Abstract
Primary prevention through the use of human papillomavirus (HPV) vaccination is expected to impact both cervical intraepithelial neoplasia (CIN) and adenocarcinoma in situ (AIS). While CIN is well described, less is known about the epidemiology of AIS, a rare cervical precancer. We identified AIS and CIN grade 3 (CIN3) cases through population-based surveillance, and analyzed data on HPV types and incidence trends overall, and among women screened for cervical cancer. From 2008 to 2015, 470 AIS and 6,587 CIN3 cases were identified. The median age of women with AIS was older than those with CIN3 (35 vs. 31 years; p < 0.01). HPV16 was the most frequently detected type in both AIS and CIN3 (57% in AIS; 58% in CIN3), whereas HPV18 was the second most common type in AIS and less common in CIN3 (38% vs. 5%; p < 0.01). AIS lesions were more likely than CIN3 lesions to be positive for high-risk types targeted by the bivalent and quadrivalent vaccines (HPV16/18, 92% vs. 63%; p < 0.01), and 9-valent vaccine (HPV16/18/31/33/45/52/58, 95% vs. 87%; p < 0.01). AIS incidence rates decreased significantly in the 21–24 year age group (annual percent change [APC] overall: −22.1%, 95% CI: −33.9 to −8.2; APC among screened: −16.1%, 95% CI: −28.8 to −1.2), but did not decrease significantly in any older age group. This report on the largest number of genotyped AIS cases to date suggests an important opportunity for vaccine prevention of AIS, and is the first to document a decline in AIS incidence rates among young women during the vaccine era.
Keywords: HPV, adenocarcinoma in situ, AIS, cervical cancer, cervical intraepithelial lesions
Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States of America (US) and persistent infection with high-risk (HR) HPV types is a necessary cause of most cervical cancers.1,2 Cervical cancer screening programs aim to identify and treat precancers before they progress to invasive cancer, and have resulted in increased detections of precancers and reductions in cervical cancers worldwide.3–5
The predominant cervical cancer histology is squamous cell carcinoma, representing 75% of all cervical carcinomas; 20–25% of cervical cancers are adenocarcinomas.6,7 While rates of squamous cell carcinomas have declined, the incidence of adenocarcinoma has increased worldwide.6,8–13 Although precursors for both squamous cell carcinomas (cervical intraepithelial neoplasia [CIN]) and adenocarcinoma (adenocarcinoma in situ [AIS]) arise most commonly at the squamocolumnar junction within the transformation zone, AIS is more likely to involve glands higher in the endocervical canal and to be multifocal.14 Thus, cervical cytology screening is more efficient for detection of CIN than AIS; 30–60% of AIS lesions are detected incidentally during follow-up for squamous abnormalities.15–17 Similarly, AIS lesions can be more difficult to visualize during colposcopy, and diagnosis may require dedicated endocervical sampling.18 As recently as the 1980s, the literature was unclear about the histologic criteria for diagnosis and subsequent behavior of AIS.19 Recognition of glandular precancers less severe than AIS (i.e., lower grade glandular abnormalities or atypia analogous to low-grade squamous intraepithelial lesions) remain controversial.14 Thus, identification, diagnosis and interpretation of findings for AIS remain a challenge. Recent cancer registry data from the US showed that CIN grade 3 (CIN3), the highest grade of CIN, is detected 8.7 times more often than squamous cell carcinomas, whereas AIS is detected 0.9 times as often as adenocarcinoma, suggesting that AIS often goes undetected, or that the natural history of oncogenic progression in glandular epithelium differs from that in squamous epithelium.20 Additionally, the stage of adenocarcinoma at diagnosis is often more advanced and prognosis worse than for squamous cell carcinomas.16,21,22
In the US, approximately 66% of all cervical cancers are attributable to HR-HPV types 16 and 18, and an additional 15% are attributable to 5 HR-HPV types (31/33/45/52/58).23,24 While HPV16 causes most squamous cell carcinomas and adenocarcinoma, HPV types 18 and 45 are found in a larger percentage of adenocarcinoma than squamous cell carcinomas.25–32 Similarly, reports indicate that the HPV types identified in CIN and AIS differ. While HPV16 is the most common type in both AIS and CIN lesions, data from several small studies found HPV18 more frequently in AIS than CIN lesions.17,26,29,33–36
HPV vaccination targeting oncogenic types is expected to impact incidence of precancers and cancers. In 2006, a quadrivalent HPV vaccine, the first vaccine targeting HR-HPV types 16 and 18, was licensed by the US Food and Drug Administration (FDA). In 2015, a 9-valent HPV vaccine (9vHPV), which targets HPV16, 18 and five additional HR-HPV types (31/33/45/52/58) was licensed; since 2017, it has been the only vaccine available in the US.37,38 Vaccine coverage has gradually increased in the US since vaccine introduction, and by 2017, 69% of adolescent females aged 13–17 years had received at least one dose.39 The incidence of cervical precancers (CIN grades 2, 2/3 or 3, or AIS, referred to as CIN2+) has declined among screened women since vaccine introduction,40–42 but trends in the incidence of AIS specifically in the vaccine era have not been described.
Since 2008, the Centers for Disease Control and Prevention (CDC) and partners have conducted active population-based laboratory surveillance for CIN2+ in five US locations via the HPV Vaccine Impact Monitoring Project (HPV-IMPACT). HPV-IMPACT data have demonstrated declines in cervical precancer incidence during the vaccine era and reported on HPV types present in precancers.43,44 In this report, we describe all AIS cases reported during the first 8 years of HPV-IMPACT surveillance, including demographic characteristics of women with AIS and HPV types present in lesions, and compare them with CIN3, the highest grade CIN lesion. In addition, we report cervical cancer screening history of AIS cases, and describe trends in AIS incidence from 2008 through 2015.
Methods
Surveillance population
Surveillance methods have been previously described.44 Briefly, active, population-based, laboratory surveillance was conducted beginning in 2008 among all women aged ≥18 years in all histology laboratories in five locations in the US. Laboratories reported all histologically confirmed diagnoses of CIN2+ in surveillance area residents aged ≥18 years. As women could have multiple diagnostic and treatment procedures as part of a single CIN2+ case, the incidence date was defined as the date of the earliest qualifying CIN2+ diagnosis for each woman, and the final diagnosis for each woman was defined as the highest grade lesion identified within the 6 months following the incidence date. Basic demographic and clinical data were ascertained for all women with CIN2+. Among women with CIN2+ aged 18–39 years, additional clinical information including screening and vaccine history were ascertained through review of medical records and vaccine registries. Data on the most recent screening event prior to diagnosis were collected, including type of screening test (Papanicolaou [Pap] and/or HPV tests) and results. The Centers for Disease Control and Prevention (CDC) determined this surveillance project was not human subjects research, therefore the CDC’s Institutional Review Board approval was not required.
AIS case classification
Two authors (AAC, JWG) reviewed all available pathology reports for each woman diagnosed with AIS; pathology reports with ambiguous interpretations were further reviewed by a CDC pathologist (ERU). If a woman had both AIS and a CIN lesion (grade 2 or higher) diagnosed during the initial 6-month period, the case was classified as “AIS + CIN.” AIS cases without CIN2 or higher were classified as “AIS only.” Thus, AIS cases were subdivided into those with AIS only and AIS + CIN.
Laboratory methods
The laboratory methods have been previously described, and have been uniform throughout the surveillance period.44 Briefly, for cases among women aged 18–39 years, an archived diagnostic specimen was requested, and a block representative of the histologic lesion with the highest diagnosis was selected, cut per CDC protocol and shipped to CDC. A pathologist at CDC (ERU) reviewed hematoxylin–eosin slides to ensure a high-grade lesion was present; those without a high-grade lesion were not processed. DNA was extracted and HPV typing was conducted using L1 consensus PCR (Linear Array [LA] HPV Genotyping Assay, Roche Diagnostics, Indianapolis, IN) which detects 37 distinct HPV types. Samples with inadequate or HPV negative LA results were retested with INNO-LiPA® HPV Genotying Extra Assay (Innogenetics, Gent, Belgium). Samples negative for both the genomic control probe and HPV in LA and INNO-LiPA were considered inadequate and omitted.
Of the 5,425 AIS or CIN3 cases in women aged 18–39 years, 4,346 (80%) had a specimen submitted to CDC for typing as of May 2018, for the years 2008–2015. Of these, 440 (AIS: 20.5% [49/239]; CIN3: 9.9% [391/3969]) were not processed after histologic review, mainly due to having no high-grade lesion present on the slide sent to CDC. All remaining 3,768 (AIS: 190, CIN3: 3,578) specimens were typed, and over 99% (AIS: 189, CIN3: 3,557) had adequate typing results.
Statistical analysis
This analysis was restricted to cases with a final diagnosis of AIS (AIS only, AIS + CIN) or CIN3 only during 2008–2015. We compared women with AIS to women with CIN3 by age group, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other and unknown), insurance status (public, private, other/unknown or not available) and vaccination status (vaccinated with ≥1 dose, not vaccinated, unknown or not age-eligible).
Among women aged 18–39 years with valid typing data available, we compared HPV types detected in tissues from AIS and CIN3 cases. We evaluated proportions of cases with single and multiple HPV types. HR-HPV types were described individually (without attribution of multiple-type infections to a single type). In addition, we evaluated the following HR-HPV type groups: HR-HPV types targeted by 4vHPV vaccine, HPV16 or HPV18 (HPV16/18); any HR-HPV types targeted by 9vHPV vaccine (16/18/31/33/45/52/58), any HR-HPV type (16/18/31/33/35/39/45/51/52/56/58/59/66/68), any type in the alpha-7 species (18/39/45/59/68/70) and any type in the alpha-9 species (16/31/33/35/52/58/67).27 The 14 types included as “any HR-HPV” were based on types detected in clinical HR-HPV tests approved by the US FDA.
To elucidate how AIS cases were detected, we evaluated cervical cancer screening tests immediately preceding AIS diagnosis (Pap, HPV, or both), and results of these screening tests, stratified by age group (21–24, 25–29, 30–39 years). Women in the 18–20 year age group were excluded from this analysis because screening in this age group was not recommended during part of the surveillance period. To describe changes in screening history over time, we grouped surveillance years into earlier (2008–2011) and later (2012–2015) time periods.
Incidence rates were calculated using annual population estimates and the estimated number of screened women in each site as the denominators as described previously.42 We calculated incidence rates per 100,000 women for age groups 21–24, 25–29, 30–39 and 40–64 years; women in the 18–20 year age group also were excluded from this analysis. We calculated incidence rates per 100,000 screened women for age groups 21–24, 25–29 and 30–39 years. Linear trends in age-specific incidence rates over the 8-year surveillance period were evaluated using Poisson regression models (i.e., to estimate the rate ratio [RR] and 95% confidence interval [CI] per 1-year increase in year). The annual percentage change (APC) was calculated as (1 − RR) × 100. For graphical presentation, average annual incidence rates were also calculated in 2-year intervals.
Statistical differences in categorical variables were identified using χ2 tests or Fisher’s exact tests. Statistical differences in age were evaluated using the Kruskal–Wallis test. In all analyses, the level of significance was set at α = 0.05. All analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC).
Results
Overall
From 2008 to 2015, 470 AIS cases (231 AIS only, 239 AIS + CIN) and 6,587 CIN3 only cases were reported (Table 1). The median age of women with AIS (35 years) was older than those with CIN3 (31 years; p < 0.01). Compared to women with CIN3, those with AIS were more frequently non-Hispanic white (69.1% vs.53.2%), more frequently had private insurance (63.4% vs. 49.2%), and more frequently were not age-eligible for vaccination (61.7% vs. 46.9%). Women with AIS only were older than those with AIS + CIN (median 37 vs. 32 years, p < 0.01), and more often were not age-eligible for vaccination (72.3% vs. 51.5%, p < 0.01).
Table 1.
Demographic characteristics of cases of adenocarcinomia in situ (AIS) and cervical intraepithelial neoplasia (CIN) grade 3, among women aged ≥18 years, by final case diagnosis (n = 7,057)
| AIS |
CIN3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total AIS |
AIS Only |
AIS + CIN |
CIN3 only |
||||||
| Demographics | n | % | n | % | n | % | n | % | p-value 1 |
| Overall | 470 | 100 | 231 | 100 | 239 | 100 | 6,587 | 100 | |
| Median age (IQR) | 35 | [29–42] | 37 | [31–43] | 32 | [28–41] | 31 | [26–38] | <0.01 |
| Ethnic | <0.01 | ||||||||
| NH white | 325 | 69.1 | 168 | 72.7 | 157 | 65.7 | 3,507 | 53.2 | |
| NH black | 24 | 5.1 | 11 | 4.8 | 13 | 5.4 | 830 | 12.6 | |
| Hispanic | 51 | 10.9 | 26 | 11.3 | 25 | 10.5 | 904 | 13.7 | |
| Asian | 32 | 6.8 | 12 | 5.2 | 20 | 8.4 | 364 | 5.5 | |
| Other | 6 | 1.3 | 0 | 0 | 6 | 2.5 | 222 | 3.4 | |
| Unknown | 32 | 6.8 | 14 | 6.1 | 18 | 7.5 | 760 | 11.5 | |
| Insurance types | <0.01 | ||||||||
| Public | 58 | 12.3 | 29 | 12.6 | 29 | 12.1 | 1,550 | 23.5 | |
| Private | 298 | 63.4 | 149 | 64.5 | 149 | 62.3 | 3,242 | 49.2 | |
| Other/unknown | 114 | 24.3 | 53 | 22.9 | 61 | 25.5 | 1,795 | 27.3 | |
| HPV vaccination status | <0.01 | ||||||||
| Vaccinated (≥1 dose) | 15 | 3.2 | 9 | 3.9 | 6 | 2.5 | 386 | 5.9 | |
| Not vaccinated | 55 | 11.7 | 18 | 7.8 | 37 | 15.5 | 993 | 15.1 | |
| Unknown | 110 | 23.4 | 37 | 16.0 | 73 | 30.5 | 2,118 | 32.2 | |
| Not age-eligible | 290 | 61.7 | 167 | 72.3 | 123 | 51.5 | 3,090 | 46.9 | |
p-value for comparison of total AIS and CIN3 by Kruskal–Wallis test (median age) and chi-square or Fisher’s exact test. Abbreviations: IQR, interquartile range; NH, non-Hispanic.
HPV typing results
We detected HPV in nearly all AIS (99%) and CIN3 (98%) samples assayed (188/189 AIS and 3491/3,557 CIN3 samples from women aged 18–39 years, Table 2). Most AIS (80%) and CIN3 (81%) were positive for a single type. Compared to CIN3 cases, AIS cases were more likely to have 4vHPV HR types HPV16/18 (92% vs. 63%; p < 0.01), and more likely to have a 9vHPV HR type (95% vs. 87%; p < 0.01). Detection of any HR-HPV was high in both groups (97 and 95%; p = 0.22). Alpha-7 types were more common in AIS, and alpha-9 types were more common in CIN3.
Table 2.
Human papillomavirus (HPV) Types, among women aged 18–39 years, by final case diagnosis (n = 3,746)
| AIS |
CIN3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total AIS |
AIS Only |
AIS + CIN |
CIN3 only |
||||||
| HPV type characteristics | n | % | n | % | n | % | n | % | p-value 1 |
| Total typed | 189 | 79 | 110 | 3,557 | |||||
| HPV positivity | |||||||||
| Positive for any HPV type | 188 | 99 | 78 | 99 | 110 | 100 | 3,491 | 98 | 0.26 |
| Positive for single type | 151 | 80 | 65 | 82 | 86 | 78 | 2,887 | 81 | |
| Positive for multiple types | 37 | 20 | 13 | 16 | 24 | 22 | 64 | 17 | |
| HPV type groups | |||||||||
| Any 16 and/or 18 | 174 | 92 | 73 | 92 | 101 | 92 | 2,224 | 63 | <0.01 |
| Any 9-valent HR-HPV2 | 180 | 95 | 74 | 94 | 106 | 96 | 3,085 | 87 | <0.01 |
| Any HR-HPV3 | 184 | 97 | 77 | 97 | 107 | 97 | 3,387 | 95 | 0.22 |
| Alpha-74 | 81 | 43 | 36 | 46 | 45 | 41 | 438 | 12 | <0.01 |
| Alpha-94 | 116 | 61 | 43 | 54 | 73 | 66 | 3,007 | 85 | <0.01 |
| HPV types targeted by 9-valent vaccine5 | |||||||||
| 16 | 108 | 57 | 42 | 53 | 66 | 60 | 2,080 | 58 | 0.72 |
| 18 | 72 | 38 | 33 | 42 | 39 | 36 | 172 | 5 | <0.01 |
| 31 | 4 | 2 | 1 | 1 | 3 | 3 | 392 | 11 | <0.01 |
| 33 | 2 | 1 | 0 | 0 | 2 | 2 | 143 | 4 | 0.03 |
| 45 | 4 | 2 | 1 | 1 | 3 | 3 | 80 | 2 | 1.00 |
| 52 | 6 | 3 | 0 | 0 | 6 | 6 | 286 | 8 | 0.01 |
| 58 | 3 | 2 | 1 | 1 | 2 | 2 | 165 | 5 | 0.05 |
| Other high-risk types5 | |||||||||
| 35 | 3 | 2 | 0 | 0 | 3 | 3 | 138 | 4 | 0.12 |
| 39 | 2 | 1 | 1 | 1 | 1 | 1 | 98 | 3 | 0.24 |
| 51 | 3 | 2 | 1 | 1 | 2 | 2 | 156 | 4 | 0.06 |
| 56 | 0 | 0 | 0 | 0 | 0 | 0 | 35 | 1 | 0.42 |
| 59 | 1 | 1 | 1 | 1 | 0 | 0 | 53 | 1 | 0.52 |
| 66 | 3 | 2 | 2 | 3 | 1 | 1 | 63 | 2 | 1.00 |
| 68 | 1 | 1 | 0 | 0 | 1 | 1 | 31 | 1 | 1.00 |
p-value comparison of total AIS and CIN3 by chi-square or Fisher’s exact test.
9-valent HR-HPV types include HPV 16, 18, 31, 33, 45, 52 and 58.
High-risk HPV types include HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68.
HPV alpha species: alpha-7 include HPV 18, 39, 45, 59, 68 or 70; alpha-9 include HPV 16, 31, 33, 35, 52, 58, or 67.
Categories are not mutually exclusive due to multiple types detected in individual cases.
Abbreviations: AIS, adenocarcinoma in situ; CIN3, cervical intraepithelial neoplasia grade 3; HR-HPV, high-risk human papillomavirus.
HPV16 was the most frequently detected individual type in both AIS and CIN3 (57% in AIS, 58% in CIN3). HPV18 was second most common type detected in AIS, but was significantly less common in CIN3 (38% vs. 5%; p < 0.01). HPV31 and HPV52 were the next most common types detected among CIN3 cases, and these were detected less often in AIS (HPV31: AIS: 2%, CIN3: 11%; p < 0.01; HPV52: AIS: 3%, CIN3: 8%; p = 0.01). HPV33 was also more common in CIN3 than AIS (AIS: 1%; CIN3: 4%, p = 0.03).
Comparing AIS only (n = 79) to AIS + CIN (n = 111), proportions with HPV16, HPV18 and most other individual types were similar. The single significant difference in individual types was that HPV52 was only detected in AIS + CIN cases (AIS: 0%, AIS + CIN: 5%, p = 0.04).
AIS incidence rates
AIS incidence rates per 100,000 women varied by age group over the surveillance period (Fig. 1, Table 3). Among all 21–24 year-olds, the number of cases per year was small (range 0–10); incidence rates declined from 4.6 in 2008 to 0.0 in 2015 (APC: −22.1%, 95% CI: −33.9 to −8.2; Table 3). There were no significant incidence trends among women in any other age group.
Figure 1.
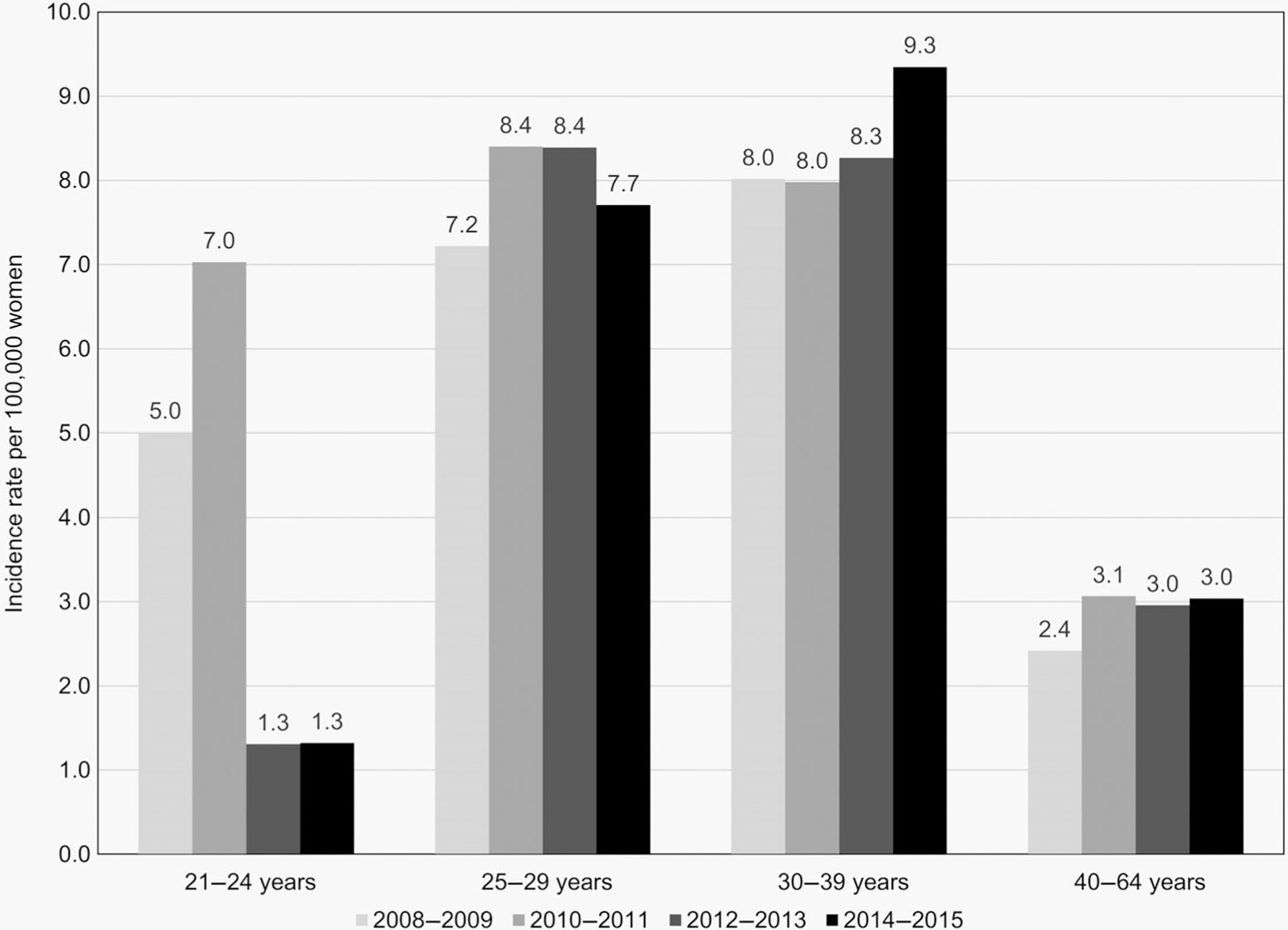
Adenocarcinoma in situ average annual 2-year incidence rates per 100,000 women, by age group. For a smoother graphical presentation, average annual incidence rates were calculated for each 2-year interval.
Table 3.
Adenocarcinoma in situ incidence rates (IR) per 100,000 women and per 100,000 screened women, by age group
| 21–24 years |
25–29 years |
30–39 years |
40–64 years |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Among all women1 |
Among screened women2 |
Among all women |
Among screened women |
Among all women |
Among screened women |
Among all women |
||||||||||||
| Year | n | IR | 95% CI | IR | 95% CI | n | IR | 95% CI | IR | 95% CI | n | IR | 95% CI | IR | 95% CI | n | IR | 95% CI |
| 2008 | 5 | 4.6 | [1.9, 11.1] | 9.5 | [4.0, 22.9] | 13 | 9.0 | [5.2, 15.5] | 17.4 | [10.1, 30.0] | 17 | 6.5 | [4.0, 10.5] | 14.7 | [9.1, 23.6] | 15 | 2.4 | [1.5, 4.0] |
| 2009 | 6 | 5.4 | [2.4, 12.0] | 11.5 | [5.2, 25.7] | 8 | 5.5 | [2.7, 10.9] | 11.4 | [5.7, 22.9] | 25 | 9.5 | [6.4, 14.1] | 22.3 | [15.1, 33.0] | 15 | 2.4 | [1.4, 4.0] |
| 2010 | 10 | 8.8 | [4.8, 16.4] | 21.7 | [11.7, 40.3] | 16 | 10.9 | [6.7, 17.8] | 24.9 | [15.3, 40.7] | 20 | 7.6 | [4.9, 11.8] | 19.9 | [12.8, 30.8] | 17 | 2.7 | [1.7, 4.3] |
| 2011 | 6 | 5.2 | [2.4, 11.7] | 13.4 | [6.0, 29.9] | 9 | 6.0 | [3.1, 11.5] | 14.2 | [7.4, 27.3] | 22 | 8.3 | [5.5, 12.7] | 21.9 | [14.4, 33.2] | 22 | 3.4 | [2.3, 5.2] |
| 2012 | 1 | 0.9 | [0.1, 6.2] | 2.4 | [0.3, 17.2] | 12 | 7.8 | [4.5, 13.8] | 20.1 | [11.4, 35.4] | 24 | 8.9 | [6.0, 13.3] | 24.7 | [16.6, 36.9] | 19 | 3.0 | [1.9, 4.6] |
| 2013 | 2 | 1.8 | [0.4, 7.0] | 5.1 | [1.3, 20.2] | 14 | 8.9 | [5.3, 15.1] | 23.6 | [13.9, 39.8] | 21 | 7.6 | [5.0, 11.7] | 21.9 | [14.2, 33.5] | 19 | 3.0 | [1.9, 4.6] |
| 2014 | 3 | 2.6 | [0.8, 8.1] | 8.6 | [2.8, 26.7] | 16 | 10.0 | [6.1, 16.2] | 28.9 | [17.7, 47.1] | 21 | 7.5 | [4.9, 11.5] | 23.4 | [15.2, 35.8] | 20 | 3.1 | [2.0, 4.8] |
| 2015 | 0 | 0.0 | – | 0.0 | – | 9 | 5.5 | [2.9, 10.6] | 16.4 | [8.5, 31.5] | 32 | 11.2 | [7.9, 15.8] | 36.5 | [25.8, 51.7] | 19 | 3.0 | [1.9, 4.6] |
| APC | −22.1 | [−33.9, −8.2] | −16.1 | [−28.8, −1.2] | −0.96 | [−9.2, 8.0] | 5.3 | [−3.4, 14.8] | 3.4 | [−3.0, 10.2] | 8.9 | [2.2, 16.0] | 3.2 | [−3.9, 10.8] | ||||
Among all women: incidence rates per 100,000 women.
Among screened women: incidence rates per 100,000 women screened.
Abbreviations: APC, annual percent change; 95% CI, 95% confidence intervals; IR, incidence rates.
Among women screened for cervical cancer, AIS incidence rates also decreased significantly in 21–24 year-olds (APC: −16.1%, 95% CI: −28.8 to −1.2), did not change in 25–29 olds (APC: 5.3%, 95% CI: −3.4 to 14.8), and increased significantly in 30–39 year-olds (APC: 8.9%, 95% CI: 2.2–16.0; Table 3).
Cervical cancer screening results
To gain insight into AIS detection during the surveillance period, we examined the screening tests that preceded AIS diagnosis among women aged 21–39 years (n = 312). Screening incorporating HPV testing increased in all age groups between 2008–2011 and 2012–2015 (21–24 year-olds: 22 to 33%; 25–29 year-olds: 35 to 55%; 30–39 year-olds: 46 to 77%). Among AIS cases with Pap test results available (n = 274), a minority (27%) indicated a glandular abnormality (atypical glandular cells, atypical glandular cells of undetermined significance, or cytologic AIS) and this varied by age group (21–24 year-olds [n = 29]: 10%; 25–29 year-olds [n = 86]: 26%; 30–39 year-olds [n = 159]: 31%). A low-grade Pap result (atypical squamous cells of unknown significance [ASCUS], low-grade squamous intraepithelial lesion) was the most frequent result preceding AIS diagnosis in all age groups (21–24 year-olds: 52%; 25–29 year-olds: 43%; 30–39 year-olds: 35%). The proportion of Pap test results with glandular abnormalities was greater for women with AIS only than for those with AIS + CIN (39% vs. 18%, p < 0.01), and the proportion with grade squamous abnormalities (atypical squamous cells cannot exclude HSIL, high-grade squamous intraepithelial lesion) was lower (15% vs. 31%, p < 0.01). Other cytology findings were similar between AIS only and AIS + CIN.
Discussion
This large descriptive epidemiological analysis of 470 AIS cases identified through population-based surveillance, including the largest number of genotyped AIS cases to date, supports the predominance of HPV types 16 and 18 in the etiology of this rare precancer and the higher percentage of HPV18 in AIS cases compared to CIN3. Consistent with previous studies, we found age at diagnosis for AIS was older than for CIN3.34,36 Additionally, we provide the first data on AIS incidence trends in the vaccine era; in contrast to the increasing AIS incidence in young women reported in 1976–2000,13 we document a decline in incidence among young women, which may be in part attributable to early vaccine impact.
In this analysis, 92% of 190 typed AIS cases tested positive for HPV types 16 or 18; this was higher than the percentage among CIN3 (63%), and due entirely to the higher prevalence of HPV18 (38% vs. 5%). These findings are similar to prior reports worldwide; several papers published in the past 15 years have provided data on HPV16/18 prevalence in AIS tissues based on small numbers of women (all less than 100 cases), with estimates ranging from 79% to 96%.17,26,29,33–36,45 In the US, the largest previous report on typing data from AIS cases (n = 61) included data from the first 2 years of HPV-IMPACT surveillance; the prevalence of HPV16/18 among AIS cases was 82%.35 Another population-based study conducted in New Mexico, USA with 51 AIS cases found that 88% had HPV16/18 (44% HPV16, 48% HPV18).34 The two largest prior studies, with 96 and 61 cases, were conducted in the Netherlands; these identified HPV16/18 in 79 and 94% of AIS cases (41 and 34% HPV16, 43 and 66% HPV18).26,36 Four additional studies ducted in a variety of settings, with <50 cases each, also found HPV16/18 in the vast majority of AIS cases (ranging from 85% to 96%, with 51–71% HPV16 and 36–39% HPV18).17,29,33,45
HPV18 and HPV45 belong to the alpha-7 species, which may have a greater tendency to cause adenocarcinoma compared to the alpha-9 species (which includes HPV16).27 However, while an association between HPV18 and AIS has been well-documented, data linking HPV45 to AIS have been less consistent. We found a similar low prevalence of HPV45 among AIS and CIN3 (2% in both). Previous typing studies reported 3–8% of AIS cases positive for HPV45.17,26,29,34–36,45,46 Few studies have directly compared the prevalence of specific HPV types between CIN3 and AIS. The largest to date found no significant difference in HPV45 prevalence between CIN2/3 and AIS.36 The New Mexico study found a higher proportion of both HPV45 and alpha-7 species among AIS compared to CIN3. The authors also noted the proportion of cases with alpha-9 species was lower among AIS than CIN3.34 Similarly, we found that alpha-9 species as a group and by individual alpha-9 types 31, 33 and 52 were identified more often in CIN3 than in AIS.
Like previous reports, we found that women with AIS were diagnosed at older ages than women with CIN3,34,36 and a larger percentage were non-Hispanic white.9,13,20 We considered whether proportions with HPV testing or proportion vaccinated could explain racial/ethnic differences; analyses were inconclusive because numbers were small (data not shown). While reasons for an racial/ethnic difference in AIS incidence are not clear, it seems unlikely that racial/ethnic differences in screening explain these differences, as black and white women had similar screening proportions within the past 3 years in a recent national survey, and rates of squamous cell carcinoma declined similarly in blacks and whites.9,13,47
Similar to prior reports, over half of the AIS cases in our study had concurrent CIN lesions.11,17,29,36 HPV type distributions were similar between AIS only and AIS + CIN, but women with AIS only were typically diagnosed 5 years older, on average, than women with AIS + CIN. A similar age difference was reported in 2003, which was speculatively attributed to a faster progression of disease for AIS + CIN than AIS alone, or incidental identification of AIS after screen-detected abnormal squamous cells.36
Importantly, we report significant declines in AIS incidence rates among 21–24 year-olds, overall and among screened women. Although numbers are small in this group, this reported decline among AIS cases is similar to recent reports of declines among all CIN2+ lesions in the US during the same time period.40,42,48 Revised cervical cancer screening recommendations have resulted in a smaller pool of women screened annually, and thus fewer are available to be diagnosed annually.49 The decline we observed among screened women in this age group suggests that reductions are not entirely due to increased screening intervals and could result, at least in part, from vaccine impact. Vaccination could have impacted AIS rates directly, by preventing the HPV infections that could have progressed to AIS, or indirectly through a reduction in squamous abnormalities leading to fewer opportunities to detect AIS incidentally.
While there were no significant changes in population-level AIS incidence rates in 25–29 or 30–39 year-olds, we did note a significant 8.9% increase in AIS incidence rates between 2008 and 2015 among 30–39 year-old screened women. This finding is similar to CIN2+ incidence trends in the US42,48 and the Netherlands.11 Van der Horst et al. suggested that increases in AIS rates from 2004 to 2013 among women aged 25–39 years may have been due to recent inclusion of HPV testing. Similarly, in the US, changes in cervical cancer screening recommendations have included the addition of HPV testing as part of a screening strategy. In 2004, HPV tests were recommended in the US as an option to be used concurrently with cytology (cotest) among women aged ≥30 years, or among all women as reflex testing after an ASCUS cytology result. In 2012, cotesting became the preferred screening strategy among women aged ≥30 years.50 We observed that HPV testing increased in all age groups between the early (2008–2011) and later (2012–2015) surveillance periods. Notably, almost all normal or low-grade cytology results preceding a CIN3 or AIS diagnosis had a positive HPV test, suggesting that the increased use of HPV testing as part of screening may have detected cases that would have been overlooked with cytology testing alone. An analysis of cervical cancer screening practices from 2003 to 2009 found that incorporating HPV testing resulted in earlier identification of women at risk for adenocarcinoma, and was more sensitive for adenocarcinoma and AIS than for squamous cell carcinoma.51 These findings suggest that increases in HPV testing may be identifying AIS before progression to invasive disease.
There were several limitations to our analysis. Typing was only performed on cases in women aged 18–39 years, and although this represented roughly 70% of cases, typing results may not be generalizable to women aged >39 years. In AIS cases in women aged >39 years, the HPV type distribution might be different; for example, HPV45, which is found in a larger percentage of adenocarcinoma than squamous cell carcinomas, might be more prevalent. However, one large retrospective international study found that HPV45-associated AIS arises at younger ages than other HPV type-associated AIS.28 Another limitation is that the proportion of cases known to have been vaccinated was low (3.2% of AIS, and 5.9% of CIN3), and we did not have sufficient numbers among vaccine-eligible age groups to examine changes in types by vaccine status.44 Finally, although women could have had multiple lesion histology types (i.e., AIS and CIN2–3), only one specimen was collected per woman. In cases where multiple lesion types were present on different specimens, the AIS lesion was preferentially selected for typing, and thus HPV type differences between AIS and AIS + CIN cases could be underestimated. One study that reported HPV types among multiple lesions found similar HPV16, HPV18 and HPV45 proportions in both AIS and concurrent non-AIS lesions, and although HPV types in AIS lesions were usually also detected in concurrent non-AIS lesions, many concurrent non-AIS lesions also had additional HPV types detected.17
This is the first report to document declines in AIS incidence among 21–24 year-old women in the US. In addition, we found increases in AIS among screened women aged 30–39 years. Our results demonstrate that a high proportion of AIS cases are vaccine preventable. Our large, population-based surveillance system includes a larger and more diverse sample of women than any previous publication on AIS, and allows for evaluation of HPV typing, incidence trends, and screening history in the same women.
The high proportion of AIS cases attributable to vaccine types indicates an important opportunity for vaccine prevention of AIS. Given the older median age of women with AIS compared to CIN, it may take longer for the HPV vaccine to have a large impact on AIS incidence. However, our data suggest vaccine impact in the youngest age group already, 13 years after introduction of the vaccination program in the US. Adding HPV testing to cervical cancer screening may result in detection of AIS that would otherwise be missed. Continued surveillance is needed to monitor changes in the vaccine era and guide local and national cervical cancer prevention activities.
What’s new?
Adenocarcinoma in situ (AIS) is a rare histologic type of cervical precancer. Little is known about the epidemiology of AIS, however, and this information is important in the era of human papillomavirus (HPV) vaccination for cervical cancer prevention. In this study, the authors report on the largest collection of genotyped AIS cases to date from the multisite U.S. population-based HPV Vaccine Impact Monitoring Project. From 2008 to 2015, 470 AIS cases were documented, compared to 6,587 cervical intraepithelial neoplasia grade 3 (CIN3) cases. AIS was diagnosed at an older age than CIN3 and was associated most frequently with HPV16 or HPV18. These are the first data on AIS incidence trends in the vaccine era; the authors document a decline in incidence among young women, which may be in part attributable to early vaccine impact.
Acknowledgments
Grant sponsor: Centers for Disease Control and Prevention; Grant numbers: U50CK000482 [California], U50CK000484 [Oregon], U50CK000486 [New York], U50CK000488 [Connecticut], U50CK000491 [Tennessee]
Abbreviations:
- 9vHPV
9-valent HPV vaccine
- 4vHPV
quadrivalent HPV vaccine
- AIS
adenocarcinoma in situ
- APC
annual percent change
- CI
confidence interval
- CIN
cervical intraepithelial neoplasia
- CIN2+
cervical intraepithelial neoplasia grades 2, 2/3, 3, or adenocarcinoma in situ
- CIN3
cervical intraepithelial neoplasia grade 3, the highest grade of CIN
- HPV
human papillomavirus
- HPV-IMPACT
HPV Vaccine Impact Monitoring Project
- HR
high-risk
- HR-HPV
high-risk human papillomavirus
- Pap
Papanicolaou
- RR
rate ratio
- SCC
squamous cell carcinoma
Footnotes
Conflict of interests: L. M. N. reports personal fees from Merck, outside the submitted work. All other authors report no potential conflicts.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control.
References
- 1.Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002;55:244–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013;40:187–93. [DOI] [PubMed] [Google Scholar]
- 3.Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer 2016;122: 3682–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller JW, Royalty J, Henley J, et al. Breast and cervical cancers diagnosed and stage at diagnosis among women served through the National Breast and cervical cancer early detection program. Cancer Causes Control 2015;26:741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrae B, Kemetli L, Sparen P, et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst 2008;100:622–9. [DOI] [PubMed] [Google Scholar]
- 6.Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Womens Health 2012; 21:1031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008;113:2855–64. [DOI] [PubMed] [Google Scholar]
- 8.Mathew A, George PS. Trends in incidence and mortality rates of squamous cell carcinoma and adenocarcinoma of cervix—worldwide. Asian Pac J Cancer Prev 2009;10: 645–50. [PubMed] [Google Scholar]
- 9.Sherman ME, Wang SS, Carreon J, et al. Mortality trends for cervical squamous and adenocarcinoma in the United States. Relation to incidence and survival. Cancer 2005;103:1258–64. [DOI] [PubMed] [Google Scholar]
- 10.Smith HO, Tiffany MF, Qualls CR, et al. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States--a 24-year population-based study. Gynecol Oncol 2000;78:97–105. [DOI] [PubMed] [Google Scholar]
- 11.van der Horst J, Siebers AG, Bulten J, et al. Increasing incidence of invasive and in situ cervical adenocarcinoma in The Netherlands during 2004–2013. Cancer Med 2017;6:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo F, Cofie LE, Berenson AB. Cervical cancer incidence in young U.S. females after human papillomavirus vaccine introduction. Am J Prev Med 2018;55:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SS, Sherman ME, Hildesheim A, et al. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer 2004;100:1035–44. [DOI] [PubMed] [Google Scholar]
- 14.Polterauer S, Reinthaller A, Horvat R, et al. Cervical adenocarcinoma in situ: update and management. Curr Obstet Gynecol Rep 2013;2: 86–93. [Google Scholar]
- 15.Munro A, Codde J, Spilsbury K, et al. Risk of persistent and recurrent cervical neoplasia following incidentally detected adenocarcinoma in situ. Am J Obstet Gynecol 2017;216:272 e1–7. [DOI] [PubMed] [Google Scholar]
- 16.Loureiro J, Oliva E. The spectrum of cervical glandular neoplasia and issues in differential diagnosis. Arch Pathol Lab Med 2014;138:453–83. [DOI] [PubMed] [Google Scholar]
- 17.Ault KA, Joura EA, Kjaer SK, et al. Adenocarcinoma in situ and associated human papillomavirus type distribution observed in two clinical trials of a quadrivalent human papillomavirus vaccine. Int J Cancer 2011;128:1344–53. [DOI] [PubMed] [Google Scholar]
- 18.Miller RA, Mody DR, Tams KC, et al. Glandular lesions of the cervix in clinical practice: a cytology, histology, and human papillomavirus correlation study from 2 institutions. Arch Pathol Lab Med 2015;139:1431–6. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins MP, Roberts JA, Schmidt RW. Cervical Adenocarcinoma In Situ. Obstet Gynecol 1988;71: 842–4. [PubMed] [Google Scholar]
- 20.Watson M, Soman A, Flagg EW, et al. Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries, United States, 2009–2012. Prev Med 2017;103:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruba S, Schoolland M, Allpress S, et al. Adenocarcinoma in situ of the uterine cervix: screening and diagnostic errors in Papanicolaou smears. Cancer 2004;102:280–7. [DOI] [PubMed] [Google Scholar]
- 22.Davy ML, Dodd TJ, Luke CG, et al. Cervical cancer: effect of glandular cell type on prognosis, treatment, and survival. Obstet Gynecol 2003;101: 38–45. [DOI] [PubMed] [Google Scholar]
- 23.Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2016; 65:661–6. [DOI] [PubMed] [Google Scholar]
- 24.Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015;107:djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellsague X, Diaz M, de Sanjose S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst 2006;98:303–15. [DOI] [PubMed] [Google Scholar]
- 26.Bulk S, Berkhof J, Bulkmans NWJ, et al. Preferential risk of HPV16 for squamous cell carcinoma and of HPV18 for adenocarcinoma of the cervix compared to women with normal cytology in The Netherlands. Br J Cancer 2005;94:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clifford G, Franceschi S. Members of the human papillomavirus type 18 family (alpha-7 species) share a common association with adenocarcinoma of the cervix. Int J Cancer 2008;122:1684–5. [DOI] [PubMed] [Google Scholar]
- 28.de Sanjose S, Quint WGV, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11: 1048–56. [DOI] [PubMed] [Google Scholar]
- 29.Holl K, Nowakowski AM, Powell N, et al. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: results from a European multinational epidemiological study. Int J Cancer 2015;137:2858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer 2011;128: 927–35. [DOI] [PubMed] [Google Scholar]
- 31.Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008;26(Suppl 10): K1–16. [DOI] [PubMed] [Google Scholar]
- 32.Alemany L, de Sanjose S, Tous S, et al. Time trends of human papillomavirus types in invasive cervical cancer, from 1940 to 2007. Int J Cancer 2014;135:88–95. [DOI] [PubMed] [Google Scholar]
- 33.Castellsagué X, Ault KA, Bosch FX, et al. Human papillomavirus detection in cervical neoplasia attributed to 12 high-risk human papillomavirus genotypes by region. Papillomavirus Res 2016; 2:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joste NE, Ronnett BM, Hunt WC, et al. Human papillomavirus genotype-specific prevalence across the continuum of cervical neoplasia and cancer. Cancer Epidemiol Biomarkers Prev 2014; 24:230–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hariri S, Unger ER, Powell SE, et al. Human papillomavirus genotypes in high-grade cervical lesions in the United States. J Infect Dis 2012;206: 1878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bekkers RL, Bulten J, Wiersma-van Tilburg A, et al. Coexisting high-grade glandular and squamous cervical lesions and human papillomavirus infections. Br J Cancer 2003;89:886–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markowitz LE, Dunne EF, Saraiya M, et al. Centers for disease C, prevention, advisory committee on immunization P. Quadrivalent human papillomavirus vaccine: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 2007;56:1–24. [PubMed] [Google Scholar]
- 38.Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015;64: 300–4. [PMC free article] [PubMed] [Google Scholar]
- 39.Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2017. MMWR Morb Mortal Wkly Rep 2018;67:909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial Neoplasia in the human papillomavirus vaccine era. JAMA Oncol 2017;3:833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flagg EW, Torrone EA, Weinstock H. Ecological Association of Human Papillomavirus Vaccination with cervical dysplasia prevalence in the United States, 2007–2014. Am J Public Health 2016;106:2211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gargano JW, Park IU, Griffin MR, et al. Trends in high-grade cervical lesions and cervical cancer screening in five states, 2008–2015. Clin Infect Dis 2018;68:1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hariri S, Bennett NM, Niccolai LM, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States—2008–2012. Vaccine 2015;33: 1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClung NM, Gargano JW, Bennett NM, et al. Trends in Human Papillomavirus Vaccine Types 16 and 18 in Cervical Precancers, 2008–2014. Cancer Epidemiol Biomarkers Prev 2019;28:602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quint KD, de Koning MNC, Geraets DT, et al. Comprehensive analysis of human papillomavirus and chlamydia trachomatis in in-situ and invasive cervical adenocarcinoma. Gynecol Oncol 2009;114: 390–4. [DOI] [PubMed] [Google Scholar]
- 46.Dahlström LA, Ylitalo N, Sundström K, et al. Pro-spective study of human papillomavirus and risk of cervical adenocarcinoma. Int J Cancer 2010; 127:1923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson M, Benard V, King J, et al. National assessment of HPV and pap tests: changes in cervical cancer screening, National Health Interview Survey. Prev Med 2017;100:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flagg EW, Datta SD, Saraiya M, et al. Population-based surveillance for cervical cancer precursors in three central cancer registries, United States 2009. Cancer Causes Control 2014;25:571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moyer VA. Screening for cervical cancer: U.S. preventive services task force recommendation statement. Ann Intern Med 2012;156:880–91. w312. [DOI] [PubMed] [Google Scholar]
- 50.Saslow D, Solomon D, Lawson HW, et al. can Cancer Society, American Society for copy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012;137:516–42. [DOI] [PubMed] [Google Scholar]
- 51.Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011;12:663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


