Abstract
Triphala is a famous triherbal drug, comprising three herb fruits, including Terminalia chebula (Haritaki), Terminalia bellirica (Bibhitaki), and Phyllanthus emblica (Amalaki). It is enriched with vitamin C, polyphenols, flavonoids, sterols, saponins, etc., and is well-documented for its potent antioxidant, anticancer, chemoprotective, antimicrobial, and anti-inflammatory effects. This research was conducted to evaluate the synergistic antioxidative and cytotoxic potential of mixtures of the individual constituents of Triphala at their nonequivalent ratios along with the chemical characterization of individual constituents of Triphala to identify and quantify individual compounds. The antioxidative potential was measured using total antioxidant capacity (TAC), DPPH free radical scavenging assay, and total phenolic content (TPC) tests. The cytotoxic potential was assessed on brain cancer cells (N4X4) using MTT assay, and phytochemical characterization was performed by GS-MS analysis. Nonequivalent ratios of Triphala constituents exhibited significantly higher synergistic antioxidant and cytotoxic potential than the equivalent ratios of them. Moreover, the nonequivalent ratio where the quantity of Amalaki was doubled than the other two constituents showed the highest synergistic antioxidant and cytotoxic effect. GC-MS analysis of individual constituents of Triphala identified and quantified the presence of a wide array of compounds, and fatty acid, fatty acid ester, triterpene, and aminoglycoside remained the predominant class of compounds. Thus, it can be inferred that the observed bioactivities can be attributed to the phytocompounds characterized and extracts at the nonequivalent ratio of Triphala constituents where Amalaki is doubled can be more effective in treating oxidative degenerative diseases and glioblastoma.
Keywords: Triphala, Nonequivalent ratio, Antioxidant, Cytotoxicity, Phytochemical characterization, GC-MS
1. Introduction
Triphala, a popular triherbal drug, has traditionally been utilized in the Indian subcontinent to cure a wide array of disorders from ancient times. It comprises three herb fruits; Phyllanthus emblica (Amalaki), Terminalia chebula (Haritaki), and Terminalia bellirica (Bibhitaki). Amalaki contains biologically active secondary metabolites including vitamin C, polyphenols, flavonoids, glycosides, terpenoids, tannins, etc. and individual phytocompounds such as phyllemblin, gallic acid, geranin, furosin, corilagin, quercetin, chebulinic acid, and many more (Hasan et al., 2016, Saini et al., 2022). Amalaki has a huge therapeutic potential due to possessing these phytoconstituents that contribute to a wide range of bioactivities, including antioxidant, anticancer, anti-inflammatory, immunomodulatory, and antimutagenic (Saini et al., 2022). Different bioactive chemical constituents of Haritaki have been identified and reported, including flavonoids, phenolic acids, tannins, and other compounds. Bioactivities of Haritaki, such as antioxidant, cytotoxic, neuroprotective, and anti-inflammatory effects, among others, have been reported (Nigam et al., 2020). Bibhitaki contains flavonoids, glycosides, glucosides, tannins, vitamin C, terpenoids, saponins, lignans, gallic acid, chebulinic acid, ellagic acid, bellaric acid, terpene acids as its major bioactive chemical constituents. Bibhitaki has enormous therapeutic potential, especially antioxidant and anticancer effects, among other bioactivities (Kumar & Khurana, 2018).
Reactive oxygen species (ROS) induces oxidative stress and lead to cellular damage or tissue injury by reacting with the endogenous molecules, including lipids, proteins, lipoproteins, and nucleic acids (Zhou et al., 2020). Most importantly, ROS-induced cellular damage is responsible for neurodegenerative diseases, cardiovascular diseases, cancer, and inflammatory diseases (Krishnaiah et al., 2011, Zhou et al., 2020). Synthetic antioxidants used in the food industry have produced cancer and liver damage (Krishnaiah et al., 2011). Therefore, it is important to discover new/novel and safe antioxidants from natural sources like plants. Plant extracts are potential antioxidants such as polyphenolic compounds, flavonoids, tannins, and phenolic acids. These compounds can neutralize excessive ROS in the body due to their possessing redox properties, metal chelating effects, singlet oxygen quenching power, and hydrogen donating ability and prevent ROS-induced diseases (Krishnaiah et al., 2011, Zhou et al., 2020).
A significant DPPH free radical scavenging was exhibited by Triphala and its constituents in a previous study conducted by Singh et al. (Singh et al., 2016). A number of researchers studied the ROS scavenging activity of Triphala and found that it obliterated X- and γ- radiation-mediated ROS production in HeLa cells and scavenged DPPH and superoxide free radicals (Prasad & Srivastava, 2020).
Brain tumors are the second most common cancer in children, and consist of around 40% glioma, about 15–25% of pediatric malignancies, and approximately 25% medulloblastomas (Hussain, 2013). Glioblastoma is one of the cancers with rapid progression and largely remains incurable despite advances in cancer treatment modalities (Hanif et al., 2017). Researchers found that herbal medicinal products are considered one of the best treatments for cancer (Kumar et al., 2012, Pham et al., 2018). Compared to synthetic drugs, these natural compounds are more readily available, cheaper, can be easily administered orally, and have negligible side effects (Seca and Pinto, 2018, Lichota and Gwozdzinski, 2018). A previous study showed that natural plant compounds could work as an anticancer agent and restore chemotherapy sensitivity. For example, during an in vivo study, the synergistic anticancer effect of doxorubicin towards resistant MCF-1/DOX cells was detected when a natural active alkaloid, tetrandrine, was used in combination with doxorubicin (Aung et al., 2017). Paclitaxel, a natural anticancer drug, was isolated from the Texus bravefolia plant. It has been most effectively used in the clinical treatment of numerous cancers, including lung, breast, and ovarian cancers (Pham et al., 2018). Moreover, identifying medicinal plants with potent cytotoxicity is the key to discovering and developing effective cancer therapy. Research is expanding day by day, searching for safe, effective, and more selective natural drugs for chemotherapy (Akter et al., 2014).
Several study findings reported significant inhibition of a vast number of cancer cells growth when the anticancer effect of Triphala was investigated against different cancer cell lines: Capan-2 pancreatic cells, gastric cancer cell, SKOV-3, HeLa, HEC-1B, colon cancer cells, PANC-1, breast cancer cells; MDA-MB-231 and MCF-7, HCT116, HCCSCs, PC-3, T47D, and DU-145, BxPC-3 and HPDE-6 and findings showed significant inhibition of all cancer cells growth (Prasad & Srivastava, 2020).
The available Triphala ayurvedic formulation used to treat various disorders contains Amalaki, Haritaki, and Bibhitaki in equal proportions. Formulation containing nonequivalent ratios of each constituent might provide a better therapeutic effect than the formulation containing three constituents at equivalent ratios due to varying in range and concentrations of phytocompounds present in each constituent. In vitro study is warranted to see whether Triphala extract comprising three constituents at their nonequivalent ratios produces a higher antioxidant effect before opting for formulation development. In addition to that, no study was performed on N4X4-brain cancer cells previously with Triphala extract. Thus, our present study selected methanol extract of Triphala for the synergistic antioxidant potential and cytotoxicity determination with N4X4 cell lines with nonequivalent ratios of its components. The positive findings of our current study might encourage formulation scientists to develop a new formulation of Triphala using nonequivalent ratios of each constituent.
2. Materials and methods
2.1. Fruit materials
Amalaki, Haritaki, and Bhibitaki fruit powders were collected from commercially available packets. Extracts of three fruits were obtained by cold maceration using methanol as a solvent. 600 gm of the coarse powder of Amalaki, Haritaki, and Bibhitaki was separately soaked in 1.2 L of methanol in three cleaned beakers and kept for seven days at ambient temperature with occasional stirring (22–25 °C) (Alam et al., 2013). After filtering, the filtrate was transferred to a round bottom flask and concentrated using a vacuum rotary evaporator (Heidolph, Germany), maintaining the temperature and speed at 30 °C and 100 rpm, respectively. The extract was preserved in the refrigerator at −20° C for further use.
2.2. Measurement of the antioxidative potential of Triphala
The antioxidative potential of Triphala was measured by employing three different tests such as DPPH free radical scavenging assay to identify its inhibitory potential on free radical scavenging, total phenolic content (TPC) test to determine the total quantity of phenols present, and total antioxidant capacity (TAC) test to assess its overall antioxidative potential. To perform TPC and TAC tests, a range of concentrations (200–1200 µg/mL) and for the DPPH assay, a range of concentrations (3.125–1200 of µg/mL) of Triphala was prepared by serial dilution.
2.2.1. DPPH free radical scavenging potential of Triphala
The antioxidant effect of Triphala extracts at different ratios of all three components, and its individual components were evaluated by its potential to scavenge 2,2-diphenyl-1-picryl-hydrazyl (DPPH) free radicals using the method described earlier (Lalhmingmawii & Jagetia, 2018). 0.5 mL of each extract solution of different concentrations was added and appropriately mixed with 1 mL of 0.1 mM methanol solution of DPPH. The solution mixture was kept in the dark for 30 min. The absorbance of the control (methanol) and sample solutions was measured at 523 nm using a UV–Vis spectrophotometer (U-2910, Hitachi High Technologies, USA), L-ascorbic acid served as the standard antioxidant. The calculation of the % inhibition of DPPH radical scavenging activity of Triphala was done utilizing the equation [(Ao-A1)/Ao] where Ao and A1 are the absorbance of the control and sample/standard, respectively. IC50 values were calculated from the percentage inhibition curves.
2.2.2. Total phenolic content assay of Triphala
Estimation of total phenolic content (TPC) of Triphala extracts at different ratios of all three components and its individual components was performed by the modified Folin-Ciocalteu method described earlier (Chandra et al., 2014). 0.2 mL of the test sample was added with 0.6 mL of water and 0.2 mL of Folin-Ciocalteu’s phenol reagent in a 1:1 ratio. 1 mL of 8% (w/v in water) saturated sodium carbonate solution was added to the previous solution after 5 min. The mixture was then kept in the dark for 30 min to complete the reaction. Absorbance was measured at 765 nm utilizing a UV–Vis Spectrophotometer (U-2910, Hitachi High Technologies, USA). Gallic acid served as the standard, and TPC was calculated by extrapolating the calibration curve prepared by different concentrations of gallic acid solutions. The determination of the phenolic compounds was conducted in triplicates. In addition to this, the TPC was expressed as milligrams of gallic acid equivalent (GAE) per gram of the dried extract (mg of GAE/g dried extract).
Gallic Acid Equivalents (GAE) were found using the following equation:
Where, C = Total content of phenolic compounds, milligrams of Gallic acid per gram of dried plant extract, expressed as Gallic acid equivalents (QE).
Gallic Acid Equivalent (GAE) c = Concentration of Gallic acid obtained from calibration curve (mg/ mL).
V = Volume of sample solution (mL), m = Weight of the sample (g).
2.2.3. Total antioxidant capacity of Triphala
Estimation of total antioxidant capacity (TAC) of Triphala extracts at different ratios of all three components and its individual components was achieved using the method mentioned earlier (Pisoschi & Negulescu, 2012). 300 µL of each of the test samples of different concentrations were taken in test tubes, and 3 mL of reagent solution (0.6 M sulfuric acid, 0.028 M sodium phosphate, and 0.004 M ammonium molybdate) was added into the test tubes and incubated at 95 °C in a water bath for 90 min. Absorbance was measured at 765 nm using a UV–Vis Spectrophotometer (U-2910, Hitachi High Technologies, USA). Ascorbic acid was utilized as the standard, and TAC was calculated by extrapolating the calibration curve prepared by different concentrations of ascorbic acid solutions. The estimation of TAC was conducted in triplicates, and the TAC was expressed as milligrams of ascorbic acid equivalent (AAE) per gram of the dried extract (mg of AAE/g dried extract).
The total antioxidant capacity, A, for each of the fractions was expressed as:
Where, C = Total antioxidant capacity, milligrams of ascorbic acid per gram of dried plant extract, expressed as ascorbic acid equivalent (AAE).
c = Concentration of ascorbic acid obtained from calibration curve (mg/mL).
V = Volume of sample (mL), m = Weight of the sample (g).
2.3. Cytotoxicity determination of Triphala by MTT assay
Cytotoxicity of Triphala extracts at the equivalent ratio (A:B:H = 1:1:1), and nonequivalent ratio (A:B:H = 2:1:1) of its individual components was determined against N4X4 (Human glioma NP2 derived) cell line utilizing the MTT [3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay.
2.3.1. Cell culture
At a temperature of 37 °C in a humidified incubator containing 95% air and 5% CO2, the human glioma NP2- derived cell line, N4X4 was cultured and kept in advanced RPMI 1640 medium and DMEM (Dulbecco’s Modified Eagle’s Medium) supplemented with 10% fetal bovine serum, 0.2% gentamycin, and 1% penicillin–streptomycin.
N4X4 cells were preserved in liquid nitrogen in cryovials, and the cryovial was taken and rapidly thawed by swirling the vial delicately using a water bath at 37 °C. Afterward, the thawed cells were centrifuged for 10 min at room temperature at 150 to 200 × g. The supernatant was discarded, and cells were washed with a fresh medium to remove DMSO. The cells were suspended in the medium gently, transferred to the culture vessels, and kept in the incubator. After 80% confluency, cultured media was washed by DMEM, and 800 μL of trypsin was added for detaching the cells from the top of the culture vessels. After watching 90% of cells separated under the microscope, 5 mL DMEM media was added to the vessels and blended using a pipette. Finally, 1 mL of this solution was taken and mixed with 4 mL of DMEM in a new vessel and kept in an incubator for the MTT assay with the samples.
2.3.2. MTT colorimetric assay
The MTT colorimetric assay was employed to assess the cytotoxicity of Triphala. This study was conducted in Dhaka, Bangladesh’s Centre for Advanced Research in Sciences (CARS). In a 5% CO2 environment, N4X4 cells were seeded into 96-well flat-bottom tissue culture plates at a density of 1.5 × 104 cells per well and incubated at 37 °C for 48 h to allow the cells to attach on the surface of the culture plate. Then, the cells were treated with ten μL of sample solution following 24-hour incubation at concentrations of 0.0025–25 mg/mL. The CellTiter 96 non-radioactive cell proliferation assay kit (Promega, USA) was used to assess cytotoxicity after 48-hour incubation. After adding the assay kit, plates were incubated for 4 h. During this incubation period, living cells converted the yellow MTT tetrazolium component of the dye solution to a purple formazan product. Finally, the absorbance was measured at a wavelength of 570 nm using a microplate reader. The absorbance is based on reducing a yellow tetrazolium salt or MTT to purple formazan crystals, and the darker the solution, the greater the number of viable, metabolically active cells. A 2% DMSO solution and cycloheximide played a negative and positive control role, respectively. Two independent experiments were conducted to generate the results, and each experiment was conducted in triplicate. The percentage of cell growth inhibition and IC50 was calculated from a concentration–response curve.
2.3.3. Gas Chromatography-Mass Spectrometry (GC-MS) analysis of Triphala extracts
GC-MS analysis of the Triphala extracts was carried out by utilizing a GC-MS-QP 2010 Ultra instrument. An AB innowax column (30 × 0.25 mm id, film thickness 0.25 m) was employed for the analysis. The column oven temperature was initially maintained at 100 °C for 1 min, which was then gradually elevated to 270 °C and maintained for 25 min. The sample injection volume was 0.5 μL for analysis. Helium was employed as the carrier gas at a flow rate of 1.15 mL/min in the splitless mode. The sample injector and detector temperatures were kept at 200 °C and 250 °C, successively, and the split ratio was kept at 200 throughout the experiment. Electrons with an energy of around 70 eV were used in the electron ionization mass spectrometry. In addition to this, for 45 min, mass spectra were finally recorded in the range of 50 m/z to 650 m/z.
2.3.4. Identification and quantification of individual phytoconstituents
The individual phytocompounds were detected by matching their retention indices and mass spectra fragmentation patterns to authentic samples available in the Wiley database and data already available in the NIST libraries (Alilou & Akssira, 2021). Additionally, each component’s relative proportion was calculated via measuring and comparing its average peak area to the total peak areas.
2.4. Statistical analysis
Each of the experiments, namely; TPC and TAC, were conducted in triplicates (n = 3), while DPPH was carried out in duplicates (n = 2). MTT colorimetric assay for cytotoxic activity screening was carried out in triplicates, and each experiment was performed in duplicates. Microsoft Office Excel (MS-Excel) 2010 was utilized for all statistical analysis, including mean and standard deviation calculation and graphical representations.
3. Results
3.1. Antioxidant activity of Triphala by DPPH free radical scavenging assay
Triphala’s antioxidant effect was evaluated using DPPH free radical scavenging assay for Amalaki, Haritaki, and Bibhitaki individually at concentrations of 3.125 to 1200 µg/mL. The findings exhibited a gradual increment of the percentage inhibition of free radicals via Bibhitaki, Amalaki, Haritaki, and the standard Ascorbic acid. It was observed that concentrations 3.125 to 100 µg/mL (Table 1). However, from 200 to 1200 µg/mL, the standard and samples values remained nearly constant. In this test, the highest concentration of 1200 μg/mL produced the highest percentage of inhibition of free radicals, which was 96.85%, 95.80%, and 96.06% for Amalaki, Bibhitaki, and Haritaki, respectively (Table 1). Moreover, using DPPH test antioxidant effect with 1:1:1 (A:B:H), 1:2:1 (A:B:H), 2:1:1 (A:B:H), and 1:1:2 (A:B:H) ratios of Triphala constituents at concentrations of 3.125 to 100 µg/mL was determined. At the highest concentration of 100 μg/mL, the percentage inhibition of DPPH free radicals was 96.59%, 97.64%, 96.46%, and 95.41% for the Triphala ratios 1:1:1 (A:B:H), 2:1:1 (A:B:H), 1:2:1 (A:B:H), and 1:1:2 (A:B:H), respectively (Table 2). It is noteworthy to mention that the percentage inhibition of the Ascorbic acid (standard) was 96.98%, and the 2:1:1 (A:B:H) Triphala ratio where Amalaki was double in quantity than Bibhitaki and Haritaki showed more inhibition than that of standard ascorbic acid which was 97.64%. From the perspective of IC50 value, 10.29, 15.93, and 12.99 μg/mL of Amalaki, Haritaki, Bibhitaki, respectively, were found to inhibit half of DPPH free radicals effectively. As the IC50 value for ascorbic acid was 12.96 μg/mL, Amalaki showed more inhibition at a lower concentration (IC50 of Amalaki: 10.29 μg/mL). The IC50 values for the Triphala ratios 1:1:1 (A:B:H), 2:1:1 (A:B:H), 1:2:1 (A:B:H), and 1:1:2 (A:B:H), were 11.28, 7.01, 8.33, and 11.03 μg/mL, respectively and it was observed that all the IC50 values were lower compared to the IC50 value of Ascorbic acid and the individual components of Triphala as well (Table 3).
Table 1.
The % inhibition of DPPH free radical scavenging by Ascorbic acid, Amalaki, Bibhitaki, and Haritaki.
| Concentration of Sample (μg/mL) | % inhibition of Ascorbic Acid | % inhibition of Amalaki | % inhibition of Bibhitaki | % inhibition of Haritaki |
|---|---|---|---|---|
| 3.125 | 20.21 | 21.52 | 15.09 | 19.82 |
| 6.25 | 40.03 | 42.52 | 41.34 | 23.49 |
| 12.5 | 59.06 | 66.40 | 63.91 | 62.86 |
| 25 | 83.07 | 88.45 | 82.42 | 76.64 |
| 50 | 93.05 | 94.36 | 93.18 | 93.70 |
| 100 | 94.88 | 96.19 | 94.49 | 94.88 |
| 200 | 96.19 | 96.33 | 95.67 | 95.01 |
| 400 | 96.19 | 96.59 | 95.72 | 95.67 |
| 800 | 96.85 | 96.72 | 95.83 | 95.93 |
| 1200 | 96.98 | 96.85 | 95.84 | 96.06 |
Table 2.
The % inhibition of DPPH free radical scavenging by different ratios of Triphala constituents.
| Concentration of Sample (μg/mL) | % inhibition by 1:1:1 (A:B:H) | % inhibition by 2:1:1 (A:B:H) | % inhibition by 1:2:1 (A:B:H) | % inhibition by 1:1:2 (A:B:H) |
|---|---|---|---|---|
| 3.125 | 25.98 | 28.08 | 17.85 | 8.92 |
| 6.25 | 44.09 | 51.44 | 48.03 | 53.54 |
| 12.5 | 57.22 | 65.62 | 77.30 | 70.21 |
| 25 | 83.33 | 86.35 | 85.70 | 80.71 |
| 50 | 92.39 | 93.04 | 96.06 | 95.14 |
| 100 | 96.59 | 97.64 | 96.46 | 95.41 |
Table 3.
In DPPH test IC50 values of Ascorbic acid, Amalaki, Bibhitaki, Haritaki and different ratios of them.
| Samples and Standard | IC50 (μg/mL) |
|---|---|
| Ascorbic acid (Standard) | 12.96 |
| Amalaki | 10.29 |
| Bibhitaki | 12.99 |
| Haritaki | 15.93 |
| 1:1:1 (A:B:H) | 11.28 |
| 2:1:1 (A:B:H) | 7.01 |
| 1:2:1 (A:B:H) | 8.33 |
| 1:1:2 (A:B:H) | 11.03 |
3.2. Total phenolic content of Triphala
Total phenolic compounds present in the individual constituents of Triphala as well as at 1:1:1 (A:B:H), 2:1:1 (A:B:H), 1:2:1 (A:B:H), and 1:1:2 (A:B:H) ratios was measured at different concentrations of 200–1200 μg/mL using TPC test and the findings were expressed in mg of GAE per gram of dried extracts. In the case of the TPC test, the total phenolic content of each component and the mixtures at equivalent and nonequivalent ratios was increased with the increasing concentrations, and Amalaki contained the highest TPC (395.09 mg GAE/g of extract) at its highest concentration of 1200 μg/mL among three fruits (Table 4). Furthermore, at the same concentration, the dried extract showed the highest phenolic content, which was 475.83, 548.9, 510.09, and 491.65 mg of GAE per gram of 1:1:1 (A:B:H), 2:1:1 (A:B:H), 1:2:1 (A:B:H), and 1:1:2 (A:B:H) ratios, respectively (Table 5).
Table 4.
Total phenolic content (TPC) of Amalaki, Bibhitaki and Haritaki is expressed in mg of gallic acid equivalent (GAE)/g of crude extract (CE).
| Concentration of Sample (μg/mL) | TPC of Amalaki (mg GAE/g CE) | TPC of Bibhitaki (mg GAE/g CE) | TPC of Haritaki (mg GAE/g CE) |
|---|---|---|---|
| 200 | 178.90 | 190.66 | 37.51 |
| 400 | 243.94 | 210.37 | 220.66 |
| 800 | 348.23 | 349.66 | 254.66 |
| 1200 | 395.09 | 368.94 | 260.65 |
Table 5.
Total phenolic content (TPC) of Amalaki, Bibhitaki, and Haritaki at different ratios is expressed in mg of gallic acid equivalent (GAE)/g of crude extract (CE).
| Concentration of Sample (μg/mL) | TPC of 1:1:1 (A:B:H) (mg GAE/g CE) | TPC of 2:1:1 (A:B:H) (mg GAE/g CE) | TPC of 1:2:1 (A:B:H) (mg GAE/g CE) |
TPC of 1:1:2 (A:B:H) (mg GAE/g CE) |
|---|---|---|---|---|
| 200 | 310.86 | 390.66 | 350.90 | 325.51 |
| 400 | 365.91 | 430.37 | 401.94 | 395.66 |
| 800 | 445.23 | 510.66 | 480.23 | 460.66 |
| 1200 | 475.83 | 548.94 | 510.09 | 491.65 |
3.3. Total antioxidant capacity of Triphala
Total antioxidant capacity of individual constituents of Triphala as well as at 1:1:1 (A:B:H), 2:1:1 (A:B:H), 1:2:1 (A:B:H), and 1:1:2 (A:B:H) ratios was carried out at the concentrations of 200–1200 μg/mL and the findings were expressed in mg of AAE per gram of crude dried extracts. In the case of the TAC test, the total antioxidant capacity of each component and the mixtures at equivalent and nonequivalent ratios was increased with the increasing concentrations, and Haritaki at its highest concentration of 1200 μg/mL had the highest TAC (559.76 mg AAE/g of extract) among three fruits (Table 6). Moreover, at the same concentration, the dried extract showed the highest antioxidant capacity, which was 535.83, 675.50, 545.32, and 689.76 mg of ascorbic acid per gram of 1:1:1 (A:B:H), 2:1:1 (A:B:H), 1:2:1 (A:B:H), and 1:1:2 (A:B:H) ratios, respectively (Table 7).
Table 6.
Total antioxidant content (TAC) of Triphala is expressed as ascorbic acid equivalent (AAE)/g of crude extract (CE).
| Concentration of Sample (μg/mL) | TAC of Amalaki (mg AAE/g CE) | TAC of Bibhitaki (mg AAE/g CE) | TAC) of Haritaki (mg AAE/g CE) |
|---|---|---|---|
| 200 | 16.95 | 8.51 | 90.08 |
| 400 | 163.20 | 121.39 | 154.76 |
| 800 | 235.95 | 197.14 | 417.00 |
| 1200 | 485.50 | 245.32 | 559.76 |
Table 7.
Total antioxidant content (TAC) of different ratios of Triphala constituents is expressed as ascorbic acid equivalent (AAE)/g of crude extract (CE).
| Concentration of Sample (μg/mL) | TAC of 1:1:1 (A:B:H) (mg AAE/g CE) | TAC of 2:1:1 (A:B:H) (mg AAE/g CE) | TAC of 1:2:1 (A:B:H) (mg AAE/g CE) |
TAC of 1:1:2 (A:B:H) (mg AAE/g CE) |
|---|---|---|---|---|
| 200 | 25.86 | 37.95 | 27.51 | 105.07 |
| 400 | 265.91 | 293.20 | 283.39 | 294.76 |
| 800 | 345.23 | 385.95 | 352.14 | 417.00 |
| 1200 | 535.83 | 675.50 | 545.32 | 689.76 |
3.4. Cytotoxicity of Triphala
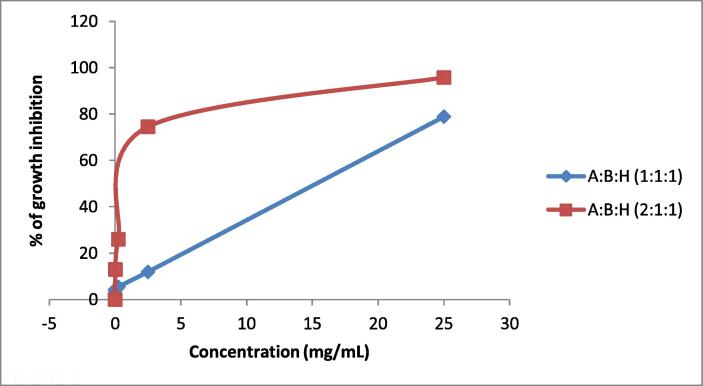
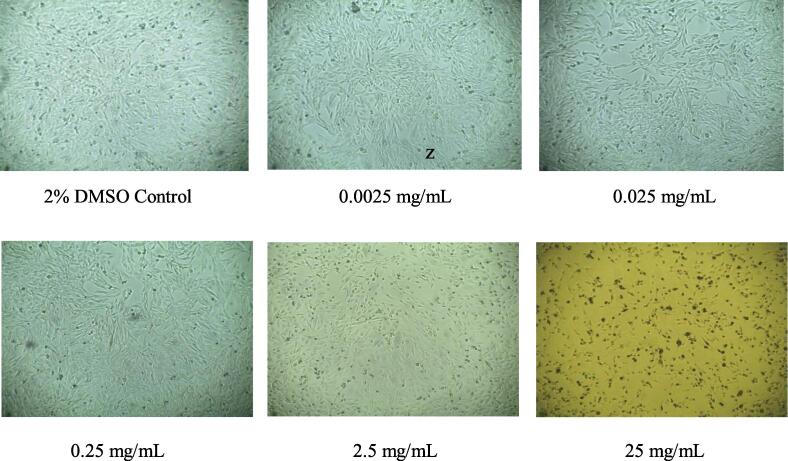
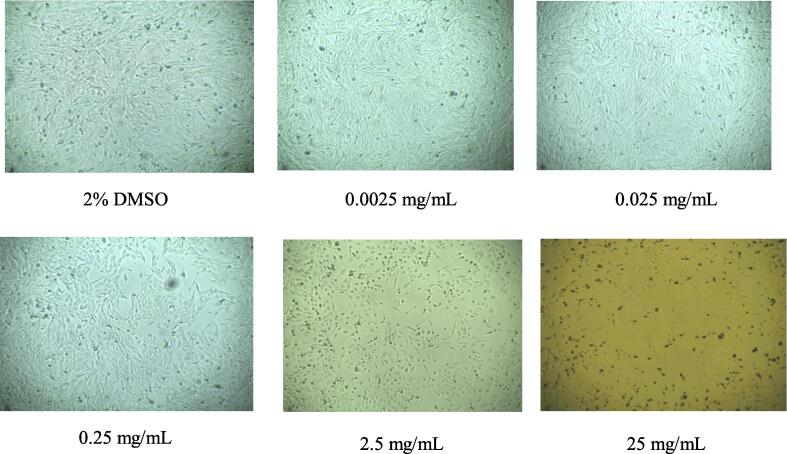
In vitro cytotoxicity test of Triphala at an equivalent ratio of 1:1:1 (A:B:H) and a nonequivalent ratio of 2:1:1 (A:B:H) was assessed using MTT colorimetric assay on N4X4-brain cancer cells. The MTT assay results demonstrated that the % of cell growth inhibition was concentration-dependent at both ratios. The highest toxicity against N4X4 cells was produced by the maximum concentration of 25 mg/mL, which was 78.75% and 95.88% cell growth inhibition for 1:1:1 (A:B:H) and 2:1:1 (A:B:H) ratios, respectively (Table 8, Fig. 3). Fig. 1 and Fig. 2 further exhibited that the number of viable cells reduced with the escalating concentration of extracts at both ratios. IC50 values of 1:1:1 (A:B:H) and 2:1:1 (A:B:H) ratios were 15.31 mg/mL and 8.29 mg/mL, respectively.
Table 8.
The % inhibition of N4X4 cell line in different concentrations along with their IC50 values at the 1:1:1(A:B:H) and 2:1:1 (A:B:H) ratios.
| Concentration of Sample (mg/mL) | % of Cell Growth Inhibition at 1:1:1 (A:B:H) ratio |
% of Cell Growth Inhibition at 2:1:1 (A:B:H) ratio |
IC50 (mg/mL) | |
|---|---|---|---|---|
| 0.0025 | 3.96 | 0.02 | 1:1:1 (A:B:H) | 2:1:1 (A:B:H) |
| 0.025 | 4.39 | 12.94 |
15.31 |
8.29 |
| 0.25 | 5.43 | 25.93 | ||
| 2.5 | 11.89 | 54.52 | ||
| 25 | 78.88 | 95.75 | ||
Fig. 3.
Cytotoxic effect of methanol crude extracts on N4X4 cell line: The % of cell growth inhibition by equivalent 1:1:1 (A:B:H) and non-equivalent 1:2:1 (A:B:H) ratios of methanol extracts of Triphala constituents.
Fig. 1.
Cell viability of methanol extract of Triphala, 1:1:1 (A:B:H) ratio, at different extract concentrations of 0.0025 mg/ml, 0.025 mg/mL, 0.25 mg/mL, 2.5 mg/mL, 25 mg/mL and 2% DMSO as control respectively after incubating 48 h in N4X4 cell line.
Fig. 2.
Cell viability of methanol extract of Triphala, 2:1:1 (A:B:H) ratio, at different extract concentrations of 0.0025 mg/ml, 0.025 mg/mL, 0.25 mg/mL, 2.5 mg/mL, 25 mg/mL and 2% DMSO as control respectively after incubating 48 h in N4X4 cell line.
3.5. Identification and quantification of individual phytoconstituents by GC-MS
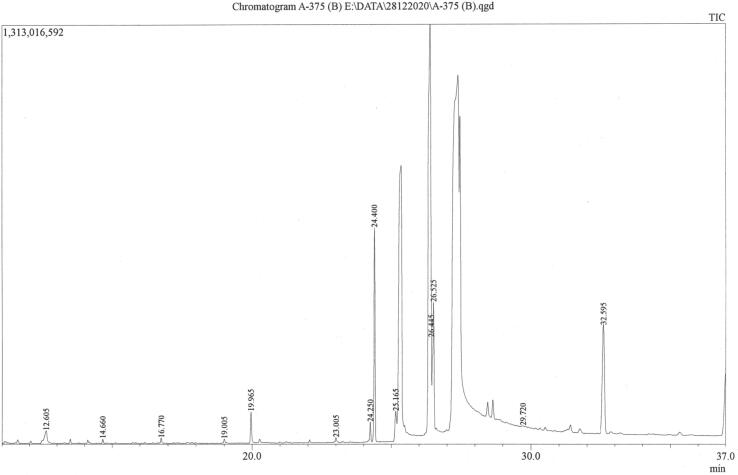
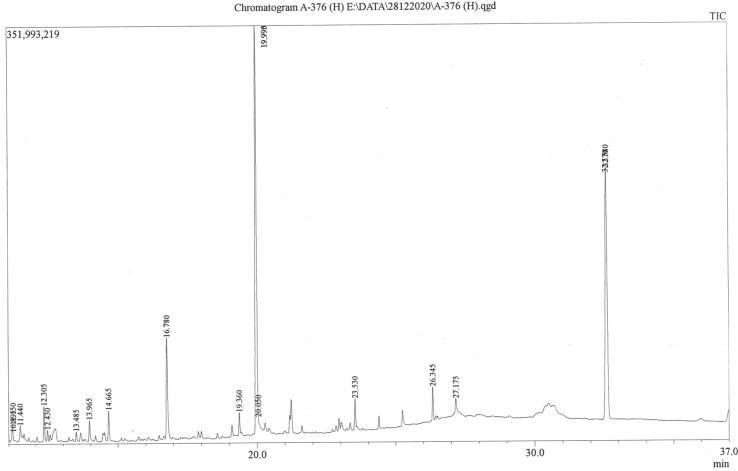
GC-MS analysis of individual constituents of Triphala led to the Identification of 17, 18, and 21 compounds in Amalaki, Bibhitaki, and Haritaki extracts, respectively (Fig. 4, Fig. 5, Fig. 6 and Table 9, Table 10, Table 11). Predominant compounds were represented by 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester (16.969%), Lanosta-7,9(11)-dien-18-oic acid, 22,25-epoxy-3beta,17,20-trihydroxy-, gamma-lactone (19.131%), and 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester (10.863%), in Amalaki, Bibhitaki, and Haritaki, respectively. 1,3-Benzenedicarboxylic acid bis(2-ethylhexyl) ester was also found in predominant concentration (12.053%) in the methanol extract of Bibhitaki. Other major compounds identified and quantified as 2,4-Dimethylfuran, Trans-2,3-Epoxyoctane, Heptanoic acid, 3-hydroxy-, methyl ester, 4-(2-Hydroxyethyl)-3-methyl-2-pyrazolin-5-one, 2-Furancarboxylic acid, 2-ethylhexyl ester, paromomycin, octanoic acid in Amalaki extract. Moreover, other principal compounds present in the extract of Bibhitaki were identified and quantified as 2-Cyclopenten-I -one, 5-hydroxy-2,3 –dimethyl, Spirohexane-1-carboxylic acid, ethyl ester, 1-Cyclohexene-1-carboxylic acid, 9-Octadecenoic acid (Z)-, methyl ester, 7-Hexadecenoic acid, methyl ester, (Z)-, 9-Hexadecenoic acid, methyl ester, (Z)-, 2-Cyclopenten-1 -one, 5-hydroxy-2,3-dimethyl, and 2-Pyrrolidinone, 5 -(hydroxymethyl)-. Furthermore, the second most abundant compounds in Haritaki extract were detected as Paromomycin, Furfural, 2-Cyclopenten-1 -one, 5-hydroxy-2,3 –dimethyl-, Carbamic acid, phenyl ester, and 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one.
Fig. 4.
GC-MS chromatogram of methanol extract of Phyllanthus emblica (Amalaki).
Fig. 5.
GC-MS chromatogram of methanol extract of Terminalia bellirica (Bibhitaki).
Fig. 6.
GC-MS chromatogram of methanol extract of Terminalia chebula (Haritaki).
Table 9.
Phytochemicals identified and quantified in the methanol extract of Amalaki.
| Sl. no | Name of the Compound | Retention time (min) | m/z | Area | Height | Conc. (%) |
|---|---|---|---|---|---|---|
| 1. | 2,4-Dimethylfuran | 8.516 | 95.00 | 42,100,441 | 8,365,517 | 14.936 % |
| 2. | 4-(2-Hydroxyethyl)-3-methyl-2-pyrazolin-5-one |
10.398 | 111.00 | 27,773,063 | 8,367,038 | 9.853 % |
| 3. | Trans-2,3-Epoxyoctane |
12.058 | 84.00 | 39,541,711 | 8,255,872 | 14.028 % |
| 4. | 2-Furancarboxylic acid, 2-ethylhexyl ester | 13.794 | 95.00 | 26,873,156 | 8,204,970 | 9.534°/o |
| 5. | Heptanoic acid, 3-hydroxy-, methyl ester |
14.187 | 71.00 | 36,400,612 | 7,612,806 | 12.914 % |
| 6. | 2-(3-Methylguanidino)ethanol | ----- | 85.00 | ------ | ------- | N.D. (Ref) % |
| 7. | d-Glycero-d-ido-heptose |
------ | 55.00 | ------- | ------- | N.D. (Ref) % |
| 8. | Paromomycin |
16.803 |
109.00 | 27,593,590 | 8,274,656 | 9.790 % |
| 9. | Octadecanoic acid |
17.645 | 55.00 | 4,779,887 | 1,067,771 | 1.696 % |
| Octadecanoic acid |
17.926 | 57.00 | 9,984,246 | 2,660,374 | 3.542 % | |
| 10. | 9,9-Dimethoxybicyclo[3.3.1 ]nona-2,4-dione |
------- | 82.00 | ------- | ------- | N.D. (Ref) % |
| 11. | n-Propyl nonyl ether | ------- | 69.00 | ------- | ------- | N.D. (Ref) % |
| 12. | Octadecanoic acid, 2-(2-hydroxyethoxy)ethyl ester |
24.536 | 60.00 | 1,885,820 | 329,949 | 0.669 % |
| 13. | n-Hexadecanoic acid |
------- | 55.00 | ------- | ------- | N.D. (Ref) % |
| 14. | Z-(13,14-Epoxy)tetradec-11 -en-1-ol acetate |
------- | 69.00 | ------- | ------- | N.D. (Ref) % |
| 15. | 6,9,12,15-Docosatetraenoic acid, methyl ester |
------- | 67.00 | ------- | ------- | N.D. (Ref) % |
| 16. | Cyclopentaneundecanoic acid |
27.170 | 55.00 | 17,104,964 | 4,791,795 | 6.068 % |
| 17. | 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | 32.587 | 67.00 | 47,829,702 | 8,251,805 | 16.969 % |
Table 10.
Phytochemicals identified and quantified in the methanol extract of Bibhitaki.
| Sl. no | Name of the Compound | Retention time (min) | m/z | Area | Height | Conc. (%) |
|---|---|---|---|---|---|---|
| 1. | 2-Cyclopenten-I -one, 5-hydroxy-2,3 -dimethyl | 19.962 | 51.00 | 32,617,077 | 8,295,690 | 7.716 |
| 2. | Spirohexane-1-carboxylic acid, ethyl ester | 19.962 | 51.00 | 32,617,077 |
8,295,690 | 7.716 |
| 3. | 1-Cyclohexene-1-carboxylic acid |
19.962 | 51.00 | 32,617,077 |
8,295,690 | 7.716 |
| 4. | 9-Octadecenoic acid (Z)-, methyl ester | 24.250 | 55.00 | 22,009,193 | 7,645,464 | 5.206 |
| 5. | 7-Hexadecenoic acid, methyl ester, (Z)- | 24.250 | 55.00 | 22,009,193 | 7,645,464 | 5.206 |
| 6. | 9-Hexadecenoic acid, methyl ester, (Z)- | 24.250 | 55.00 | 22,009,193 | 7,645,464 | 5.206 |
| 7. | Lanosta-7,9(11)-dien-18-oic acid, 22,25-epoxy-3beta,17,20-trihydroxy-, gamma-lactone | 26.312 | 67.00 | 80,875,411 | 7,915,536 | 19.131 |
| 8. | Pentaborane(11) | -------- | 61.00 | ------- | ------- | N.D. (Ref) |
| 9. | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6 methyl- | 14.659 | 55.00 | 7,868,536 | 2,970,696 | 1.861 |
| 10. | Hydroxy methyl furfural | 16.765 | 97.00 | 8,661,673 | 3,536,218 | 2.049 |
| 11. | 2-Pyrrolidinone, 5 -(hydroxymethyl)- | 19.006 | 84.00 | 22,437,120 | 7,414,633 | 5.308 |
| 12. | 2-Cyclopenten-1 -one, 5-hydroxy-2,3-dimethyl | 19.962 | 51.00 | 32,617,077 | 8,295,690 | 7.716 |
| 13. | n-Hexadecanoic acid | 23.002 | 60.00 | 6,849,035 | 2,557,141 | 1.620 |
| 14. | Octadecanoic acid, 2-(2-hydroxyethoxy)ethyl ester | 24.402 | 157.00 | 17,928,441 | 8,396,404 | 4.241 |
| 15. | 0leicAcid | -------- | 55.00 | ------- | ------- | N.D. (Ref) |
| 16. | 1,2,4-Trioxolane-2-octanoic acid, 5-octyl-, methyl ester | -------- | 77.00 | ------- | ------- | N.D. (Ref) |
| 17. | Cyclopentaneundecanoic acid | -------- | 55.00 | ------- | ------- | N.D. (Ref) |
| 18. | 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl | 32.598 | 50,953,031 | 8,357,116 | 12.053 |
Table 11.
Phytochemicals identified and quantified in the methanol extract of Haritaki.
| Sl. no | Name of the Compound | Retention time (min) | m/z | Area | Height | Conc. (%) |
|---|---|---|---|---|---|---|
| 1. | 1,2,4-Benzenetriol | ------- | 52.00 | ------- | ------- | N.D. (Ref) |
| 2. | 2-Cyclopenten-1 -one, 5-hydroxy-2,3 -dimethyl. | 16.776 | 69.00 | 32,220,591 | 8,244,314 | 6.755 |
| 3. | Paromomycin | 19.992 | 57.00 | 21,209,765 | 8,108,614 | 4.446 |
| 4. | 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl | 32.588 | 167.00 | 51,817,366 | 8,382,485 | 10.863 |
| 5. | Carbonocyanidic acid, ethyl ester |
7.770 |
54.00 | 17,087,391 | 6,804,018 | 3.582 |
| 6. | Furfural |
8.518 | 95.00 | 40,835,307 | 8,388,693 | 8.561 |
| 7. | Cyclohexanone |
10.408 | 55.00 | 7,084,690 | 2,567,521 | 1.485 |
| 8. | 4-Nonanol |
10.891 | 55.00 | 8,082,150 | 3,402,005 | 1.694 |
| 9. | 2-Furancarboxaldehyde, 5-methyl- |
11.148 | 53.00 | 9,810,133 | 3,950,420 | 2.057 |
| 10. | Cyclobut-1 -enylmethanol |
11.435 | 55.00 | 10,502,255 | 3,775,573 | 2.202 |
| 11. | Carbamic acid, phenyl ester |
12.303 | 94.00 | 19,774,147 | 7,875,633 | 4.146 |
| 12. | Tetrahydroeyclopenta[ 1,3]dioxin-4-one |
12.431 | 68.00 | 15,328,408 | 5,993,338 | 3.214 |
| 13. | Thymine | 13.483 | 55.00 | 9,012,657 | 3,577,835 | 1.889 |
| 14. | 2,3-Dimethylfumaric acid | 13.964 | 55.00 | 12,171,251 | 4,830,716 | 2.552 |
| 15. | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6 methyl- |
14.662 | 55.00 | 13,752,481 | 5,271,781 | 2.883 |
| 16. | 2-Propenoic acid, 3-phenyl- |
19.358 |
51.00 | 6,462,997 | 2,515,742 | 1.355 |
| 17. | Paromomycin |
19.993 | 97.00 | 41,308,023 | 8,285,082 | 8.660 |
| 18. | 1,2,3,5-Cyclohexanetetrol, (1.alpha.,2.beta.,3.alpha.,5.beta.)- | 23.527 | 60.00 | 15,238,417 | 4,925,917 | 3.195 |
| 19. | Cyclopropanepentanoic acid, 2-undecyl-, methyl- | 26.345 | 55.00 | 9,445,985 | 3,855,825 | 1.980 |
| 20. | Cyclopentaneundecanoic acid |
------- | 55.00 | ------- | ------- | N.D. (Ref) |
4. Discussion
Reactive oxygen species (ROS) have been implicated in the oxidative damage of cell membrane, cellular protein, DNA which in turn lead to aging and a wide range of ailments, including cancer, cardiovascular diseases, mild cognitive impairment, Alzheimer’s disease, Parkinson’s disease, and atherosclerosis (Syed Ahmed et al., 2018; Liu et al., 2018, Uddin et al., 2014). Natural antioxidants such as polyphenolic compounds flavonoids can combat these diseases by quenching ROS in the biological system (Pisoschi & Negulescu, 2011).
The antioxidant effect of extracts of Triphala and its individual constituents was assessed by DPPH free radical scavenging assay, TPC, and TAC determination tests. It is noteworthy to mention that our study is reporting the synergistic DPPH free radical scavenging effect, TPC, and TAC of nonequivalent ratios of Triphala components for the first time.
The findings of our study demonstrated that the nonequivalent ratios of Tripahal constituents showed higher synergistic DPPH free radical scavenging, TPC, and TAC than that of equivalent ratios of individual constituents. Furthermore, it is important to mention that Amalaki alone and the nonequivalent ratios of three Triphala constituents where Amalaki was doubled in quantity (2:1:1 = A:B:H), showed the highest synergistic antioxidant potential and the IC50 (7.01 μg/mL) of the nonequivalent ratio was much lower than the IC50 of the standard antioxidant, ascorbic acid which was 12.96 μg/mL. In our study at 100 μg/mL concentration, Amalaki showed a slightly higher % of free radical inhibition (96.19%) than that of standard ascorbic acid (94.88%), Bibhitaki (94.49%), and Haritaki (94.88%) (Table 1). Our results are supported by previous study findings where Amalaki showed a bit higher DPPH free radical scavenging (94.747%) compared to Bibhitaki (94.406%) and Haritaki (94.482%) at the concentration of 500 µg/mL (Parveen et al., 2018). However, Amalaki produced only 46.8% inhibition, and ascorbic acid showed 65.6% inhibition of DPPH free radicals at the same concentration (100 μg/mL) in a previous study (Singh et al., 2016). It is important to mention that at concentrations higher than 100 μg/mL DPPH scavenging effect produced was not linear with the increasing concentration ranging from 400 to 1200 μg/mL in the present study (Table 1). In the TPC and TAC assessment, at the concentration of 400 μg/mL, the nonequivalent ratios of Amalaki, Bibhitaki, and Haritaki had significantly higher TPC and TAC than the equivalent ratios of these constituents. The TPC of A:B:H (2:1:1), A:B:H (1:2:1), A:B:H (1:1:2) were found to have 430.37, 401.94, and 395.66 mg GAE/g crude extracts, respectively whereas A:B:H (1:1:1) was found to have 365.91 mg GAE/g crude extracts at the concentration of 400 μg/mL (Table 5). Similarly the TAC of A:B:H (2:1:1), A:B:H (1:2:1), A:B:H (1:1:2) were 293.20, 283.39, and 294.76 mg AAE/g crude extracts, respectively while the TAC of A:B:H (1:1:1) was 265.91 AAE/g crude extract at the same concentration (Table 7). It was evident that the quantity/ratio of Amalaki was a major contributing factor to the DPPH free radical scavenging effect and TPC of Triphala.
This highest antioxidant effect of Amalaki might be attributed to the presence of octadecanoic acid and n-hexadecanoic identified in this extract by our GC-MS analysis, as well as its highest Vit-C content. The fruit juice of Amalaki has been reported to contain the highest amount of vitamin C (478.56 mg/100 mL) in a previous study (Shastri Brhamashankar & Bhavaprakasha, 1969). This highest TPC of Amalaki could be ascribed to its highest gallic acid content. HPLC analysis of Triphala detected that Amalaki, Bibhitaki, and Haritaki contain 0.081%, 0.005%, and 0.024% w/w of gallic acid, respectively (Pundareekaksha, 2017). Another study reported the TPC of individual fruits of Triphala, and they found that the TPC in terms of gallic acid equivalent varies from 33 to 44% (Naik et al., 2005). Furthermore, the TPC of the crude extracts of Triphala ranging from 195.3 to 296.4 mg of GAE/gm of GAE/gm dry extracts was reported previously (Jayajothi et al., 2004). The findings of a previous study demonstrated the total phenolic content in ethanol extract of Triphala was 254 ± 8.3 mg GAE/g of crude extracts (Babu et al., 2013). This highest TAC of Haritaki could be attributed to the compound, 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6 methyl- which was detected in the GC-MS analysis of Haritaki extract. This compound has been reported as a strong antioxidant in a previous study (Yu, Zhao, Liu, Zeng, & Hu, 2013) as well as due to its highest ascorbic acid content.
In vitro cytotoxicity test of Triphala at both nonequivalent ratio of 2:1:1 (A:B:H) and equivalent ratio; 1:1:1 (A:B:H) was measured by MTT assay against glioblastoma cells, N4X4. This cancer cell line was selected since no previous study was performed using Triphala extracts and with a hope to find a natural cure for this cancer as glioblastoma is one of the rapidly growing and mostly incurable brain cancers despite advances in cancer treatment modalities (Hanif et al., 2017).
Among three nonequivalent ratios; 2:1:1 (A:B:H), 1:2:1 (A:B:H), and 1:1:2 (A:B:H), the nonequivalent ratio of 2:1:1 (A:B:H) was selected for assessing the cytotoxic effect because of its maximum DPPH radical scavenging potential and TPC estimated in our antioxidant activity study. Moreover, GC-MS-employed characterization of Amalaki extracts divulged the presence of a compound named 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester in the highest concentration of (16.969%). Notably, antitumor/antioxidant activity of the compound has been reported against numerous cancer cell lines, including prostate cancer (PC3), breast cancer (MCF), colon cancer (HCT-116), lung cancer (A549), and pancreatic cancer (MIA PaCa-2) cell lines in a previously conducted study (Save, 2015). Also, Amalaki is more easily edible and contains more gallic acid than Bibhitaki and Haritaki (Pundareekaksha, 2017, Kaur et al., 2005). Importantly, gallic acid is a well-documented polyphenol for its cytotoxic potential (Marienfeld, 2003). Further to mention that gallic acid is one of the key constituents present in Triphala and has the potential to arrest cancer cell proliferation recommending the major factor contributing to the antimutagenic and cytotoxic effects of Triphala (Kaur et al., 2005).
The nonequivalent ratio of Triphala constituents showed significantly higher synergistic cytotoxic activity in N4X4 cells. Thus, the nonequivalent ratio of Triphala composition exhibited a notable difference in synergistic cytotoxic effect produced by 2:1:1 (A:B:H) ratio where the quantity of Amalaki was doubled with an IC50 value of 8.29 mg/mL compared to 1:1:1 (A:B:H) ratio with an IC50 value of 15.31 mg/mL where Amalaki was in equal quantity as of other two constituents. In the previous study, Triphala showed a strong inhibitory effect on gynecological cancer cells with IC50 values ranging 98.28–101.23 µg/mL against three different cancer cells, including ovarian (SKOV-3), cervical (HeLa), and endometrial (HEC-1B) cancer cells. Several studies reported the cytotoxic effect of Triphala where it showed significant cell growth inhibition with an IC50 value of 50 µg/mL and induced apoptosis in pancreatic cancer cells (Capan-2) (Prasad and Srivastava, 2020, Shi et al., 2008). Prasad & Srivastava reported the dose-dependent antiproliferative effect of Triphala methanol extract (TME) on human carcinoma stem cells (HCCSC) and colon cancer cells (HCT116). They also reported TME-induced apoptosis properties in HCCSCs (Prasad & Srivastava, 2020). Triphala-induced apoptosis was characterized by enhanced expression of p53 level and Bax (B-cell lymphoma protein 2-associated x)/Bcl-2 (B-cell lymphoma protein 2) ratio via activation of the mitochondrial apoptotic signaling pathway. Since TME exhibited a remarkable anticancer effect against colon cancer cells, it can be taken simultaneously with the conventional chemotherapeutic agents to treat and manage colon cancer (Vadde et al., 2015). Our study also suggested that Triphala has strong cytotoxicity against glioblastoma cells. More importantly, the nonequivalent ratio where the quantity of Amalaki was doubled exhibited significantly higher activity against N4X4-brain cancer cell line than the equivalent ratio of Triphala composition.
In our study, GC-MS analysis of individual constituents of Triphala led to the characterization and quantification of a significant number of compounds belonging to fatty acid, fatty acid ester, alcohol, aminoglycoside, triterpene, etc. in the Amalaki, Bibhitaki, and Haritaki extracts, the components of Triphala. Of those, some compounds have been reported with huge potential to exert antioxidant and cytotoxic effects. Octadecanoic acid, n-Hexadecanoic acid, 1,2,3, Benzenetriol, 9-Octadecenoic acid (Z)-, methyl ester, 9-Hexadecenoic acid, methyl ester, (Z)-, 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6 methyl-, Lanosta-7,9(11)-dien-18-oic acid, 22,25-epoxy-3beta,17,20-trihydroxy-, gamma-lactone, and 7-Hexadecenoic acid, methyl ester, (Z)- and have been reported to produce an antioxidant effect (Ganesh, 2017, Lim et al., 2016, Reza et al., 2021, Xia et al., 2014, Yu et al., 2013). 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester and Lanosta-7,9(11)-dien-18-oic acid, 22,25-epoxy-3beta,17,20-trihydroxy-, gamma-lactone have been reported to exert anticancer/antitumor activity (Save, 2015, Xia et al., 2014). More specifically, 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester has been reported with remarkable anticancer activity against PC3, MCF, HCT-116, A549, and MIAPACA cancer cell lines (Save, 2015). Therefore, these GC-MS identified individual compounds along with other polyphenolic compounds and vitamin C might be responsible for the synergistic antioxidant and cytotoxic effect exhibited by the nonequivalent ratios of the Triphala constituents.
5. Conclusion
Our findings revealed the significantly higher synergistic antioxidant activity of mixtures of the individual constituents of Triphala at their nonequivalent ratios than that of an equivalent ratio. It is noteworthy to mention that the nonequivalent ratio at which the quantity of Amalaki was double than other two constituents showed the highest synergistic antioxidant effect. Cytotoxic activity determination on N4X4-brain cancer cells, nonequivalent ratios of individual components of Triphala showed a notable difference in synergistic cytotoxicity compared to an equivalent ratio of them. GC-MS analysis of the three individual constituents of Triphala led to the Identification and quantification of a wide array of compounds. Importantly some of them with antioxidant and cytotoxic potential could be contributing to the exhibited synergistic antioxidant and cytotoxic effects. Thus, it can be inferred that the methanol extract of Triphala at nonequivalent ratios of its three constituents where the quantity of Amalaki is double is expected to be more effective in treating oxidative degenerative diseases and glioblastoma (brain cancer). A new formulation can be developed using this nonequivalent ratio where Amalaki is doubled in quantity, and a clinical trial can be performed to estimate/prove its higher therapeutic benefits.
Funding
This research did not receive any funds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2022.103287.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Syed Ahmed Z., Nowrin T., Hemayet Ho M.d., Nasrin T., Akter R. Metabolite profiling of Crotalaria verrucosa leaf extract and evaluation of its antioxidant and cytotoxic potency. Research Journal of Phytochemistry. 2018;12(2):60–70. doi: 10.3923/rjphyto.2018.60.70. [DOI] [Google Scholar]
- Alam M.N., Bristi N.J., Rafiquzzaman M.d. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceutical Journal. 2013;21(2):143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilou H., Akssira M. Chemical composition, antibacterial, antioxidant and insecticidal activities of moroccan Thapsia transtagana essential oil. Saudi Journal of Biological Sciences. 2021;28(12):6756–6764. doi: 10.1016/j.sjbs.2021.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter R., Uddin S.J., Grice I.D., Tiralongo E. Cytotoxic activity screening of Bangladeshi medicinal plant extracts. J. Nat. Med. 2014;68(1):246–252. doi: 10.1007/s11418-013-0789-5. [DOI] [PubMed] [Google Scholar]
- Aung T.N., Qu Z., Kortschak R.D., Adelson D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017;18:656. doi: 10.3390/ijms18030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu D., Gurumurthy P., Borra S.K., Cherian K.M. Antioxidant and free radical scavenging activity of triphala determined by using different in vitro models. Journal of Medicinal Plant Research. 2013;7:2898–2905. doi: 10.5897/JMPR2013.5124. [DOI] [Google Scholar]
- Chandra S., Khan S., Avula B., Lata H., Yang M.H., ElSohly M.A., Khan I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evid.-Based Complementary and Alternative Medicine. 2014;2014:1–9. doi: 10.1155/2014/253875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif, F., Muzaffar, K., Perveen, K., Malhi, S.M., & Simjee, S. (2017). Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pacific Journal of Cancer Prevention : APJCP, 18(1), 3–9. https://doi.org/10.22034/APJCP.2017.18.1.3 [DOI] [PMC free article] [PubMed]
- Hussain S.M.A. Comprehensive update on cancer scenario of Bangladesh. South Asian Journal of Cancer. 2013;02(04):279–284. doi: 10.4103/2278-330X.119901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayajothi E., Elavarasu T., Hamsaveni M., Sridhar S.K. Antioxidant activity and total phenolic content of Triphala churna. Natural Product Science. 2004;10:16–19. [Google Scholar]
- Kaur S., Michael H., Arora S., Härkönen P.L., Kumar S. In vitro cytotoxic and apoptotic activity of Triphala–an Indian herbal drug. J. Ethnopharmacol. 2005;97:15–20. doi: 10.1016/j.jep.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Kumar D.R.N., George V.C., Suresh P.K., Kumar R.A. Cytotoxicity, apoptosis induction and anti-metastatic potential of Oroxylum indicum in human breast cancer cells. Asian Pac. J. Cancer Prev. 2012;13(6):2729–2734. doi: 10.7314/apjcp.2012.13.6.2729. [DOI] [PubMed] [Google Scholar]
- Lalhminghlui K., Jagetia G.C. Evaluation of the free-radical scavenging and antioxidant activities of Chilauni, Schima wallichii Korth in vitro. Future Sci. OA. 2018;4(2):FSO272. doi: 10.4155/fsoa-2017-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichota A., Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018;19:3533. doi: 10.3390/ijms19113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Ren Z., Zhang J., Chuang C.-C., Kandaswamy E., Zhou T., Zuo L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018;9(477) doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marienfeld C., Tadlock L., Yamagiwa Y., Patel T. Inhibition of cholangiocarcinoma growth by tannic acid. Hepatology. 2003;37:1097–1104. doi: 10.1053/jhep.2003.50192. [DOI] [PubMed] [Google Scholar]
- Naik G.H., Priyadarsini K.I., Bhagirathi R.G., Mishra B., Mishra K.P., Banavalikar M.M., Mohan H. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother. Res. 2005;19(7):582–586. doi: 10.1002/ptr.1515. [DOI] [PubMed] [Google Scholar]
- Parveen R., Shamsia T.N., Singhb G., Athara T., Fatima S. Phytochemical analysis and in-vitro biochemical characterization of aqueous and methanolic extract of Triphala, a conventional herbal remedy. Biotechnol. Rep, 2018;17:126–136. doi: 10.1016/j.btre.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham H.N.T., Sakoff J.A., Bond D.R., Vuong Q.V., Bowyer M.C., Scarlett C.J. In vitro antibacterial and anticancer properties of Helicteres hirsuta Lour. leaf and stem extracts and their fractions. Mol. Biol. Rep. 2018;45(6):2125–2133. doi: 10.1007/s11033-018-4370-x. [DOI] [PubMed] [Google Scholar]
- Pisoschi A.M., Negulescu G.P. Methods for total antioxidant activity determination: a review. Biochemical & Analytical Biochemistry. 2011;1:1. doi: 10.4172/2161-1009.1000106. [DOI] [Google Scholar]
- Prasad S., Srivastava S.K. Oxidative stress and cancer: chemopreventive and therapeutic role of Triphala. Antioxidants. 2020;9:72. doi: 10.3390/antiox9010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pundareekaksha R.P. Antioxidant effect of Triphala-critical review. Journal of Ayurveda and Integrated Medical Sciences. 2017;1:213–219. doi: 10.21760/jaims.v2i1.7513. [DOI] [Google Scholar]
- Seca A.M., Pinto D.C. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018;19:1–22. doi: 10.3390/ijms19010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Sahu R.P., Srivastava S.K. Triphala inhibits both in vitro and in vivo xenograft growth of pancreatic tumor cells by inducing apoptosis. BMC Cancer. 2008;8:294–1235. doi: 10.1186/1471-2407-8-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G., Choudhary P., Yaqoob S.A., Rawat R.S., Jat D.B.L. Antibacterial and antioxidant activity of Triphala extracts. International Journal of Current Research. 2016;8:38335–38348. [Google Scholar]
- Uddin R., Saha M., Subhan N., Hossain H., Jahan I.A., Akter R., Islam A. HPLC-analysis of polyphenolic compounds in Gardenia jasminoids and determination of antioxidant activity by using free radical. Advanced Pharmaceutical Bulletin. 2014;4:273–281. doi: 10.5681/apb.2014.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadde R., Radhakrishnan S., Reddivari L., Vanamala J.K.P. Triphala extract suppresses proliferation and induces apoptosis in human colon cancer stem cells via suppressing c-Myc/Cyclin D1 and elevation of Bax/Bcl-2 ratio. Biomed Res. Int. 2015;2015:1–12. doi: 10.1155/2015/649263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh M. Extraction and identification of bioactive components in Sida cordata (Burm.f.) using gas chromatography–mass spectrometry. J. Food Sci. Technol. 2017;54 doi: 10.1007/s13197-017-2744-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.Y., Kim C.-M., Rhee J.H., Kim Y.R. Effects of Pyrogallol on Growth and Cytotoxicity of Wild-Type and katG Mutant Strains of Vibrio vulnificus. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0167699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza, A. S. M. A., Haque, M. A., Sarker, J., Nasrin, M. S., Rahman, M. M., Tareq, A. M., Alam, A. K., 2021. Antiproliferative and antioxidant potentials of bioactive edible vegetable fraction of Achyranthes ferruginea Roxb. in cancer cell line. Food Science & Nutrition, 9, 3777-3805. 10.1002%2Ffsn3.2343. [DOI] [PMC free article] [PubMed]
- Save S. Determination of 1, 2-Benzenedicarboxylic acid, bis (2-ethylhexyl) ester from the twigs of Thevetia Peruviana as a Colwell Biomarker. Journal of Innovations in Pharmaceuticals and Biological Sciences. 2015;2:349–362. [Google Scholar]
- Xia Q., Zhang H., Sun X., Zhao H., Wu L., Zhu D., She G. A Comprehensive Review of the Structure Elucidation and Biological Activity of Triterpenoids from Ganoderma spp. Molecules. 2014;19:17478–17535. doi: 10.3390/molecules191117478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Zhao M., Liu F., Zeng S., Hu J. Identification of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose–histidine Maillard reaction products. Food Res. Int. 2013;51:397–403. doi: 10.1016/j.foodres.2012.12.044. [DOI] [Google Scholar]
- Zhou J., Yang Q., Zhu X., Lin T., Hao D., Xu J. Antioxidant activities of Clerodendrum cyrtophyllum Turcz leaf extracts and their major components. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0234435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89:217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- Saini R., Sharma N., Oladeji O.S., Sourirajan A., Dev K., Zengin G., El-Shazly M., Kumar V. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022;282 doi: 10.1016/j.jep.2021.114570. [DOI] [PubMed] [Google Scholar]
- Hasan M.R., Islam M.N., Islam M.R. Phytochemistry, pharmacological activities and traditional uses of Emblica officinalis: A review. International Current Pharmaceutical Journal. 2016;5:14–21. [Google Scholar]
- Nigam M., Mishra A.P., Adhikari-Devkota A., Dirar A.I., Hassan M.M., Adhikari A., Belwal T., Devkota H.P. Fruits of Terminalia chebula Retz.: A review on traditional uses, bioactive chemical constituents and pharmacological activities. Phytother. Res. 2020;34:2518–2533. doi: 10.1002/ptr.6702. [DOI] [PubMed] [Google Scholar]
- Kumar N., Khurana S.M.P. Phytochemistry and medicinal potential of the Terminalia bellirica Roxb. (Bahera) Indian Journal of Natural Products and Resources. 2018;9:97–107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.