Abstract
Compared to the parental SARS-CoV-2 virus, infections by the now dominant Delta variant of SARS-CoV-2 appear to be more common and more severe in pregnant women. The need for a robust, cheap, and quick method for diagnosing placental infection by SARS-CoV-2 has thus become more acute. Here, we describe a highly sensitive and specific immunohistochemical assay for SARS-CoV-2 nucleocapsid protein for routine use in placental pathology practice.
Keywords: SARS-CoV-2, COVID-19, immunohistochemistry, nucleocapsid protein, spike protein, placenta, electron microscopy, ultrastructure
Introduction
In the last few months, the Delta variant (B.1.617.2) of SARS-CoV-2 has become the predominant variant in many countries.1-4 This has been accompanied by a greater number of infections among pregnant women and a greater proportion of severe infection and worse pregnancy outcomes compared to the period of SARS-CoV-2 infection prior to the Delta variant dominance.4,5 This has increased the request for evaluation of placentas to look for evidence of SARS-CoV-2 infection as a first step to determining transplacental transmission. The histopathologic features of SARS-CoV-2 infection have now been well-documented and include histiocytic intervillositis, intervillous fibrin, and trophoblast necrosis.6-9 However, none of these histologic features by themselves are diagnostic of SARS-CoV-2 infection of the placenta. For example, while plasmacellular villitis is characteristic of cytomegalovirus (CMV) infection, a diagnosis of CMV placentitis can be confidently made only by the demonstration of CMV cytopathic change and/or immunohistochemistry for CMV proteins. Likewise, a diagnosis of placental infection by SARS-CoV-2 can only be made by demonstration of the virus by electron microscopy, in-situ hybridization or polymerase chain reaction for viral RNA, or immunohistochemistry for viral proteins.10 Of these techniques, immunohistochemistry is the most accessible, the easiest and quickest to perform, and the cheapest option for pathology laboratories across the world. Here we describe the validation of a robust SARS-CoV-2 immunohistochemistry assay in placental tissue.
Methods
After an initial trial of 4 antibodies – 2 against SARS-CoV-2 spike protein and 2 against SARS-CoV-2 nucleocapsid protein (Table 1), we selected mouse monoclonal antibody to SARS-CoV/SARS-CoV-2 nucleocapsid protein (SinoBiological, Wayne, PA, USA) for its robust staining in our hands. Using a dilution of 1:4000 and heat-induced epitope retrieval at high pH, the antibody was optimized on an autostainer (Dako Omnis, Agilent, Santa Clara, CA, USA). The optimization was performed on formalin-fixed and paraffin-embedded (FFPE) sections of lung tissue from adult patients who died of SARS-CoV-2 infection as previously reported.11
Table 1.
Immunohistochemical Antibodies Against SARS-CoV-2.
| Target | Host | Clonality | Vendor | Catalog # |
|---|---|---|---|---|
| SARS-CoV/SARS-CoV-2 Nucleocapsid Protein | Mouse | Monoclonal | SinoBiological | 40143-MM05 |
| SARS-CoV/SARS-CoV-2 Spike Protein | Rabbit | Polyclonal | SinoBiological | 40150-T62-COV2 |
| SARS-CoV-2 Nucleocapsid Protein | Rabbit | Monoclonal | ABclonal | A20021 |
| SARS-CoV-2 Spike Protein | Rabbit | Monoclonal | ABclonal | A20022 |
Results and Discussion
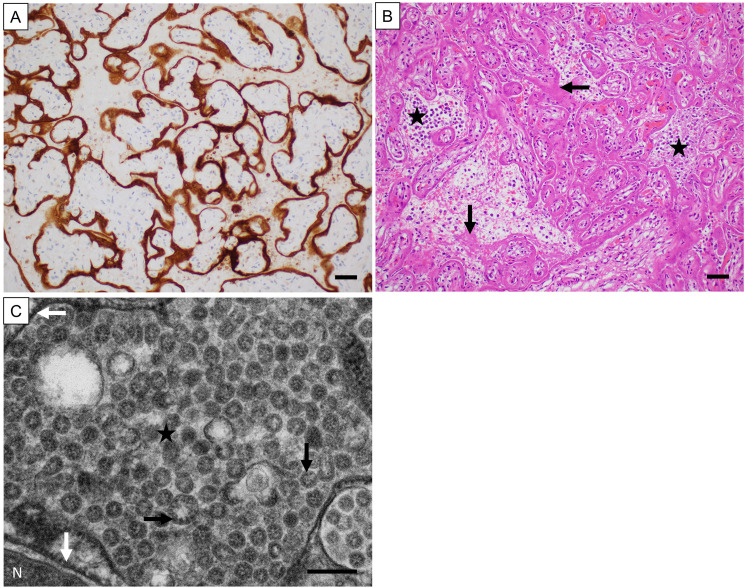
The sensitivity of the optimized immunostain was assessed in FFPE sections of placental tissues from 9 patients with positive nasopharyngeal SARS-CoV-2 PCR and characteristic clinical and histopathologic features totaling 30 sections including replicates (2-10 different blocks per case). Positive staining was typically granular to chunky and usually intracytoplasmic in the trophoblast, although in areas of trophoblast necrosis, extracytoplasmic granular to chunky staining was also considered positive. All 9 unique placental tissue sections showed robust focal/multifocal (4/9, 44%) or diffuse (5/9, 56%) staining (Figure 1 A). All replicates showed identical staining patterns. Importantly, all placenta specimens with positive staining showed histopathologic features that are now known to be characteristic for SARS-CoV-2 infection of the placenta, including histiocytic intervillositis, intervillous fibrin, and trophoblast necrosis (Figure 1 B).6,7,12 Further, as the gold standard test, in the first 4 positive placenta specimens, the presence of virions in the trophoblast was confirmed by electron microscopy, which showed characteristic 70 to 100 nm viral particles containing cross-sections of the nucleocapsid most readily identified in dilated cisternae of rough endoplasmic reticulum (Figure 1 C).
Figure 1.
Validation of immunohistochemical staining of SARS-CoV-2 nucleocapsid protein in formalin-fixed and paraffin-embedded placental tissue. (A) immunoperoxidase staining of the infected placentas showed granular/chunky staining (brown) associated with the trophoblast. 200x original magnification, magnification bar = 50 microns. (B) hematoxylin and eosin staining of the placentas with positive immunoperoxidase staining showed characteristic histologic features. Shown here is diffuse trophoblast necrosis with histiocytes and fibrin in the intervillous spaces. The horizontal arrow points to an area of trophoblast necrosis; the asterisks mark two areas of histiocytic intervillositis; and the vertical arrow points to a focus of intervillous fibrin. 200x original magnification, magnification bar = 50 microns. (C) electron microscopy performed on four of the placentas with positive immunoperoxidase staining showed characteristic SARS-CoV-2 virions. The white arrows point to the rough endoplasmic reticulum; the asterisk is surrounded by many virions within the vacuole formed by the distended rough endoplasmic reticulum; and the black arrows point to cross-sections of the helical viral nucleocapsid (tiny black dots). N = nucleus. 40,000x original magnification, magnification bar = 200 nm.
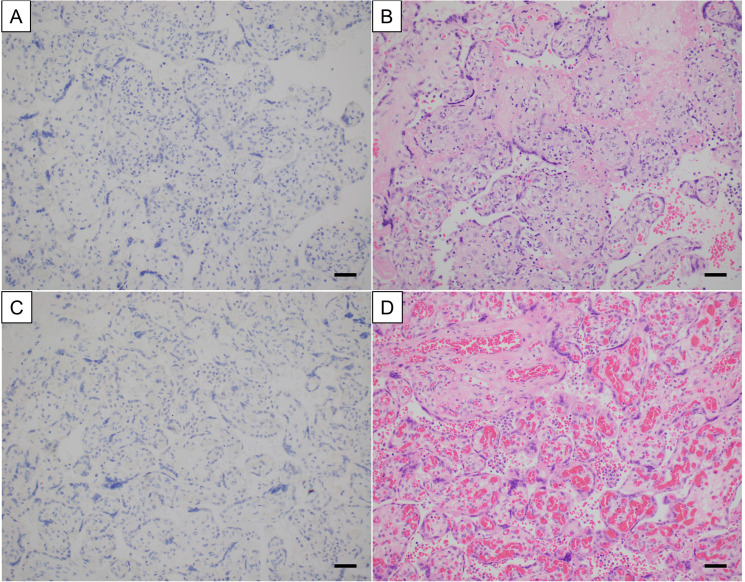
The specificity of the optimized immunostain was assessed in pediatric/perinatal tissues from 4 groups: 1) autopsy lung specimens from patients who died prior to the pandemic with histologic findings of diffuse alveolar damage, necrosis, and/or pneumonia of non-viral etiology (n = 8); 2) various tissues known to be positive for other viruses including CMV, herpes simplex virus (HSV) 1/2, adenovirus, respiratory syncytial virus (RSV), and varicella zoster virus (VZV) and either negative for SARS-CoV-2 by nasopharyngeal PCR or from prior to the pandemic (n = 9); 3) appendectomy specimens from patients with asymptomatic pre-operative nasopharyngeal PCR-positivity for SARS-CoV-2 (n = 6); and 4) placenta specimens from mothers who were nasopharyngeal PCR positive for SARS-CoV-2 but clinically asymptomatic or recovered and without characteristic histologic features suggesting SARS-CoV-2 placental infection (n = 27). All these tissues were negative for SARS-CoV-2 nucleocapsid protein by immunohistochemistry (Figure 2). While there was some background nonspecific blush in a few tissues, such as staining of intraluminal material in appendix specimens, it was not difficult to distinguish background from specific staining.
Figure 2.
Examples of placentas negative for immunoreactivity with SARS-CoV-2 nucleocapsid protein antibody. The top panel shows a placenta with chronic villitis (villitis of unknown etiology) and the bottom panel shows a placenta with mild histiocytic intervillositis without trophoblast necrosis or intervillous fibrin. (A) and (C) immunoperoxidase staining. 200x original magnification, magnification bar = 50 microns. (B) and (D) hematoxylin and eosin staining. 200x original magnification, magnification bar = 50 microns.
After initial validation of the immunohistochemical stain by one pathologist (DR), all positively staining and negatively staining cases (single glass slides or whole slide scans, no replicates) were reviewed by another pathologist (CT) without clinical context. There was 100% concordance.
In summary, we have described an immunohistochemical assay for SARS-CoV-2 nucleocapsid protein that is highly sensitive, specific, and robust, allowing routine use in placental pathology practice.
Footnotes
Author Contributions: DR and CFT reviewed the histology slides; DR and SEM reviewed the ultramicrographs; DR, CFT, SEM, and SM wrote the paper; DC and DR optimized and validated the immunostain; KT, DR, ES, LMB, EJD, and SM identified material for immunostain optimization and validation; all authors reviewed and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical Approval: The UT Southwestern Human Research Protection Program (HRPP) reviewed the project and determined that it does not meet the definition of research under 45 CFR 46.102 and therefore does not require IRB approval or oversight.
Informed Consent: This article describes an immunostain method. The optimization and validation were performed on de-identified formalin-fixed and paraffin-embedded tissue sections.
Trial Registration: This article does not contain any clinical trials.
ORCID iD: Dinesh Rakheja https://orcid.org/0000-0001-6888-7902
References
- 1.Ito K, Piantham C, Nishiura H. Predicted dominance of variant Delta of SARS-CoV-2 before Tokyo Olympic Games, Japan, July 2021. Euro Surveill. Jul 2021;26(27):2100570. doi: 10.2807/1560-7917.ES.2021.26.27.2100570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra S, Mindermann S, Sharma M, et al. Changing composition of SARS-CoV-2 lineages and rise of Delta variant in England. EClinicalMedicine. Sep 2021;39:101064. doi: 10.1016/j.eclinm.2021.101064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thangaraj JWV, Yadav P, Kumar CG, et al. Predominance of delta variant among the COVID-19 vaccinated and unvaccinated individuals, India, May 2021. J Infect. Aug 6 2021. doi: 10.1016/j.jinf.2021.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adhikari EH, SoRelle JA, McIntire DD, Spong CY. Increasing severity of COVID-19 in pregnancy with Delta (B.1.617.2) variant surge. Am J Obstet Gynecol. Sep 14 2021. doi: 10.1016/j.ajog.2021.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vousden N, Ramakrishnan R, Bunch K, et al. Impact of SARS-CoV-2 variant on the severity of maternal infection and perinatal outcomes: data from the UK obstetric surveillance system national cohort. medRxiv. 2021. doi: 10.1101/2021.07.22.21261000 [DOI] [Google Scholar]
- 6.Sisman J, Jaleel MA, Moreno W, et al. Intrauterine transmission of SARS-COV-2 infection in a preterm infant. Pediatr Infect Dis J. Sep 2020;39(9):e265-e267. doi: 10.1097/INF.0000000000002815 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz DA, Baldewijns M, Benachi A, et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch Pathol Lab Med. May 1 2021;145(5):517-528. doi: 10.5858/arpa.2020-0771-SA [DOI] [PubMed] [Google Scholar]
- 8.Bouachba A, Allias F, Nadaud B, et al. Placental lesions and SARS-Cov-2 infection: diffuse placenta damage associated to poor fetal outcome. Placenta. Sep 1 2021;112:97-104. doi: 10.1016/j.placenta.2021.07.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husen MF, van der Meeren LE, Verdijk RM, et al. Unique severe COVID-19 placental signature independent of severity of clinical maternal symptoms. Viruses. Aug 23 2021;13(8):1670. doi: 10.3390/v13081670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts DJ, Edlow AG, Romero RJ, et al. A standardized definition of placental infection by SARS-CoV-2, a consensus statement from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development SARS-CoV-2 placental infection workshop. Am J Obstet Gynecol. Dec 2021;225(6):593 e1-593 e9. doi: 10.1016/j.ajog.2021.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. May 5 2020;153(6):725-733. doi: 10.1093/ajcp/aqaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins JC, Torous VF, Roberts DJ. Defining severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis. Arch Pathol Lab Med. Nov 1 2021;145(11):1341-1349. doi: 10.5858/arpa.2021-0246-SA [DOI] [PubMed] [Google Scholar]