Highlights
-
•
Structural language impairment present in individuals with a current or past autism diagnosis.
-
•
Heightened leftward neural lateralization of language function related to language impairment.
-
•
Behavioral and neural patterns reflect language impairment distinct from autism features.
Abbreviations: LAD, Loss of Autism Diagnosis; LI, structural language impairment; LN, normal-range structural language
Keywords: Autism Spectrum Disorder, Loss of Autism Diagnosis, Language, Lateralization, Functional magnetic resonance imaging
Abstract
This study probed for structural language impairment using behavioral and functional neuroimaging methods in individuals with Autism Spectrum Disorder (ASD) and those diagnosed with ASD in childhood who no longer meet criteria for ASD, referred to as Loss of Autism Diagnosis (LAD1). Participants were drawn from Fein et al. (2013): ASD (n = 35), LAD (n = 31), and Neurotypical (NT; n = 34). Criteria for structural language impairment were: Scores ≤ 82 on Clinical Evaluation of Language Fundamentals-4 (CELF) Core Language, an omnibus measure of language; and scores ≤ 7 on CELF Recalling Sentences, a clinical marker of structural language impairment. Task-based fMRI examined lateralization of significantly activated language-related brain regions in groups with structural language impairment (LI2) versus normal-range language (LN3), collapsed across ASD, LAD1, and NT status. Results showed no ASD versus LAD group differences in the proportion of participants with structural language impairment according to either metric (Recalling Sentences or Core Language). Functional MRI results indicated greater left hemisphere lateralization within significantly activated regions in the LI2 group. Structural language abilities were not meaningfully associated with either social abilities or lifetime ADHD symptoms in LI2 subgroups, further suggesting the presence of structural language impairment. Findings indicate the presence of persistent structural language difficulty even in the absence of ASD symptoms in some individuals within the LAD1 group and unique patterns of language-related neural specialization for language function in LI2 relative to LN3.
1. Introduction
Autism Spectrum Disorder (ASD) is characterized by difficulty with social communication and restricted and repetitive behaviors and interests (RRBs) which cause lifelong functional difficulties in most cases (American Psychological American Psychiatric Association, 2013, Landa and Kalb, 2012, Mukaddes et al., 2014). A subgroup of individuals diagnosed with ASD in childhood (e.g., based on gold-standard ASD diagnostic assessments, early language delay) goes on to no longer meet criteria for ASD in adolescence or adulthood. This group, referred to as a Loss of Autism Diagnosis (LAD) group, appears to comprise 3–25% of individuals who receive an ASD diagnosis in childhood (Anderson et al., 2014, Fein et al., 2013, Helt et al., 2008). The presence of structural language impairment (i.e., syntax deficits relative to same-age controls) has been documented in a subgroup of individuals with a current ASD diagnosis (e.g., Kjelgaard and Tager-Flusberg, 2001, Riches et al., 2010, Wittke et al., 2017). The current study asks whether current LAD status precludes current structural language impairment and examines neural circuitry associated with structural language deficits.
Several studies of a particular sample of youth with LAD reported no structural language differences in LAD compared to neurotypical (NT) peers according to standardized clinical assessments (e.g., Fein et al., 2013, Orinstein et al., 2015a, Tyson et al., 2014). In these reports, while group differences in standardized clinical assessments may not have been significant, there was a wide range of standard scores, suggesting that at least some individual LAD participants might fall below the clinical cutoff for language impairment. Additionally, there is evidence from functional brain imaging (fMRI) during a language task that LAD status is associated with activation patterns that are similar to those of ASD peers in a subset of activated regions, but most broadly, by compensatory (heightened) activation relative to both ASD and NT peers (Eigsti et al., 2016). That is, language-relevant neural activity in LAD youth appears to reflect their history of ASD, as well as the recruitment of a range of additional brain regions (e.g., anterior and posterior cerebellum; on the right, motor regions, middle and superior frontal gyrus, supramarginal gyrus, superior temporal and parahippocampal gyrus; and on the left, precentral and inferior temporal gyrus, precuneus, superior temporal gyrus, and occipital gyrus). Thus, compensatory activation was evident in frontal regions important in cognitive control and in right hemisphere regions that serve as homologues of typically left-lateralized areas that are critical to language processing. These patterns were presumed to be relevant to the history of language difficulty in LAD. Further examination of structural language skills in LAD at the individual level is needed in order to determine the extent to which current structural language impairment is present in LAD, and to characterize neural signatures of that impairment.
1.1. Structural language in ASD and LAD
Acquisition of spoken language is a critical factor in long-term outcomes for individuals with ASD (Mukaddes et al., 2014) and may be more resistant to typical behavioral treatment of ASD (e.g., Applied Behavior Analysis) than behavioral and cognitive skills (Sallows and Graupner, 2005). Language ability in ASD is highly heterogeneous, similar to other areas of functioning, and is associated with social communication, a core area of difficulty in ASD (Blume et al., 2021, Gibson et al., 2013, Kjelgaard and Tager-Flusberg, 2001, Riches et al., 2010). Studies have shown poorer language performance in individuals with ASD relative to NT and LAD peers, such as on standardized measures of vocabulary (i.e., Peabody Picture Vocabulary Test-3; Kelley et al., 2010) and composite language skills (i.e., Core Language on the Clinical Evaluation of Language Fundamentals (CELF-4; Fein et al., 2013, Tyson et al., 2014).
Other work shows overlapping structural language profiles in subgroups of individuals with ASD and Developmental Language Disorder groups. Developmental Language Disorder is characterized by deficits in structural language in the absence of biomedical conditions, particularly in grammar relative to vocabulary in the school-age years (Bishop et al., 2017). In a study of 89 children with ASD ages 4–14 years, most with nonverbal IQs within the typical range, Kjelgaard and Tager-Flusberg (2001) identified a subgroup with structural language difficulty as defined by Core Language composite scores of 84 and lower on the CELF. This subgroup also struggled on nonword repetition, a task used as a clinical marker of Developmental Language Disorder, relative to the ASD-subgroup with normal-range language performance on the CELF (note that the subgroup with normal-range structural language likely had other language difficulties, such as in pragmatics, rather than no language difficulty). Extending these findings, Wittke et al. (2017) examined spontaneous language samples and found a subgroup of children with ASD (33%) who had relatively intact vocabulary but poor grammatical skills, as well as a subgroup (25%) with depressed overall language performance (see also Loucas et al., 2008, Riches et al., 2010; but see Williams et al., 2008). These findings suggest that some individuals with ASD have poor overall language ability and may have language profiles similar to those found in Developmental Language Disorder. Furthermore, there are similarities in patterns of performance between ASD and Developmental Language Disorder in other cognitive domains, such as executive function (Ellis Weismer et al., 2017, Larson et al., 2021).
Whereas some prior studies of LAD (e.g., Anderson et al., 2014, Fein et al., 2013, Fountain et al., 2012, Zachor and Ben-Itzchak, 2020) have not used structural language skills to rule participants in or out of LAD group membership, others excluded potential LAD group participants with current language impairment (e.g., Mukaddes et al., 2014). Even when LAD criteria do not include measures of structural language, the relationship between social communication (e.g., greetings, requesting a social routine) and structural language would suggest that structural language may facilitate or moderate gains in social communication (Blume et al., 2021, Loucas et al., 2008, Whitehouse et al., 2009). Thus, no longer meeting social criteria for ASD might depend, in part, on intact structural language skills.
Some work suggests that LAD performance on spontaneous language (i.e., narrative) tasks lies at an intermediate level of ability relative to ASD and NT peers, not differing significantly from either group, even when ASD and NT groups differ (Canfield et al., 2016, Suh et al., 2014; see also Kelley et al., 2006). Another study using standardized language measures (CELF Core Language composite scale and Formulated Sentences subscale) reported that LAD scores were significantly better than ASD peers, and in the average range; however, scores were significantly lower than that of NT peers (Fein et al., 2013). These mixed patterns of results appear to depend on the particular measure of language used and occur in the context of normal-range performance on average in LAD and ASD groups, and higher than normal-range performance (e.g., mean scaled score of 117 on the CELF score) in the NT group (Canfield et al., 2016, Fein et al., 2013, Suh et al., 2014; see also Tyson et al., 2014 for additional evidence from this research group). The significant differences among LAD, ASD, and NT groups in this research do not reflect clinically impaired language abilities on average.
That said, there is variance in language abilities in the LAD group, which might suggest a previously undescribed subgroup of LAD individuals with structural language impairment. For instance, on the CELF, the range of standardized language scores reported in Fein et al. (2013) was 79 to 126 on Core Language in the LAD group. The range of scaled scores (i.e., mean of 10, standard deviation of 3) was 4–14 on Recalling Sentences (Fein et al., 2013). These ranges indicate that at least some LAD individuals fall below the clinical cutoff of 82 on the Core Language and below 7 on the Recalling Sentences scales, even though ASD features are no longer present. LAD may be associated with persistent language difficulty that requires ongoing speech-language services (Turner and Stone, 2007).
It may be useful to relate behavioral language performance with in vivo language processes through neuroimaging methods. As described above, Eigsti et al. (2016) reported functional neural activation similarities during a language task in LAD and ASD, as well as additional activation in the LAD group beyond that of both NT and ASD peers. The authors argued that these patterns reflected the LAD group’s history of ASD and compensatory processes relative to NT peers. Sridhar et al. (preprint) reported evidence of atypical, or possibly compensatory, mechanisms in brain function during a lexical processing task in ASD. Specifically, there was greater reconfiguration of resting-state versus language-task-based brain function (i.e., change in functional activation between rest and task states) in the ASD relative to the NT group. In an ASD subgroup with language abilities similar to NT peers, but not an ASD subgroup with poorer language abilities, greater reconfiguration was associated with better scores on the CELF Word Classes subscale, suggesting differences in the links between brain function and language in individuals with high versus low language abilities.
Early childhood is characterized by significant language-related activation in both left and right hemispheres; over the course of development, activation is increasingly lateralized to the left hemisphere, in the absence of lesions or other brain atypicalities (Olulade et al., 2020). Individuals with ASD exhibit reduced language-related left hemisphere functional lateralization (Jouravlev et al., 2020). Atypical lateralization in language and language-homologue regions suggests reduced specialization for language, potentially reflecting less efficient neural processing of language. Two studies that examined neural lateralization in ASD and structural language impairment groups reported that youth with ASD and with Developmental Language Disorder had similar patterns of structural brain lateralization and differed from their NT peers (De Fossé et al., 2004, Herbert et al., 2005). In these studies, the clinical groups had more symmetrical volumes of language-relevant brain regions, where those regions were asymmetrical in NT groups. Other work suggests reduced left hemisphere lateralization of language function in Developmental Language Disorder relative to NT peers (Badcock et al., 2012, De Guibert et al., 2011). However, little prior work has examined functional neural lateralization in language and language-homologue regions in ASD structural language impairment subgroups or in LAD. Examining the neural signatures of language processes in ASD and LAD may clarify the degree to which subtle or frank structural language impairment is present in these populations.
1.2. Social Communication, Attention, and language in ASD and LAD
Another goal of this study was to probe for factors that might relate to ongoing language difficulties in LAD, focusing specifically on social communication and attention. In LAD, mild and isolated differences in social skills relative to NT peers have been observed, such as less insight into social relationships (Orinstein et al., 2015a) and poorer pragmatic language use in narratives (e.g., providing redundant information, fewer mentions of character goals; Kelley et al., 2006; see also Suh et al., 2014). However, individuals with LAD as a group have also been rated as more engaged, friendly, and approachable than NT peers (Orinstein et al., 2015a), and lab-based participant samples often involve above-average NT performers (e.g., on the Test for Auditory Comprehension of Language, 3rd edition, Elaborated Sentences subscale; Kelley et al., 2006). In LAD, Attention-Deficit/Hyperactivity Disorder (ADHD) may occur at a higher rate than in NT peers but at a similar rate as ASD peers (Orinstein et al., 2015b). Elevated rates of attentional difficulty in LAD and ASD relative to NT peers have been documented and may be characteristic of both LAD and ASD groups (Kelley et al., 2010, Troyb et al., 2014). In fact, Suh et al. (2016) showed higher ratings of some traits related to the Broader Autism Phenotype for LAD than NT peers (i.e., less emotional stability, off-topic conversational behavior) which reflected ADHD-like profiles to a greater degree than Broader Autism Phenotype-like profiles. This evaluation was based on conversational skills, such as being off-topic, that could reflect attention or pragmatic language difficulty. Notably, these conversational ratings indicated greater warmth and extraversion in the LAD group compared to NT peers, suggesting that any social differences in LAD are quite subtle and do not reflect the presence of ASD features (Suh et al., 2016). Examining interrelationships among language, social communication, and attention in LAD may further clarify these patterns in general, providing evidence regarding the processes involved in acquiring structural language skills.
1.3. The current study
This study tested three pre-registered hypotheses (https://osf.io/aysxh). A primary aim was to test for the presence of structural language impairment in LAD and ASD. We had two separate criteria for structural language impairment: (1) scaled scores ≤ 7 on the CELF-4 Recalling Sentences subscale (i.e., a subscale thought to be a clinical marker of structural language impairment; Archibald and Joanisse, 2009, Oetting et al., 2016, Redmond, 2005) or (2) standard scores ≤ 82 on the CELF-4 Core Language composite scale (Nitido and Plante, 2020). These criteria echoed prior work examining language impairment in ASD, drawing on criteria for Developmental Language Disorder (e.g., grammatical impairment, Wittke et al., 2017; and more general language impairment, Kjelgaard and Tager-Flusberg, 2001; see also Loucas et al., 2008, and Riches et al., 2010; i.e., to reflect limitations of omnibus standardized measures in measuring grammatical skills; Ebert and Scott, 2014, Klatte et al., 2022). We hypothesized the presence of structural language impairment in LAD participants, but to a lesser degree than in peers with ASD. A second aim was to evaluate the degree to which structural language impairment represented a distinct deficit, or was associated with subtle, residual social communication or attentional deficits. We hypothesized that, in LAD and ASD participants meeting criteria for language impairment, lower language scores would be associated with more impaired social communication and attentional skills; and that the magnitude of these relationships would be greater in LAD than in ASD, reflecting bootstrapping among these skills. A third aim was to examine the degree of lateralization in a priori defined language-related neural networks using a language comprehension task during fMRI. All participants across diagnostic groups (LAD, ASD, or NT) meeting either criterion for language impairment were included in a “Language Impairment” (LI) group. We hypothesized that structural language impairment would be linked with diminished functional brain lateralization relative to peers with normal-range structural language, and the magnitude of this difference would be greater after controlling for social communication and attentional skills regardless of ASD diagnostic status based on behavioral evidence of associations between language and social communication and attention (Blume et al., 2021, Riches et al., 2010, Smolak et al., 2020).
2. Methods
Participants were drawn from Fein et al. (2013) and fMRI data were drawn from Eigsti et al. (2016), though the current study is not a direct follow-up study to this prior work. Inclusion criteria were verbal, nonverbal, and full-scale IQ standard scores > 77 (i.e., less than 1.5 SD below the mean). Additional eligibility criteria for the LAD group (n = 31) included: a documented ASD diagnosis prior to age 5 years; early language delay (no words by 18 months or no phrases by 24 months); parent report of NT friends; not meeting criteria for ASD on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1994); standard scores > 77 on the Socialization and Communication scales of the Vineland Adaptive Behavior Scales (VABS; Sparrow et al., 1985); and full inclusion in regular education classroom. Additional eligibility criteria for the ASD group (n = 35) included: meeting criteria for ASD on the ADOS and clinical judgment of ASD status. Additional eligibility criteria for the NT group (n = 34) included: no first-degree relative with ASD; not meeting criteria for ASD on the ADOS; and standard scores > 77 on the Socialization and Communication scales of the VABS. Participants ranged in age from 8 to 21 years and groups did not differ significantly in age (p’s > 0.17). See Table 1 for participant information.
Table 1.
Participant demographic characteristics and standardized assessment scores.
| Participant Characteristics | NT (n = 34) |
LAD (n = 31) |
ASD (n = 35) |
p-value |
|---|---|---|---|---|
| Sex assigned at birth | 3:31 | 7:24 | 3:32 | NT-ASD p =.96 |
| (F:M) | NT-LAD p =.11 | |||
| ASD-LAD p =.10 | ||||
| Handedness | 5:29 | 2:28† | 4:31 | NT-ASD p =.31 |
| (L:R) | NT-LAD p =.70 | |||
| ASD-LAD p =.52 | ||||
| Race/Ethnicity | ||||
| Asian or Pacific Islander | 0 | 3 | 0 | |
| African American | 0 | 0 | 0 | |
| Caucasian | 31 | 28 | 34 | |
| More than one race | 2 | 0 | 0 | |
| Latino, not Puerto Rican | 0 | 0 | 1 | |
| Age (years) | 13.88(2.59) | 12.90(3.42) | 13.32(2.68) | NT-ASD p =.43 |
| NT-LAD p =.17 | ||||
| ASD-LAD p =.59 | ||||
| ADOS Communication | 0.41(0.56) | 0.48(0.63) | 3.57(1.44) | NT < ASD p <.001*** |
| NT-LAD p =.70 | ||||
| ASD > LAD p <.001*** | ||||
| ADOS Social | 0.50(0.75) | 1.16(1.34) | 6.91(2.32) | NT < ASD p <.001*** |
| NT-LAD p =.30 | ||||
| ASD > LAD p <.001*** | ||||
| ADOS Restricted | 0.03(0.17) | 0.19(0.48) | 1.23(1.21) | NT < ASD p <.001*** |
| Repetitive Behavior | NT-LAD p =.39 | |||
| ASD > LAD p <.001*** | ||||
| Core Language | 117(7) | 110(11) | 99(13) | NT > ASD p <.001*** |
| (CELF-4) | NT > LAD p <.05* | |||
| ASD < LAD p <.001*** | ||||
| Recalling Sentences | 11.97(1.77) | 10.53(2.79) | 9.44(2.89) | NT > ASD p <.001*** |
| (CELF-4) | NT > LAD p <.05* | |||
| ASD-LAD p =.08 | ||||
| Nonverbal IQ | 113(11) | 112(14) | 111(14) | NT-ASD p =.49 |
| (WISC-IV) | NT-LAD p =.82 | |||
| ASD-LAD p =.63 | ||||
| Social Communication | 1.55(1.25) | 17.10(6.68) | 22.65(6.15) | NT < ASD p <.001*** |
| (SCQ Ever) | NT < LAD p <.001*** | |||
| ASD > LAD p <.05* | ||||
| K-SADS ADHD Ever | NT < ASD p <.001*** | |||
| Meets criteria for ADHD | 4 | 17 | 27 | NT < LAD p <.001*** |
| Does not meet criteria for ADHD | 29 | 13 | 5 | ASD > LAD p <.05* |
Note. Handedness = Edinburgh Hand Dominance (Oldfield, 1971); ADOS = Autism Diagnostic Observation Schedule; CELF-4 = Clinical Evaluation of Language Fundamentals, fourth edition; WISC-IV = Wechsler Intelligence Scale – Children, fourth edition; SCQ = Social Communication Questionnaire; K-SADS = Schedule for Affective Disorders and Schizophrenia for School-Aged Children; ADHD = Attention-Deficit/Hyperactivity Disorder symptomology scale from the K-SADS; Recalling Sentences = Subscale from the CELF-4, scaled scores; Core Language = Composite score from the CELF-4, standardized scores. †one participant missing handedness preference; *p <.05; **p <.01; ***p <.001.
Participants completed the following standardized assessments: (1) CELF-4 (Semel, Wiig, & Secord, 2003), a standardized structural language assessment yielding scaled scores for subtests [e.g., Recalling Sentences; M(SD) = 10(3)] and standardized composite score [e.g., Core Language; M(SD) = 100(15)]; (2) the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Lifetime version (KSADS; Kaufman et al., 1997) to assess ADHD status (i.e., has met/has not met criteria for ADHD at any point in development); and (3) the Social Communication Questionnaire – Lifetime version (SCQ; Rutter et al., 2003), a parent report measure of social communication and social interaction (e.g., conversational turn taking, speaking to be friendly), as well as restricted and repetitive behaviors, yielding total scores (0–39) and a cutoff indicating possible ASD (i.e., a score of 15). These three measures yielded the key variables that were included in our statistical models.
2.1. Task-Based fMRI
A subgroup of participants completed fMRI scanning (ASD n = 22; LAD n = 14; NT n = 19). Note that five participants contributed fMRI data but had too much missing behavioral data to be included in behavioral analyses; these individuals were included only in fMRI analyses. See Supplementary Materials 1, Tables 5, 6, and 7, for participant characteristics, group comparisons, and information on missing data. A 3 T Siemens Allegra scanner at the Olin Neuropsychiatric Center at the Institute of Living was used to collect MRI data, and safety screening and training in a mock scanner was completed prior to placing participants on the scanner bed. Participants were provided MRI-compatible headphones and earplugs, and custom cushions inside the head coil to inhibit head movement (no participants were removed due to head motion). BOLD signal change during the task was measured via an echo-planar image gradient pulse sequence (TR/TE 1500/28 ms, flip angle 65 degrees, FOV 24 × 24 cm, 64 × 64 matrix, 3.4 × 3.4 mm in plane resolution, 5 mm effective slice thickness, 30 slices). We collected 225 images and use the first six images only for T1 stabilization. We collected 225 images and use the first six images only for T1 stabilization. The data were prepared for analysis using fMRIPrep 20.2.3 (Esteban et al., 2019b, Esteban et al., 2019a), then converted into CIFTI format compatible with Human Connectome Project (HCP) cortical atlas (Glasser et al., 2016) using the Ciftify package version 2.3.3 (Dickie et al., 2019). Cortical regions deemed significantly active in task conditions were based on 1-sample t-tests done separately for each sub-sample (Benjamini-Hochberg FDR correction q = 0.05; FDR cutoff p = 0.0345). That is, we conducted 360 t-tests and obtained an uncorrected p-value, and then subjected those p-values to FDR. See Supplementary Materials 1 for complete details on the fMRIPrep processing steps. Note that this study represents a re-analysis of previously published fMRI data (Eigsti et al., 2016).
2.1.1. fMRI language task
Participants completed a sentence comprehension task during scanning, adapted from Kana et al. (2006; see also Eigsti et al., 2016). Sentences were declarative and involved 12 high-imagery (e.g., The number eight when rotated 90 degrees looks like a pair of eyeglasses), 12 low-imagery (e.g., Addition, subtraction, and multiplication are all math skills), and 24 control (e.g., “LLLL” or “RRRR”) conditions (note that the control condition was not included in analyses as it may engage language processing; see Limitations). Participants read sentences and made a true/false value judgment via button press for the experimental condition and participants made a left/right button press for the control condition. The current study examined activation during the sentence comprehension task collapsed across imagery conditions (see Eigsti et al., 2016 for additional results related to the sentence comprehension task) in a priori defined functional language networks (see next section) in order to capture activation specific to language function. See Supplementary Materials 1, p. 12 for functional data activation analyses.
2.1.2. Laterality index and ROIs
Clusters representing language-related neural networks were chosen based on Baker et al.’s (2018) body of work, “A Connectomic Atlas of the Human Cerebrum,” using parcels defined in the Human Connectome Project cortical atlas. In Chapter 18 from Baker et al.’s (2018) larger project, Briggs et al. (2018) presents a detailed characterization of connectivity, as well as functional significance of HCP brain regions, justifying their determination of language-related neural networks. Frontal Cluster ROIs included: 44, 45, 47 medial (47 m), posterior 47 rostral (p47r), anterior 47 rostral (a47r), 47 lateral (47 l), 47 s, 6 rostral (6r), interior frontal sulcus posterior (IFSp), interior frontal sulcus anterior (IFSa), interior frontal junction anterior (IFJa), and interior frontal junction posterior (IFJp). Temporal Cluster ROIs included: superior temporal sulcus dorsal anterior (STSda), superior temporal sulcus dorsal posterior (STSdp), superior temporal sulcus ventral anterior (STSva), superior temporal sulcus ventral posterior (STSvp), temporal area 1 anterior (TE1a), temporal area 1 medial (TE1m), temporal area 1 posterior (TE1p), temporal area 2 anterior (TE2a), and PHT. A Semantic Cluster reflected a global semantic network including 44, 45, 55b, IFJa, 8C, superior longitudinal fasciculus (SFL), supplementary cingulate eye field (SCEF), 8BM, STSdp, STSvp, anterior intra-parietal area (AIP), parietal area F medial (PFm), TE1p, PHT, and Para-belt complex (PBelt); note that these clusters were partially overlapping (e.g., Broca’s). See Baker et al. (2018) for further information on the HCP regions described here (i.e., relative spatial location and their functional and connectivity patterns) and Supplementary Materials, Fig. 2, for visualization of regions comprising the language networks.
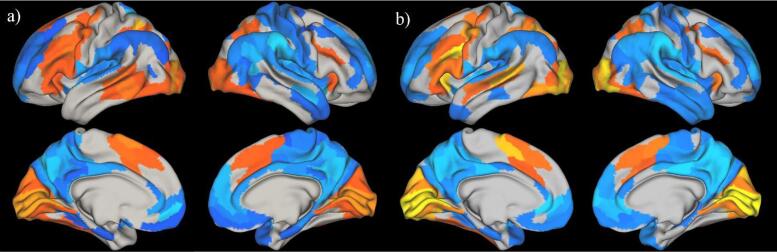
Fig. 2.
Significant functional activation in all language-related ROIs in the left hemisphere and right homologue regions in the right hemisphere for the (a) structural language impairment (LI) and (b) normal-range structural language (LN) groups. Note. Cortical regions were deemed significantly active based on thresholded p-values from 1-sample t-tests (Benjamini-Hochberg FDR correction q = 0.05), warmer colors indicate task-related activation t-values and colder colors indicate task-related de-activation t-values. See Supplementary Materials 1 Fig. 2 for cluster visualization.
Lateralization was calculated for all regions that were significantly active at the group/subgroup level (see Supplementary Materials Tables 9 and 10 for a list of significantly active regions by group and cluster), using the following formula: (Left – Right average activation) / (absolute value of Left + Right average activation); see Jansen et al. (2006 and van der Haegen and Brysbaert (2018). In this calculation, Lateralization Index values ranged from + 1 (purely left lateralization) to −1 (purely right lateralization). We calculated lateralization values for each cluster at the participant level and conducted a post-hoc analysis of lateralization values for all ROIs to increase our sensitivity and account for language-related lateralization across language networks. See Rivera-Figueroa (2020) for a similar approach with NT participants. These lateralization values were statistically compared between ASD, LAD, and NT, and the normal-range (LN) versus impaired structural language (LI) groups. We collapsed across ASD-LI and LAD-LI groups, Table 2, to best capture the neural underpinnings of behavioral language features and to maximize subgroup sample sizes (ASD-LI N = 7; LAD-LI N = 3; see Limitations for additional information).
Table 2.
Participant characteristics for Recalling Sentences language-impaired ASD versus LAD groups.
| Participant Characteristics | LAD-LI (n = 7) |
ASD-LI (n = 8) |
p-value | p-value vs. NT |
|---|---|---|---|---|
| Sex assigned at birth | 4:3 | 0:8 | p <.05* | ASD-LI p =.41 |
| (F:M) | LAD-LI p <.01 | |||
| Handedness | 1:5† | 3:5 | p =.50 | ASD-LI p =.15 |
| (L:R) | LAD-LI p =.93 | |||
| Age | 12.97(2.82) | 14.09(3.71) | p =.40 | ASD-LI p =.72 |
| LAD-LI p =.37 | ||||
| Recalling Sentences | 6.29(1.25) | 5.62(1.41) | p =.28 | ASD-LI p <.001 |
| (CELF-4) | LAD-LI p <.001 | |||
| Core Language | 97(12) | 91(17) | p =.60 | ASD-LI p <.001 |
| (CELF-4) | LAD-LI p <.001 | |||
| Receptive Vocabulary | 104(10) | 96(14) | p =.30 | ASD-LI p <.001 |
| (PPVT) | LAD-LI p <.001 | |||
| Nonverbal IQ | 105(13) | 108(7) | p =.56 | ASD-LI p =.12 |
| (WISC-IV) | LAD-LI p =.06 | |||
| Social Communication | 16(9) | 23(7) | p =.18 | ASD-LI p <.001 |
| LAD-LI p <.001 | ||||
| K-SADS ADHD Ever | ||||
| Meets criteria for ADHD | 4 | 8 | p <.05* | ASD-LI p <.001 |
| Does not meet criteria for ADHD | 3 | 0 | LAD-LI p <.01 |
Note. Handedness = Edinburgh Hand Dominance (Oldfield, 1971); CELF-4 = Clinical Evaluation of Language Fundamentals, fourth edition; WISC-IV = Wechsler Intelligence Scale – Children, fourth edition; SCQ = Social Communication Questionnaire; K-SADS = Schedule for Affective Disorders and Schizophrenia for School-Aged Children; ADHD = Attention-Deficit/Hyperactivity Disorder symptomology scale from the K-SADS; Recalling Sentences = Subscale from the CELF-4, scaled scores; Core Language = Composite score from the CELF-4, standardized scores. †one participant missing handedness preference; *p <.05; **p <.01; ***p <.001.
2.2. Statistical analyses
2.2.1. Analytical approach
Group characteristics were analyzed using t-tests for the full sample and non-parametric Mann-Whitney U test for the ASD-LI and LAD-LI subgroup comparisons. Missing behavioral data were imputed using predictive mean matching; see Table 1, Table 2 and Supplementary Materials 1. Research questions were addressed using the imputed data and a Bayesian framework. Bayesian statistical approaches offer several advantages over frequentist-based null hypothesis significance testing. Results of prior research are incorporated directly into Bayesian statistical modeling to yield posterior distributions (e.g., posterior probability intervals), whereas null hypothesis significance testing offers a binary test to reject the null hypothesis (i.e., which is subject to multiple-comparison corrections, unlike Bayesian approaches; Kaplan, 2014, van de Schoot et al., 2014). Bayesian statistics may also be more effectively implemented with small samples (e.g., samples involving low-prevalence clinical populations) than frequentist statistics due in part to null hypothesis significance testing being associated with a bias toward extreme results more so than “true” results for small samples (Button et al., 2013, Wasserstein and Lazar, 2016). We have included information on the results from prior research that we included in our statistical models (i.e., priors) in Supplementary Materials 1, Table 1.
2.2.2. Bayesian statistical results
The Bayesian framework utilizes the 95% posterior probability interval (PPI) rather than frequentist-based p-values. In contrast to frequentist-based 95% confidence intervals, which indicate that 95 percent of intervals constructed the same way (i.e., according to long-run frequency probability) will contain the true value, a 95% PPI is interpreted as the 95% probability that the true value lies in that range. Bayesian statistical output yields b-estimates and standard error, similar to null hypothesis significance testing results, yet these values represent the posterior distribution of priors plus the data. The posterior distribution, therefore, includes information from prior literature directly in the results. As is common under a Bayesian framework (e.g., Larson et al., 2020), we interpreted an effect to be important if zero was not contained within the 95% PPI and we used the term “important” to identify these effects. See Supplementary Materials 2 for all model convergence information which describes the model fit of the model predictions with the data.
3. Results
3.1. Language impairment subgroups – behavioral data analyses
Using the criterion of Core Language standard scores ≤ 82 on the CELF-4 (Nitido and Plante, 2020), 4/35 (11.4%) participants in the ASD group and 1/31 (3.2% for the imputed data) participants in the LAD group were classified with structural language impairment. There was no significant ASD versus LAD group difference in the percent of participants meeting this criterion (two-tailed Fishers exact p =.36). Using the CELF-4 Recalling Sentences ≤ 7 criterion, 8/35 (22.9%) participants in the ASD group and 7/31 (22.6%) participants in the LAD group were classified with structural language impairment; this proportion did not differ between the ASD and LAD groups (two-tailed Fishers exact p = 1). No NT participants met either criterion and one participant with ASD met criteria using the Core Language criterion but not the Recalling Sentences criterion. See Fig. 1 for a violin plot of Recalling Sentences scores as a function of group. We chose Recalling Sentences scaled scores ≤ 7 as our criterion for subsequent analysis of language impairment subgroups due to its relatively greater specificity in probing morphosyntactic skills compared to the Core Language composite measure, which may capture a broader set of cognitive abilities (Archibald and Joanisse, 2009, Oetting et al., 2016, Redmond, 2005; see also Eigsti et al., 2016). However, we retained Core Language scores for additional analyses to test the degree to which Core Language captures additional cognitive abilities, and whether these patterns differ depending on group status.
Fig. 1.
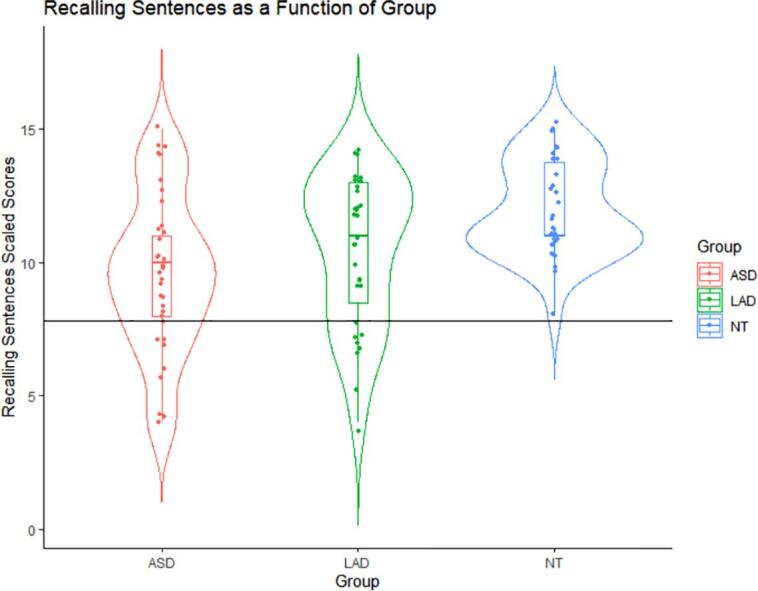
Recalling Sentences scaled scores as a function of Autism Spectrum Disorder (ASD), Loss of Autism Diagnosis (LAD), and Neurotypical (NT) group. All data points below the horizontal line fall in our LI clinical range of ≤ 7 scaled scores. See Supplementary Materials Fig. 1 for a similar visualization for Core Language standard scores. Note. Data points are jittered. The horizontal line markes the cutoff for LI.
3.1.1. ASD-LI and LAD-LI status validation
To put these LI scores into a broader context, we examined relevant clinical and demographic characteristics of LI and non-LI groups; Table 2 summarizes these data. Relative to NT peers, the ASD-LI and LAD-LI groups both had significantly poorer Recalling Sentences, Core Language, receptive vocabulary (Peabody Picture Vocabulary Test, 4th edition; Dunn and Dunn, 2007), and social communication-repetitive behaviors (SCQ), and a significantly greater number of participants meeting ADHD criteria (KSADS; p’s less than 0.01). The ASD-LI and LAD-LI groups did not differ significantly from the NT group on nonverbal ability (WASI NVIQ; p =.56).
3.1.2. ASD-LI vs. LAD-LI
There were no significant differences between the ASD-LI and LAD-LI groups in receptive vocabulary (p =.30) and Core Language (p =.61). There were no significant differences between groups in SCQ scores (p =.18) or in nonverbal ability (p =.56). The ASD-LI group had significantly more participants meeting criteria for ADHD than the LAD-LI group (p <.05). These patterns suggest that ASD-LI and LAD-LI did not differ across measures of social communication-repetitive behaviors and nonverbal skills, but that attentional difficulties may be greater in ASD-LI than LAD-LI.
3.1.3. Language and social communication in LI vs. LN
Collapsing across LAD, ASD, and NT groups, we examined the role of social communication-repetitive behaviors (SCQ) in language abilities. For Recalling Sentences, there were no important SCQ or Group by SCQ interaction effects. For Core Language, there was an important SCQ effect (b = −0.373; SD = 0.156; 95% PPI[−0.685, −0.072]), indicating that a history of relatively more social communication and repetitive behavior difficulty was associated with relatively poorer Core Language, and there were no important Group by SCQ interaction effects.
3.1.4. Language and ADHD in LI vs. LN
Collapsing across LAD, ASD, and NT groups, we examined the role of ADHD status (KSADS) in language abilities. For Recalling Sentences, there were no important ADHD status or Group by ADHD interaction effects. For Core Language, there was an important ADHD status effect (b = 10.615; SD = 3.524; 95% PPI[3.617, 17.513]), indicating that meeting ADHD criteria was associated with relatively poorer Core Language skills, and there were no important Group by ADHD interaction effects. See Supplementary Materials, Table 4 for complete results.
Table 4.
Functional lateralization of activated regions by cluster, group, and language status subgroups.
| Group | Frontal Cluster | Temporal Cluster | Semantic Cluster |
|---|---|---|---|
| LN (n = 45) | |||
| n Regions = | 9 | 8 | 14 |
| M(SD) | 0.40(0.36) | 0.40(0.33) | 0.36(0.32) |
| LI (n = 10) | |||
| n Regions = | 8 | 5 | 11 |
| M(SD) | 0.62(0.38) | 0.71(0.49) | 0.41(0.38) |
Note. n Regions = Total number of regions activated; M = Mean activation across regions within a cluster; SD = Standard deviation across regions within a cluster.
3.2. Language and social communication by ASD status – Behavioral data analyses
3.2.1. Recalling sentences
There were no important ASD-LAD, ASD-NT, or LAD-NT Group comparison, SCQ, or Group by SCQ interaction effects.
3.2.2. Core language
There were important ASD-LAD and ASD-NT Group comparison effects, but no important LAD-NT Group comparison, SCQ, or Group by SCQ interaction effects. The ASD group had poorer Core Language than the LAD group (b = 13.700; SD = 6.972; 95% PPI[0.590, 27.672]), and the ASD group had poorer Core Language than the NT group (b = 20.299; SD = 5.903; 95% PPI[8.793, 31.972]). See Supplementary Materials Table 4 for complete results.
3.3. Language and ADHD by ASD status – behavioral data analyses
3.3.1. Recalling sentences
There were no important ASD-LAD or ASD-NT Group comparison, ADHD status, or Group by ADHD status interaction effects. There was an important LAD-NT Group comparison effect, indicating that the LAD group had poorer Recalling Sentences than the NT group (b = 1.560; SD = 0.760; 95% PPI[0.109, 3.074]).
3.3.2. Core language
There were important ASD-LAD and ASD-NT Group comparison effects, but not LAD-NT Group comparison or Group by Social Communication interaction effects. The ASD group had poorer Core Language than the LAD group (b = 8.750; SD = 3.431; 95% PPI[1.675, 15.221]), and meeting ADHD criteria was associated with poorer Core Language skills (b = 4.572; SD = 3.671; 95% PPI[2.544, 11.742]) in this model. The ASD group also had poorer Core Language than the NT group (b = 11.682; SD = 3.592; 95% PPI[4.285, 18.204]). There were no other important ADHD status effects. See Table 3 for a summary of results and Supplementary Materials Table 4 for complete results.
Table 3.
Summary of behavioral analysis important effects.
| Model | Group Effect | Other Effects |
|---|---|---|
| Core Language ∼ ASD-LAD*Social Communication | ASD < LAD | None |
| Core Language ∼ ASD-NT*Social Communication | ASD < NT | None |
| Recalling Sentences ∼ LAD-NT*ADHD status | LAD < NT | None |
| Core Language ∼ ASD-LAD*ADHD status | ASD < LAD | ADHD status |
| Core Language ∼ ASD-NT*ADHD status | ASD < NT | None |
Note. Other effects = the Social Communication or ADHD status predictor and the interaction term.
3.4. Neural lateralization – fMRI data analyses
See Supplementary Materials 1 for fMRI participant characteristics (Tables 5, 6, and 7), complete list of significantly activated regions (Tables 9 and 10), complete statistical output (Table 11), and ASD, LAD, and NT neural lateralization group comparisons (Table 8). See Table 4 for descriptive Lateralization results.
3.4.1. Frontal cluster
There was an important Group effect (b = -0.317; SD = 0.135; PPI [−0.569, −0.034]) when accounting for (i.e., covarying) the SCQ effect (b = −0.010; SD = 0.005; PPI[−0.018, −0.001] ]), which indicated relatively greater left hemisphere lateralization for the LI than LN group and relatively greater left hemisphere lateralization for individuals with a history of relatively more social communication and repetitive behavior difficulty. There was no important effect of Group when accounting for ADHD status.
3.4.2. Temporal cluster
There was an important Group effect (b = -0.310; SD = 0.127; PPI [−0.573, −0.073]), indicating greater left hemisphere lateralization for the LI than LN group. The Group effect remained important when accounting for the SCQ effect (b = -0.010; SD = 0.005; PPI [−0.019, −0.000]), which indicated relatively greater left hemisphere lateralization for individuals with a history of relatively more social communication and repetitive behavior difficulty. The group effect remained important when accounting for ADHD status.
3.4.3. Semantic cluster
There was no important Group or ADHD status effects, but there was an important SCQ effect (b = -0.011; SD = 0.004; PPI [−0.019, −0.003]), indicating relatively greater left hemisphere lateralization for individuals with relatively more social communication and repetitive behavior difficulty.
3.4.4. All clusters (post-hoc)
There was an important Group effect (b = -161; SD = 0.133; PPI-0.294, −0.021]), indicating greater left hemisphere lateralization for the LI than LN group. The Group effect remained important when accounting for the SCQ effect (b = -0.010; SD = 0.003; PPI [−0.016, −0.006]) and when accounting for SCQ and ADHD status. However, there was no important Group effect when accounting for only ADHD status. See Table 5 for a summary and Fig. 2 for visualization of neural activation.
Table 5.
Important effects: Left relative to right hemisphere lateralization.
| Model | Lateralization Effect | Cluster |
|---|---|---|
| Group | LI > LN | Temporal, All Clusters |
| Group + ADHD status | None | Frontal, Temporal, Semantic, Across All Clusters |
| Group + Social Communication | LI > LN; Social Communication | Frontal, Temporal, Across All Clusters |
| Group + Social Communication | Social Communication | Semantic |
| Group + Social Communication + ADHD status | LI > LN; Social Communication | Temporal, All Clusters |
| Group + Social Communication + ADHD status | Social Communication | Frontal, Semantic |
Note. Social Communication effect = history of social communication (SCQ) difficulty associated with relatively greater left than right hemisphere lateralization.
4. Discussion
4.1. Structural language impairment in ASD and LAD
The current pre-registered study examined possible structural language impairment in individuals with ASD and in individuals with a history of ASD who no longer meet criteria for an ASD diagnosis, a Loss of Autism Diagnosis (LAD) group, relative to NT peers. It was important to probe for a LI in the LAD group because, while group-level data suggest age-appropriate structural language skills, little work to date has examined structural language abilities in these individuals. Additionally, no prior work has examined functional lateralization in language and language-homologue regions in ASD structural language impairment subgroups or in LAD.
Although we hypothesized that more ASD participants would meet criteria for structural language impairment than LAD participants, our findings indicated that a similar proportion of ASD (22.9%) and LAD (22.6%) participants met our clinical marker criterion (i.e., ≤7 scaled score for Recalling Sentences) for structural language impairment. There was also no significant difference in the number of ASD-LI and LAD-LI participants who fell in the clinical range based on a composite language criterion (i.e., Core Language on the CELF). No NT participants met either criterion for structural language impairment. Additionally, the ASD-LI and LAD-LI groups differed from the NT group on all language measures, Social Communication Questionnaire scores (i.e., social communication-repetitive behavior), and ADHD status, but not nonverbal ability.
Taken together, these results suggest that structural language impairment presents similarly in ASD and LAD, even in the absence of current ASD diagnostic features in LAD. This finding aligns with prior work demonstrating subtle, yet inconsistent deficits in language in LAD relative to NT peers (i.e., in context of normal-range language skills on average; Canfield et al., 2016, Fein et al., 2013, Orinstein et al., 2015a, Suh et al., 2014, Turner and Stone, 2007). Inconsistencies in language findings in LAD may reflect, in part, a subgroup of individuals with LAD-LI that were previously unaccounted for. This interpretation is consistent with previous work suggesting that structural language impairment is characteristic of a substantial proportion of individuals with ASD (i.e., may be viewed as common in ASD; Blume et al., 2021, Kjelgaard and Tager-Flusberg, 2001, Riches et al., 2010, Wittke et al., 2017). This finding may reflect the fact that language problems are less responsive to intervention relative to behavioral and cognitive skills, at least via behavioral interventions most commonly used with young children with ASD (Sallows and Graupner, 2005).
4.2. Structural language impairment distinct from related skills in ASD and LAD
We further examined the association between structural language impairment and domains that represent mild and isolated differences in LAD relative to NT peers – social communication and attention (Orinstein et al., 2015; Kelley et al., 2006, Suh et al., 2016; note, however, that lab-based participant samples often involve above-average NT performers in these and other domains). There was no evidence of an important relationship between Recalling Sentences scores (i.e., our marker of structural language impairment) and SCQ scores or ADHD status within our LAD, ASD, or NT groups. There was also little evidence that the relationships between language and these measures differed among ASD, LAD, and NT groups.
However, there was evidence that the composite language measure, CELF Core Language, was associated with both SCQ scores and ADHD status when comparing LN and LI groups collapsed across ASD-status groups. Results showed that SCQ and ADHD status were each associated with general language performance to a greater degree than language performance more specific to morphosyntax (recalling sentences may also involve a high memory load; Archibald and Gathercole, 2006). The fact that social communication may be associated with general language performance but not with the test specific to morphosyntax may suggest that overall language level is related to social communication, perhaps with a threshold effect, and that social communication may depend on more social behaviors (e.g., affect, conversational turn-taking, prosody) than grammatical skill. This relationship may have been driven by the LN group; therefore, it is also possible that social communication and attention are more closely associated with language abilities for individuals with normal-range structural language, compared to individuals with impaired structural language (Blume et al., 2021). Consistent with this suggestion, a comparison of these relationships within ASD-LI and LAD-LI subgroups revealed no evidence of language/social communication-repetitive behavior or language/ADHD relationships.
Collectively, behavioral findings suggest the presence of structural language impairment in a subgroup of individuals with ASD and LAD, and that this impairment is not simply a reflection of lifetime history of difficulties with social communication and repetitive behaviors or lifetime history of difficulties with attention at the individual level. In LAD, these findings may suggest a dissociation of structural language from gains in social communication and attention across development, and therefore less potential bootstrapping of early language delay, at least in a meaningful subset of individuals. Thus, this group may be susceptible to continued language difficulty even though they no longer present with ASD features. This result has an important clinical implication; even if youth appear to have resolved most or all of their earlier social communication difficulties, they should nonetheless be evaluated by a speech-language pathologist or a psychologist for ongoing speech-language challenges.
4.3. Neural lateralization and structural language impairment
We hypothesized that structural language impairment would be linked with diminished degree of left hemisphere lateralization of language function (i.e., greater symmetry) relative to peers with intact structural language in the three a priori defined language-related neural networks that we examined – frontal, temporal, and global semantic clusters. This hypothesis was based on prior work demonstrating neural specialization of language function to the left rather than right hemisphere in neurotypical individuals across development (Olulade et al., 2020) and diminished left hemisphere lateralization of language function in individuals with ASD (Jouravlev et al., 2020). Results robustly contradicted this expectation. We found greater left hemisphere lateralization of language function for the LI than LN group for regions significantly activated in the frontal cluster and across all clusters when accounting for social communication-repetitive behaviors (SCQ). In other words, when accounting for variance in history of social communication and repetitive behavior difficulty, an individual with structural language impairment had relatively greater left hemisphere lateralization of language function in significantly activated language-related brain regions than an individual with normal-range structural language. Conversely, when accounting for variance related to LI versus LN status, an individual with a history of social communication and repetitive behavior difficulty had relatively greater left hemisphere lateralization of language function in significantly activated language-related brain regions than an individual without a history of social communication and repetitive behavior difficulty. We also found greater left hemisphere lateralization of language function for the LI than LN group in the temporal cluster regardless of social communication. We did not observe a group difference in lateralization for the global semantic cluster, although the LN group had numerically greater left hemisphere lateralization. Additionally, there was greater left than right hemisphere lateralization across groups and clusters.
Some of our findings may appear to be inconsistent with prior work that has shown greater left hemisphere lateralization in NT individuals over development (Olulade et al., 2020), in NT compared to ASD peers (Jouravlev et al., 2020), and in LI groups with and without ASD (De Fossé et al., 2004, Herbert et al., 2005). However, those prior studies that included individuals with ASD and LI subgroups examined volumetric differences between brain regions in the left versus right hemisphere (De Fossé et al., 2004, Herbert et al., 2005), rather than language-related functional activation differences between brain regions as in the current study. We only examined lateralization in regions significantly activated within each group, thus there were differences between the LN and LI groups in the set of ROIs contributing to lateralization values. For instance, the LN group had more language-related regions significantly activated than the LI group (LN N = 31 ROIs; LI N = 21 ROIs) and more regions significantly activated bilaterally (LN N = 10 ROIs; LI N = 2 ROIs; Table 4). However, these differences likely reflect differing statistical power within each group and future work should examine these patterns in more balanced LN and LI samples.
Prior work examining language-related functional lateralization in language impairment groups without ASD suggests diminished lateralization in left inferior frontal regions (Badcock et al., 2012, De Guibert et al., 2011) and in left superior temporal regions (de Guibert et al., 2011). Convergingly, in the current study, several key language regions that were significantly activated in the LN group were not significantly activated in the LI group, including inferior frontal (44, 45) and superior temporal (STSvs, STSda, STSdp) regions in the left hemisphere (see Supplementary Materials 1 Tables 8 and 9; note that leftward lateralization was greater at the cluster level). We also found regions in the right hemisphere with significant deactivation in the LN, but not LI, group, such as superior and inferior temporal regions (TE1m, TE1a, TE2a, STSvp). Taken together, there may be differences in brain regions involved in language function in individuals experiencing atypical language development that have not been revealed in prior work on neural structure in LI and that cannot be captured by prior work on neural function in NT populations. Importantly, future work should examine these patterns using balanced sample sizes as significantly activated regions may reflect differing statistical power in the LN and LI groups.
Our region-specific findings are broadly consistent with those of Badcock et al., 2012, De Guibert et al., 2011. Differences in lateralization findings between these two prior studies and the current study may reflect ASD or LAD status of participants in our LN and LI groups (e.g., diminished left hemisphere lateralization in ASD, Jouravlev et al., 2020, and compensatory activation of additional brain regions in LAD relative NT peers; Eigsti et al, 2016). In fact, we conducted additional lateralization analyses of ASD, LAD, and NT groups which suggested that left hemisphere lateralization differed between groups and clusters (e.g., numerical left hemisphere lateralization: semantic cluster NT > LAD > ASD; temporal cluster LAD = NT > ASD; frontal cluster NT > LAD > ASD; Supplementary Materials 1 Table 8 for descriptive data and Tables 12 and 13 for statistical comparisons). Statistical comparisons suggested that the NT group had greater left hemisphere lateralization than the ASD and LAD groups in the semantic cluster, which is also the only cluster for which lateralization did not differ between the LN and LI groups (See Supplementary Materials 1). It is likely that ASD and LAD status relates to lateralization patterns in the current study in addition to LI status.
We also examined the relationship between lateralization and two domains relevant to language functioning – social communication-repetitive behavior and attention. We hypothesized that the magnitude of diminished left hemisphere lateralization in the LI relative to LN group would be greater after controlling for social communication-repetitive behavior and attentional skills regardless of ASD diagnostic status. Counter to this prediction, the magnitude of heightened left hemisphere lateralization in the LI relative to the LN group was greater for the frontal, temporal, and across all clusters when accounting for social communication-repetitive behavior. This effect did not hold when accounting for ADHD status or when accounting for social communication-repetitive behavior and ADHD status. These patterns also align with prior evidence of associations between social communication and language (Blume et al., 2021, Gibson et al., 2013, Riches et al., 2010) and our behavioral findings of a link between social communication and general language ability. Although these findings appear to reflect more typical left hemisphere lateralization in the presence of fewer social communication deficits, consistent with prior work, they may also reflect heightened, or compensatory, lateralization in the presence of language and social communication difficulty.
In fact, accounting for past difficulty in social communication-repetitive behavior did not make lateralization patterns in the LN and LI groups more similar. Given that a key difference between LN and LI groups was social communication-repetitive behavior, due in part to the LI group including only ASD and LAD participants and the LN group including NT, ASD, and LAD participants, this finding may be a nuanced version of ASD status and may allow for lateralization differences between the LN and LI groups to be more readily detectable. However, this hypothesis requires further examination, such as in individuals with structural language impairment without a history of ASD (e.g., Developmental Language Disorder), as well as in prospective work with LN and LI groups balanced in sample size. Another hypothesis is that language and social communication difficulty may be associated with compensatory activation patterns, such as heightened activation in left hemisphere language regions or heightened de-activation in right hemisphere homologue regions. For instance, Eigsti et al. (2016) showed language-related compensatory activation in several language-related brain regions in LAD relative to ASD and NT peers (see also Sridhar et al., preprint). Taken together, compensatory lateralization and reduced lateralization (Jouravlev et al., 2020) may underlie inefficient language processing, as well as reflect disruption in typical language development, potentially underscoring an optimal range for language-related lateralization. However, Eigsti et al. (2016) also showed language-related compensatory activation in several brain regions not typically associated with language in LAD relative to ASD and NT peers, such as regions in the left and right anterior and posterior cerebellum. These cerebellar regions have been implicated in semantic processing and metalinguistic skills in lesion studies and studies of aphasia, although they are typically associated with motor control (Eigsti et al., 2016). Thus, it is possible that conducting whole-brain analysis will be necessary to capture language-related compensatory activation and lateralization in populations experiencing atypical language development or language-related brain injury.
5. Limitations
Due in part to the retrospective nature of this study, structural language impairment subgroups included small sample sizes. This may also reflect the relatively high cognitive abilities in participants, which limited our ability to detect effects that are relevant to structural language impairment and invariant to ASD status. The ASD-LI and LAD-LI groups were well matched on behavioral measures, providing justification for collapsing across ASD status for LI. Regardless, the small LI group sample size may have limited our ability to detect statistically significant brain region activation. Given that we analyzed our research questions using a Bayesian framework, we examined model convergence rather than power, finding acceptable levels of model convergence in all comparisons. However, generalizability of our findings remains a concern and future work would benefit from increasing sample size and prospectively recruiting structural language impairment group participants. Lastly, future work should analyze lateralization in individuals with ASD-LN and LAD-LN separately from individuals with ASD-LI and LAD-LI to fully dissociate the role of ASD status versus the role of structural language impairment status.
There are multiple methods for measuring lateralization, such as language tasks, brain regions, and voxel count versus average activation (e.g., Barth et al., 2012, Bradshaw et al., 2017). Our approach involved a commonly used language task, brain regions identified to be robustly involved in language function (Baker et al., 2018), and a lateralization measure that accounted for the degree of activation. This approach does not address differences in the individual brain regions recruited during language processing, such as those observed in prior work on LAD relative to ASD and NT peers (Eigsti et al., 2016). Future work may benefit from analyzing brain regions identified to be labile to atypical development to account for compensatory neural activation to a greater degree. Additionally, we conducted post-hoc re-analysis of lateralization covarying handedness preference (which did not differ significantly in any by-group comparisons, e.g., Supplementary Materials 1 Table 6) and found one change in results. The group effect in the temporal cluster was no longer important when covarying handedness (see Supplementary Materials 1 Table 11). However, the numerical pattern of greater left-hemisphere lateralization in the LI than LN group was consistent across comparisons.
We excluded our control condition (i.e., “LLLL” or “RRRR” presentation) from analyses given that this condition may have engaged language processing (e.g., reading circuitry). Future work may benefit from employing an experimental task that is validated to activate nonlinguistic perceptual brain regions during fMRI to provide a more refined test of language-related function than in the current study (e.g., Wilson et al., 2018). Additional measures of structural language, social communication, and attention may further clarify the characteristics of structural language subgroups (e.g., morphological errors) and developmental trajectories of lateralization (e.g., trajectories specific to language versus generally related to social contexts in which language is learned).
5.1. Conclusions
In behavioral analyses, the current study demonstrated the presence of structural language impairment in LAD at a similar rate as peers with ASD using a clinical marker of structural language impairment. We demonstrated similarities in the LAD-LI and ASD-LI subgroups in the relationship between language and two skills that represent areas of subtle residual weakness in LAD – social communication-repetitive behavior and attention – even though ASD features were no longer present in the LAD group. Furthermore, structural language abilities were not associated with social communication-repetitive behavior or ADHD status in either subgroup, suggesting the presence of structural language impairment distinct from related skills. Findings suggest the possibility of persistent language difficulty across development in some LAD individuals.
In neural analyses, there was evidence of greater left hemisphere lateralization in a group of ASD and LAD individuals with structural language impairment than in a group of ASD, LAD, and NT peers with normal-range structural language skills. Specifically, there was greater left hemisphere lateralization in regions that were significantly activated, and the magnitude of this difference was greater when accounting for history of difficulty with social communication and repetitive behavior. These patterns underscore important differences in the trajectory of language-related neural specialization in individuals with structural language impairment relative to peers with normal-range structural language skills. However, future work should further investigate potential group differences in brain regions contributing to lateralization values and the degree to which heightened lateralization of language function reflects compensatory processes in individuals with structural language impairment.
CRediT authorship contribution statement
Caroline Larson: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Karla Rivera-Figueroa: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. Hannah R. Thomas: Conceptualization, Methodology, Validation, Writing – review & editing. Deborah Fein: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. Michael C. Stevens: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. Inge-Marie Eigsti: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are very grateful to the participants and their families, to Dr. Lynn Brennan and Harriet Levin for their help with recruitment, to Drs. Molly Helt, Michael Rosenthal, and Kathryn Tyson for their help in testing the children, and to many invaluable undergraduate research assistants. We gratefully acknowledge our funding from the National Institutes of Health R01MH076189 and R01MH112687-01A1.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103043.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders-Fifth Edition. American Psychiatric Association.
- Anderson D.K., Liang J.W., Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J. Child Psychol. Psychiatry. 2014;55(5):485–494. doi: 10.1111/jcpp.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald L.M.D., Joanisse M.F. On the sensitivity and specificity of nonword repetition and sentence recall to language and memory impairments in children. J. Speech, Language, Hearing Res. 2009;52(4):899–914. doi: 10.1044/1092-4388(2009/08-0099). [DOI] [PubMed] [Google Scholar]
- Badcock N.A., Bishop D.V.M., Hardiman M.J., Barry J.G., Watkins K.E. Co-localisation of abnormal brain structure and function in specific language impairment. Brain Lang. 2012;120(3):310–320. doi: 10.1016/j.bandl.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, C. M., Burks, J. D., Briggs, R. G., Conner, A. K., Glenn, C. A., Sali, G., McCoy, T. M., Battiste, J. D., O’Donoghue, D. L., & Sughrue, M. E., 2018. A Connectomic Atlas of the Human Cerebrum-Chapter 1: Introduction, Methods, and Significance. Operative Neurosurgery (Hagerstown, Md.), 15(1), 1–9. [DOI] [PMC free article] [PubMed]
- Barth J.M., Boles D.B., Giattina A.A., Penn C.E. Preschool child and adult lateralisation and performance in emotion and language tasks. Laterality: Asymmetries of Body, Brain and Cognition. 2012;17(4):412–427. doi: 10.1080/1357650X.2011.626435. [DOI] [PubMed] [Google Scholar]
- Bishop D.V.M., Snowling M.J., Thompson P.A., Greenhalgh T. Phase 2 of CATALISE : A multinational and multidisciplinary Delphi consensus study of problems with language development: Terminology. J. Child Psychol. Psychiatry. 2017;10:1068–1080. doi: 10.1111/jcpp.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume J., Wittke K., Naigles L., Mastergeorge A.M. Language Growth in Young Children with Autism: Interactions between Language Production and Social Communication. J. Autism Dev. Disord. 2021;51(2):644–665. doi: 10.1007/s10803-020-04576-3. [DOI] [PubMed] [Google Scholar]
- Briggs, R. G., Conner, A. K., Baker, C. M., Burks, J. D., Glenn, C. A., Sali, G., Battiste, J. D., O’Donoghue, D. L., & Sughrue, M. E., 2018. A Connectomic Atlas of the Human Cerebrum-Chapter 18: The Connectional Anatomy of Human Brain Networks. Operative Neurosurgery (Hagerstown, Md.), 15(1), S470–S480. https://doi.org/10.1093/ons/opy272. [DOI] [PMC free article] [PubMed]
- Bradshaw A.R., Bishop D.V., Woodhead Z.V. Methodological considerations in assessment of language lateralisation with fMRI: a systematic review. PeerJ. 2017;5 doi: 10.7717/peerj.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P.A., Mokrysz C., Nosek B.A., Flint J., Robinson E.S.J., Munafò M.R. Power failure: why small sample size undermines the reliability of neuroscience. Power failure: why small sample size undermines the reliability of neuroscience. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Canfield A.R., Eigsti I.-M., de Marchena A., Fein D. Story Goodness in Adolescents With Autism Spectrum Disorder (ASD) and in Optimal Outcomes From ASD. J. Speech, Language, Hearing Res. 2016;24(2):1–14. doi: 10.1044/2015_JSLHR-L-15-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fossé L., Hodge S.M., Makris N., Kennedy D.N., Caviness V.S., McGrath L., Steele S., Ziegler D.A., Herbert M.R., Frazier J.A., Tager-Flusberg H., Harris G.J. Language-association cortex asymmetry in autism and specific language impairment. Ann. Neurol. 2004;56(6):757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- De Guibert C., Maumet C., Jannin P., Ferré J.C., Tréguier C., Barillot C., Le Rumeur E., Allaire C., Biraben A. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia) Brain. 2011;134(10):3044–3058. doi: 10.1093/brain/awr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie E.W., Anticevic A., Smitha D.E., Coalson T.S., Manogaran M., Calarco N., Viviano J.D., Glasser M.F., Van Essen D.C., Voineskos N.A. Ciftify: A framework for surface-based analysis of legacy MR acquisitions. NeuroImage. 2019;197:818–826. doi: 10.1016/j.neuroimage.2019.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L.M., Dunn D.M. fourth ed. Pearson Assessments; Minneapolis, MN: 2007. Peabody Picture Vocabulary Test. [Google Scholar]
- Ebert K.D., Scott C.M. Relationships Between Narrative Language Samples and Norm-Referenced Test Scores in Language Assessments of School-Age Children. Language, Speech, and Hearing Services in Schools. 2014;45(4):337–350. doi: 10.1044/2014_LSHSS-14-0034. [DOI] [PubMed] [Google Scholar]
- Eigsti I.M., Stevens M.C., Schultz R.T., Barton M., Kelley E., Naigles L., Orinstein A., Troyb E., Fein D.A. Language comprehension and brain function in individuals with an optimal outcome from autism. NeuroImage: Clinical. 2016;10:182–191. doi: 10.1016/j.nicl.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis Weismer S., Davidson M.M., Gangopadhyay I., Sindberg H., Roebuck H., Kaushanskaya M. The role of nonverbal working memory in morphosyntactic processing by children with specific language impairment and autism spectrum disorders. J. Neurodevelopmental Disorders. 2017;9(1) doi: 10.1186/s11689-017-9209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Blair R.W., Nielson D.M., Varada J.C., Marrett S., Thomas A.G., Poldrack R.A., Gorgolewski K.J. Crowdsourced MRI quality metrics and expert quality annotations for training of humans and machines. Sci. Data. 2019;6(1):1–7. doi: 10.1038/s41597-019-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A., Kent J.D., Goncalves M., DuPre E., Snyder M., Oya H., Ghosh S.S., Wright J., Durnez J., Poldrack R.A., Gorgolewski K.J. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods. 2019;16(1):111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein D., Barton M., Eigsti I.M., Kelley E., Naigles L., Schultz R.T., Stevens M., Helt M., Orinstein A., Rosenthal M., Troyb E., Tyson K. Optimal outcome in individuals with a history of autism. J. Child Psychol. Psychiatry. 2013;54(2):195–205. doi: 10.1111/jcpp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain C., Winter A.S., Bearman P.S. Six developmental trajectories characterize children with autism. Pediatrics. 2012;129(5):e1112–e1120. doi: 10.1542/peds.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J., Adams C., Lockton E., Green J. Social communication disorder outside autism? A diagnostic classification approach to delineating pragmatic language impairment, high functioning autism and specific language impairment. J. Child Psychol. Psychiatry. 2013;54(11):1186–1197. doi: 10.1111/jcpp.12079. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., Coalson T.S., Robinson E.C., Hacker C.D., Harwell J., Yacoub E., Ugurbil K., Andersson J., Beckmann C.F., Jenkinson M., Smith S.M., Van Essen D.C. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helt M., Kelley E., Kinsbourne M., Pandey J., Boorstein H., Herbert M., Fein D. Can children with autism recover? If so, how? Neuropsychol. Rev. 2008;18(4):339–366. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- Herbert M.R., Ziegler D.A., Deutsch C.K., O’Brien L.M., Kennedy D.N., Filipek P.A., Bakardjiev A.I., Hodgson J., Takeoka M., Makris N., Caviness V.S. Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain. 2005;128(1):213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Jouravlev O., Kell A.J.E., Mineroff Z., Haskins A.J., Ayyash D., Kanwisher N., Fedorenko E. Reduced language lateralization in autism and the broader autism phenotype as assessed with robust individual-subjects analyses. Autism Res. 2020;13(10):1746–1761. doi: 10.1002/aur.2393. [DOI] [PubMed] [Google Scholar]
- Kana R.K., Keller T.A., Cherkassky V.L., Minshew N.J., Just M.A. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. Guilford Press; New York: 2014. Bayesian Statistics for the Social Sciences. [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. The Schedule for Affective Disorders and Schizophrenia for School-Age Children – Lifetime version. J. Acad. Academic Child Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kelley E., Paul J.J., Fein D., Naigles L.R. Residual language deficits in optimal outcome children with a history of autism. J. Autism Dev. Disord. 2006;36(6):807–828. doi: 10.1007/s10803-006-0111-4. [DOI] [PubMed] [Google Scholar]
- Kelley E., Naigles L., Fein D. An in-depth examination of optimal outcome children with a history of autism spectrum disorders. Res. Autism Spectrum Disorders. 2010;4(3):526–538. [Google Scholar]
- Kjelgaard M.M., Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16(2–3):287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatte I.S., van Heugten V., Zwitserlood R., Gerrits E. Language Sample Analysis in Clinical Practice: Speech-Language Pathologists’ Barriers, Facilitators, and Needs. Language, Speech, and Hearing Services in Schools. 2022;53(January):1–16. doi: 10.1044/2021_LSHSS-21-00026. [DOI] [PubMed] [Google Scholar]
- Jansen A., Menke R., Sommer J., Förster A.F., Bruchmann S., Hempleman J., Weber B., Knecht S. The assessment of hemispheric lateralization in functional MRI—robustness and reproducibility. Neuroimage. 2006;33(1):204–217. doi: 10.1016/j.neuroimage.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Landa R.J., Kalb L.G. Long-term outcomes of toddlers with autism spectrum disorders exposed to short-term intervention. Pediatrics. 2012;130(Supplement_2):S186–S190. doi: 10.1542/peds.2012-0900Q. [DOI] [PubMed] [Google Scholar]
- Larson C., Gangopadhyay I., Prescott K., Kaushanskaya M., Ellis Weismer S. Planning in Children with Autism Spectrum Disorder: The Role of Verbal Mediation. J. Autism Dev. Disord. 2021;51(7):2200–2217. doi: 10.1007/s10803-020-04639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson C., Kaplan D., Kaushanskaya M., Ellis Weismer S. Language and Inhibition: Predictive Relationships in Children with Language Impairment Relative to Typically Developing Peers. J. Speech, Language Hearing Res. 2020;63(4):1115–1127. doi: 10.1044/2019_JSLHR-19-00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Loucas T., Charman T., Pickles A., Simonoff E., Chandler S., Meldrum D., Baird G. Autistic symptomatology and language ability in autism spectrum disorder and specific language impairment. J. Child Psychol. Psychiatry. 2008;49(11):1184–1192. doi: 10.1111/j.1469-7610.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- Mukaddes N.M., Tutkunkardas M.D., Sari O., Aydin A., Kozanoglu P. Characteristics of Children Who Lost the Diagnosis of Autism: A Sample from Istanbul, Turkey. Autism Res. Treatment. 2014;2014:1–10. doi: 10.1155/2014/472120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitido H., Plante E. Diagnosis of Developmental Language Disorder in Research Studies. J. Speech, Language Hearing Res. 2020;63(8):2777–2788. doi: 10.1044/2020_JSLHR-20-00091. [DOI] [PubMed] [Google Scholar]
- Oetting J.B., McDonald J.L., Seidel C.M., Hegarty M. Sentence Recall by Children with SLI Across Two Nonmainstream Dialects of English. J. Speech, Language, Hearing Res. 2016;59(1):183–194. doi: 10.1044/2015_JSLHR-L-15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olulade O.A., Seydell-Greenwald A., Chambers C.E., Turkeltaub P.E., Dromerick A.W., Berl M.M., Gaillard W.D., Newport E.L. The neural basis of language development: Changes in lateralization over age. PNAS. 2020;117(38):23477–23483. doi: 10.1073/pnas.1905590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orinstein A.J., Suh J., Porter K., De Yoe K.A., Tyson K.E., Troyb E., Barton M.L., Eigsti I.M., Stevens M.C., Fein D.A. Social Function and Communication in Optimal Outcome Children and Adolescents with an Autism History on Structured Test Measures. J. Autism Dev. Disord. 2015;45(8):2443–2463. doi: 10.1007/s10803-015-2409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orinstein A., Tyson K.E., Suh J., Troyb E., Helt M., Rosenthal M., Barton M.L., Eigsti I.M., Kelley E., Naigles L., Schultz R.T., Stevens M.C., Fein D.A. Psychiatric Symptoms in Youth with a History of Autism and Optimal Outcome. J. Autism Dev. Disord. 2015;45(11):3703–3714. doi: 10.1007/s10803-015-2520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond S.M. Differentiating SLI from ADHD using children’s sentence recall and production of past tense morphology. Clinical Linguist. Phonetics. 2005;19(2):109–127. doi: 10.1080/02699200410001669870. [DOI] [PubMed] [Google Scholar]
- Riches N.G., Loucas T., Baird G., Charman T., Simonoff E. Sentence repetition in adolescents with specific language impairments and autism: An investigation of complex syntax. Int. J. Language Commun. Disorders. 2010;45(1):47–60. doi: 10.3109/13682820802647676. [DOI] [PubMed] [Google Scholar]
- Rivera-Figueroa, K., 2020. Language hemispheric dominance and language abilities; an individual differences., surface-based fMRI approach [Master’s thesis, University of Connecticut]. The University of Connecticut Library. https://archives.lib.uconn.edu/islandora/object/20002%3A860651055.
- Rutter M., Bailey A., Lord C. Western Psychological Services; Los Angeles: 2003. Social Communication Questionnaire – Lifetime version. [Google Scholar]
- Sallows G.O., Graupner T.D. Intensive behavioral treatment for children with autism: Four-year outcome and predictors. Am. J. Mental Retardat. 2005;110(6):417. doi: 10.1352/0895-8017(2005)110[417:IBTFCW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Semel E., Wiig E.H., Secord W.A. fourth ed. Harcourt Assessment; San Antonio, TX: 2003. Clinical Evaluation of Language Fundamentals. [Google Scholar]
- Smolak E., McGregor K.K., Arbisi-Kelm T., Eden N. Sustained Attention in Developmental Language Disorder and Its Relation to Working Memory and Language. J. Speech, Language, Hearing Res. 2020;63(12):4096–4108. doi: 10.1044/2020_JSLHR-20-00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S.S., Balla D.A., Cicchetti D.V. Interview Edn. American Guidance Service; Circle Pines, MN: 1985. Vineland Adaptive Behavior Scales. [Google Scholar]
- Suh J., Eigsti I.M., Naigles L., Barton M., Kelley E., Fein D. Narrative performance of optimal outcome children and adolescents with a history of an Autism Spectrum Disorder (ASD) J. Autism Dev. Disord. 2014;44(7):1681–1694. doi: 10.1007/s10803-014-2042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J., Orinstein A., Barton M., Chen C.M., Eigsti I.M., Ramirez-Esparza N., Fein D. Ratings of Broader Autism Phenotype and Personality Traits in Optimal Outcomes from Autism Spectrum Disorder. J. Autism Dev. Disord. 2016;46(11):3505–3518. doi: 10.1007/s10803-016-2868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar, A., Keehn, R., Jao, J., Wilkinson, M., Gao, Y., Olson, M., Mash, L. E., Alemu, K., Manley, A., Marinkovic, K., Linke, A., & Müller, R.-A. (preprint). Increased heterogeneity and task-related reconfiguration of functional connectivity within a lexicosemantic network in autism. 1–50. https://www.biorxiv.org/content/10.1101/2021.11.22.469604v1.
- Troyb E., Orinstein A., Tyson K., Helt M., Eigsti I.M., Stevens M., Fein D. Academic abilities in children and adolescents with a history of autism spectrum disorders who have achieved optimal outcomes. Autism. 2014;18(3):233–243. doi: 10.1177/1362361312473519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L.M., Stone W.L. Variability in outcome for children with an ASD diagnosis at age 2. J. Child Psychol. Psychiatry. 2007;48(8):793–802. doi: 10.1111/j.1469-7610.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- Tyson K., Kelley E., Fein D., Orinstein A., Troyb E., Barton M., Eigsti I.M., Naigles L., Schultz R.T., Stevens M., Helt M., Rosenthal M. Language and verbal memory in individuals with a history of autism spectrum disorders who have achieved optimal outcomes. J. Autism Dev. Disord. 2014;44(3):648–663. doi: 10.1007/s10803-013-1921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Schoot R., Kaplan D., Denissen J., Asendorpf J.B., Neyer F.J., van Aken M.A.G. A Gentle Introduction to Bayesian Analysis: Applications to Developmental Research. Child Dev. 2014;85(3):842–860. doi: 10.1111/cdev.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstein R.L., Lazar N.A. The ASA statement on p-values: Context, process, and purpose. Am. Statistician. 2016;70:129–133. doi: 10.1080/00031305.2016.1154108. [DOI] [Google Scholar]
- Whitehouse A.J.O., Watt H.J., Line E.A., Bishop D.V.M. Adult psychosocial outcomes of children with specific language impairment, pragmatic language impairment and autism. Int. J. Language Commun. Disorders. 2009;44(4):511–528. doi: 10.1080/13682820802708098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D., Botting N., Boucher J. Language in Autism and Specific Language Impairment: Where Are the Links? Psychol. Bull. 2008;134(6):944–963. doi: 10.1037/a0013743. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Yen M., Eriksson D.K. An adaptive semantic matching paradigm for reliable and valid language mapping in individuals with aphasia. Hum. Brain Mapp. 2018;39(8):3285–3307. doi: 10.1002/hbm.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittke K., Mastergeorge A.M., Ozonoff S., Rogers S.J., Naigles L.R. Grammatical language impairment in autism spectrum disorder: Exploring language phenotypes beyond standardized testing. Front. Psychol. 2017;8(APR):1–12. doi: 10.3389/fpsyg.2017.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachor D.A., Ben-Itzchak E. From Toddlerhood to Adolescence, Trajectories and Predictors of Outcome: Long-Term Follow-Up Study in Autism Spectrum Disorder. Autism Research. 2020;13(7):1130–1143. doi: 10.1002/aur.2313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.