previous studies revealed that dipeptides can promote milk protein synthesis (Yang et al., 2015; Wang et al., 2018), we propose the activation of mTOR signaling pathway might increase milk protein synthesis by enhancing the dipeptide uptake in BMEC.
Whether mTORC1 and mTORC2 play roles in the regulation of PepT2 in BMEC was investigated by Raptor and Rictor silencing; the essential components of mTORC1 and mTORC2, respectively. Our results revealed that both mTORC1 inhibition by Raptor knockdown and mTORC2 inhibition by Rictor knockdown resulted in a marked decrease in plasma membrane expression of PepT2 as well as the uptake of β-Ala-Lys-AMCA. These observations indicated that both mTORC1 and mTORC2 are involved in the modulation of PepT2 function in BMEC.
Ubiquitination regulates the degradation of plasma membrane nutrient transporters by the ubiquitin proteasome pathway and controls subcellular localization of the transporters (Staub and Rotin, 2006). Nedd4-2 conjugate ubiquitin to the lysine residues of target proteins for degradation (Scheffner and Kumar, 2014). Nedd4-2 has been shown to participate in the regulation of nutrient transporters, such as cationic amino acid transporter 1 (CAT1), organic anion transporter 3 (OAT3), dopamine transporter 8 (DAT8) and glutamate transporter 1 (GLT-1) (Sorkina et al., 2006; Vina-Vilaseca et al., 2011; Garcia-Tardon et al., 2012). The transport activity of excitatory amino acid transporter was down-regulated by the Nedd4-2 (Boehmer et al., 2006). Nedd4-2-mediated ubiquitination enhanced the trafficking of glutamate transporter between the membrane and cytoplasm and stimulated the transport activity of glutamate transporters (Zhang et al., 2017). In the present study, Nedd4-2 knockdown significantly increased membrane expression of PepT2 and inhibited the uptake of β-Ala-Lys-AMCA in BMEC, indicating that Nedd4-2 also participates the regulation of peptide uptake by modulating the membrane expression of PepT2 in BMEC.
In the present study, although both mTORC1 and mTORC2 are regulators of PepT2, the underlying mechanisms are different. Nedd4-2 knockdown compromised the decrease of membrane expression of PepT2 and peptide transport activity in BMEC induced by mTORC1 inhibition, but not by mTORC2 inhibition, indicating that Nedd4-2 is only involved in the regulation of PepT2 by mTORC1, not mTORC2. This is consistent with previous observations that the regulation of SNAT2 and LAT1 by mTORC1 is mediated by the Nedd4-2 in primary human trophoblast cells (Rosario et al., 2016). The mechanism behind the regulation of PepT2 by mTORC2 in BMEC remains to be studied.
5. Conclusions
In summary, mTOR signaling regulates peptide uptake by modulating the membrane expression of PepT2 and Nedd4-2 mediates the regulation of PepT2 by mTORC1, but not mTORC2, in BMEC. Our study provides new insight into molecular mechanisms of PepT2 regulation and milk protein synthesis by mTOR in BMEC.
Author contributions
Caihong Wang: Conceptualized the research, Conducted the experiments, Analyzed the data and wrote the paper. Fengqi Zhao: Revised the paper. Jianxin Liu: Revised the paper. Hongyun Liu: Revised the paper and had primary responsibility for the final content. All authors read and approved final manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was funded by the National Natural Science Foundation of China 32072756 and 31872989.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2021.11.008.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Acconcia F., Sigismund S., Polo S. Ubiquitin in trafficking: the network at work. Exp Cell Res. 2009;315(9):1610–1618. doi: 10.1016/j.yexcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Bequette B.J., Backwell F.R., Kyle C.E., Calder A.G., Buchan V., Crompton L.A., et al. Vascular sources of phenylalanine, tyrosine, lysine, and methionine for casein synthesis in lactating goats. J Dairy Sci. 1999;82(2):362–377. doi: 10.3168/jds.S0022-0302(99)75243-4. [DOI] [PubMed] [Google Scholar]
- Boehmer C., Palmada M., Rajamanickam J., Schniepp R., Amara S., Lang F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4-2 is impacted by SGK kinases. J Neurochem. 2006;97(4):911–921. doi: 10.1111/j.1471-4159.2006.03629.x. [DOI] [PubMed] [Google Scholar]
- Burgos S.A., Dai M., Cant J.P. Nutrient availability and lactogenic hormones regulate mammary protein synthesis through the mammalian target of rapamycin signaling pathway. J Dairy Sci. 2010;93:153–161. doi: 10.3168/jds.2009-2444. [DOI] [PubMed] [Google Scholar]
- Foot N., Henshall T., Kumar S. Ubiquitination and the regulation of membrane proteins. Physiol Rev. 2017;97(1):253–281. doi: 10.1152/physrev.00012.2016. [DOI] [PubMed] [Google Scholar]
- Frias M.A., Thoreen C.C., Jaffe J.D., Schroder W., Sculley T., Carr S.A., et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Tardon N., Gonzalez-Gonzalez I.M., Martinez-Villarreal J., Fernandez-Sanchez E., Gimenez C., Zafra F. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination but not phosphorylation. J Biol Chem. 2012;287(23):19177–19187. doi: 10.1074/jbc.M112.355909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert E.R., Wong E.A., Webb K.E. Peptide absorption and utilization: implications for animal nutrition and health. J Anim Sci. 2008;86(9):2135–2155. doi: 10.2527/jas.2007-0826. [DOI] [PubMed] [Google Scholar]
- Groneberg D.A., Döring F., Theis S., Nickolaus M., Fischer A., Daniel H. Peptide transport in the mammary gland: expression and distribution of PepT2 mRNA and protein. Am J Physiol Endocrinol Metab. 2002;282(5):E1172–E1179. doi: 10.1152/ajpendo.00381.2001. [DOI] [PubMed] [Google Scholar]
- Huang K.Z., Fingar D.C. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol. 2014;36:3679–3690. doi: 10.1016/j.semcdb.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J.L., Bonenfant D., et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Mabjeesh S.J., Kyle C.E., Macrae J.C., Hanigan M.D., Bequette B.J. Vascular sources of amino acids for milk protein synthesis in goats at two stages of lactation. J Dairy Sci. 2002;85(4):919–929. doi: 10.3168/jds.S0022-0302(02)74150-7. [DOI] [PubMed] [Google Scholar]
- Peterson T.R., Laplante M., Thoreen C.C., Sancak Y., Kang S.A., Kuehl W.M., et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–876. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos S., Jansson N., Palmberg I., Säljö K., Powell T.L., Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Phycol. 2007;582(1):449–459. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario F.J., Dimasuay K.G., Kanai Y., Powell T.L., Jansson T. Regulation of amino acid transporter trafficking by mTORC1 in primary human trophoblast cells is mediated by the ubiquitin ligase Nedd4-2. Clin Sci. 2016;130(7):499–512. doi: 10.1042/CS20150554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario F.J., Kanai Y., Powell T.L., Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol. 2013;591:609–625. doi: 10.1113/jphysiol.2012.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton R.A., Sabatini D.M. mTOR Signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Kumar S. Mammalian HECT ubiquitin-protein ligases: biological and pathophysiological aspects. Biochim Biophys Acta. 2014;1843(1):61–74. doi: 10.1016/j.bbamcr.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sorkina T., Miranda M., Dionne K.R., Hoover B.R., Zahniser N.R., Sorkin A. RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis. J Neurosci. 2006;26(31):8195–8205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub O., Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev. 2006;86:669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- Vina-Vilaseca A., Bender-Sigel J., Sorkina T., Closs E.I., Sorkin A. Protein kinase C-dependent ubiquitination and clathrinmediated endocytosis of the cationic amino acid transporter CAT-1. J Biol Chem. 2011;286(10):8697–8706. doi: 10.1074/jbc.M110.186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.H., Sun Y.L., Zhao F.Q., Liu J.X., Liu H.Y. Functional characterization of peptide transporters in bovine mammary epithelial cells. J Agric Food Chem. 2019;67:213–219. doi: 10.1021/acs.jafc.8b05637. [DOI] [PubMed] [Google Scholar]
- Wang C.H., Zhao F.Q., Liu J.X., Liu H.Y. Dipeptide (methionyl-methionine) transport and its effect on β-casein synthesis in bovine mammary epithelial cells. Cell Physiol Biochem. 2018;49:479–488. doi: 10.1159/000492987. [DOI] [PubMed] [Google Scholar]
- Wenzel D.M., Stoll K.E., Klevit R.E. E2s: structurally economical and functionally replete. Biochem J. 2011;433(1):31–42. doi: 10.1042/BJ20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X.X., Shu R.N., Yu C.N., Fu Z.W., Li Z.Z. Mammalian AKT, the emerging roles on mitochondrial function in diseases. Aging Dis. 2022;13 doi: 10.14336/AD.2021.0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.X., Wang C.H., Xu Q.B., Zhao F.Q., Liu J.X., Liu H.Y. Methionyl-methionine promotes α-s1 casein synthesis in bovine mammary gland explants by enhancing intracellular substrate availability and activating JAK2-STAT5 and mTOR mediated signaling pathways. J Nutr. 2015;145:1748–1753. doi: 10.3945/jn.114.208330. [DOI] [PubMed] [Google Scholar]
- Zhang Y., He X., Meng X., Wu X., Tong H., Zhang X., et al. Regulation of glutamate transporter trafficking by Nedd4-2 in a Parkinson's disease model. Cell Death Dis. 2017;8(2) doi: 10.1038/cddis.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Lu K. Substrates of the human oligopeptide transporter hPepT2. BioSci Trends. 2015;9(4):207–213. doi: 10.5582/bst.2015.01078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.