This cohort study attempts to delineate test characteristics of the pediatric Sequential Organ Failure Assessment score for predicting in-hospital mortality among pediatric emergency department patients.
Key Points
Question
How does the pediatric Sequential Organ Failure Assessment (pSOFA) score perform in predicting mortality in the emergency department setting?
Findings
In this large cohort study of nearly 4 million pediatric emergency department visits, pSOFA scores of 2 or more were uncommon but associated with increased hospital mortality. Conversely, children with pSOFA scores of less than 2 were at very low risk of death, with high specificity and negative predictive value.
Meaning
In this study, pediatric patients with increasing pSOFA scores had increased risk of death; however, pSOFA is not adequately sensitive to function as a screening tool in the emergency department.
Abstract
Importance
Pediatric sepsis definitions have evolved, and some have proposed using the measure used in adults to quantify organ dysfunction, a Sequential Organ Failure Assessment (SOFA) score of 2 or more in the setting of suspected infection. A pediatric adaptation of SOFA (pSOFA) showed excellent discrimination for mortality in critically ill children but has not been evaluated in an emergency department (ED) population.
Objective
To delineate test characteristics of the pSOFA score for predicting in-hospital mortality among (1) all patients and (2) patients with suspected infection treated in pediatric EDs.
Design, Setting, and Participants
This retrospective cohort study took place from January 1, 2012, to January 31, 2020 in 9 US children’s hospitals included in the Pediatric Emergency Care Applied Research Network (PECARN) Registry. The data was analyzed from February 1, 2020, to April 18, 2022. All ED visits for patients younger than 18 years were included.
Exposures
ED pSOFA score was assigned by summing maximum pSOFA organ dysfunction components during ED stay (each 0-4 points). In the subset with suspected infection, visit meeting criteria for sepsis (suspected infection with a pSOFA score of 2 or more) and septic shock (suspected infection with vasoactive infusion and serum lactate level >18.0 mg/dL) were identified.
Main Outcomes and Measures
Test characteristics of pSOFA scores of 2 or more during the ED stay for hospital mortality.
Results
A total of 3 999 528 (female, 47.3%) ED visits were included. pSOFA scores ranged from 0 to 16, with 126 250 visits (3.2%) having a pSOFA score of 2 or more. pSOFA scores of 2 or more had sensitivity of 0.65 (95% CI, 0.62-0.67) and specificity of 0.97 (95% CI, 0.97-0.97), with negative predictive value of 1.0 (95% CI, 1.00-1.00) in predicting hospital mortality. Of 642 868 patients with suspected infection (16.1%), 42 992 (6.7%) met criteria for sepsis, and 374 (0.1%) met criteria for septic shock. Hospital mortality rates for suspected infection (599 502), sepsis (42 992), and septic shock (374) were 0.0%, 0.9%, and 8.0%, respectively. The pSOFA score had similar discrimination for hospital mortality in all ED visits (area under receiver operating characteristic curve, 0.81; 95% CI, 0.79-0.82) and the subset with suspected infection (area under receiver operating characteristic curve, 0.82; 95% CI, 0.80-0.84).
Conclusions and Relevance
In a large, multicenter study of pediatric ED visits, a pSOFA score of 2 or more was uncommon and associated with increased hospital mortality yet had poor sensitivity as a screening tool for hospital mortality. Conversely, children with a pSOFA score of 2 or less were at very low risk of death, with high specificity and negative predictive value. Among patients with suspected infection, patients with pSOFA-defined septic shock demonstrated the highest mortality.
Introduction
Sepsis is a major cause of morbidity and mortality in children, resulting in more than 7000 annual pediatric deaths in the US and resulting in more than $7 billion in annual health care costs.1,2 The early detection of sepsis can prevent morbidity and mortality through fluid resuscitation and timely antibiotics.3,4,5,6 The definition of sepsis has evolved over time and now many advocate defining sepsis by organ dysfunction rather than by a systemic inflammatory response. Specifically, the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) stresses that sepsis is a function of 4 variables, namely, (1) threat to life, (2) organ dysfunction, (3) dysregulated host response, and (4) presence of highly suspected or documented infection.7,8,9,10
The Sequential Organ Failure Assessment (SOFA) score is the basis of organ dysfunction determination in the Sepsis-3.7,8,9,10 Age-adjusted SOFA has been assessed in critically ill children in Australia and New Zealand,11 and the SOFA score was recently adapted and validated for pediatric patients (pSOFA) and tested in a population of critically ill children in a single US academic center.12 To our knowledge, the performance of the pSOFA score has not been evaluated in a more general population of children in an emergency department (ED) and the Sepsis-3 criteria have not yet been applied to children prior to intensive care unit (ICU) admission.
Most children at risk for sepsis in the US are initially evaluated in the ED. In this setting, many pediatric patients present with fever and undifferentiated mild illness, making it difficult to detect the rare child with sepsis. A score that has demonstrated promise in risk stratification for children in the ICU may play a role in early prognostication outside of the ICU setting. As pediatrics moves toward consideration of new sepsis definitions,13 it is important to assess the pediatric adaptation of adult Sepsis-3 definitions in the broader population of children seeking care in the ED. Given the limited physiologic information available in the ED relative to the ICU, it may be necessary to modify ICU scores for ED use to provide adequate diagnostic discrimination early in patients’ care.
We aimed to measure the test characteristics of a pSOFA score of 2 or more in predicting hospital mortality in a multicenter cohort of pediatric ED patients using a comprehensive all-visit ED electronic health record (EHR) registry. In addition, we evaluated mortality rates in pediatric ED patients with suspected infection, sepsis, and septic shock defined using pSOFA-based organ dysfunction criteria.
Methods
Patients and Data Collection
We performed a multicenter, retrospective cohort study using the Pediatric Emergency Care Applied Research Network Registry, an all-visit comprehensive data warehouse with data automatically extracted monthly from the EHR at each site, transformed to common data elements, and loaded into a centralized registry, as previously described.14 Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines were used. Four academic children’s hospitals with 3 satellite EDs contributed data for visits from January 1, 2012, to January 31, 2020; 2 additional academic children’s hospitals contributed data from January 1, 2016, to January 31, 2020. All ED visits by patients younger than 18 years were eligible for inclusion. We excluded visits with an ED disposition of left without being seen by a physician, nurse practitioner, or physician assistant, left against medical advice, transferred, or missing. In the primary analysis, we excluded visits during which the patient died in the ED, as our primary outcome of in-hospital mortality would be precluded by ED death. However, we included a secondary analysis in which ED deaths were included. Complex chronic conditions were defined by International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10), as previously described.15 We defined the subset of patients with suspected infection as those who had an EHR order placed during the ED visit for any bacterial, viral, or fungal testing, or for a chest radiograph (list in eTable 1 in Supplement 1). The study was approved by the institutional review boards of all study sites and the data coordinating center with a waiver of informed consent.
pSOFA Score
We calculated pSOFA, as previously described, being the sum of 6 component organ system scores (respiratory, coagulation, hepatic, cardiovascular, neurologic, kidney), each of which range from 0 to 4 (eTable 2 in Supplement 1).4,5,6 We adopted a previously described modification to the Pao2/Fio2 ratio, which uses the Spo 2 oxygen saturation (Spo2) to Fio2 ratio, since arterial blood gas measurements are rarely obtained in pediatric EDs.12 Of note, we considered supplemental oxygen by face mask or nasal cannula to be equivalent to room air (Spo2, 21%). We modified the cardiovascular subscore to account for vasoactive orders with missing or unclear dosing data as follows: epinephrine and norepinephrine infusion administrations with missing dose data were assigned a cardiovascular score of 3; dopamine and dobutamine with missing dose data were assigned a cardiovascular score of 2; and vasoactive infusions with dosing data available were used as previously published. We considered any missing values to be normal, and assigned zero points for that category.10,12 We determined the maximal pSOFA score for each ED visit, using previously described methods,10,12 assigning each subscore the worst (highest) value present during the entirety of the ED visit (ranging from 0-4 points). The sum of the subscores resulted in a pSOFA score (ranging from 0-24 points, with higher scores indicating a worse outcome).
Assessment of Sepsis-3 Definitions
We followed adult Sepsis-3 conventions,7 defining sepsis as patients with suspected infection and a pSOFA score of 2 or more, and septic shock as patients with sepsis plus need for vasoactive medication and an elevated serum lactate level of 18.0 mg/dL or more (to convert to universal units, multiply by 0.111). In addition, we conducted a sensitivity analysis with an alternate septic shock definition that did not require elevated lactate, because we expected it to be measured less often in the pediatric ED population, potentially rendering the original adult criteria less useful.
Outcomes
The primary outcome was all-cause, in-hospital mortality. Secondary outcomes included in-hospital mortality rates in patients with suspected infection, sepsis, and septic shock.
Statistical Analysis
We examined associations between categorical and continuous or ordinal variables using the Kruskal-Wallis test. We used χ2 tests to examine the association between categorical variables. We used the Cochran-Armitage trend test to evaluate the trend of increasing mortality rate with increasing pSOFA score. The level of significance was P<.05. We used a 2-tailed P-value for the Cochran-Armitage test, and the Kruskal-Wallis and χ2 tests were 1-tailed. We assessed the ability of pSOFA scores of 2 or more to predict in-hospital death using sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio, negative likelihood ratio, and the area under the receiver operating characteristic curve (AUC). For each of these statistics, we computed cluster-robust 95% CIs with the sandwich standard errors used in generalized estimating equations to account for within-patient clustering.16 We conducted a secondary analysis considering the outcome of in-hospital death at 48 hours and 1 week after ED admission in the suspected infection subpopulation. CIs and point estimates for AUCs were examined to test for differences in the pSOFA’s ability to predict deaths within 48 hours and 1 week of ED admission compared with later deaths.
Results
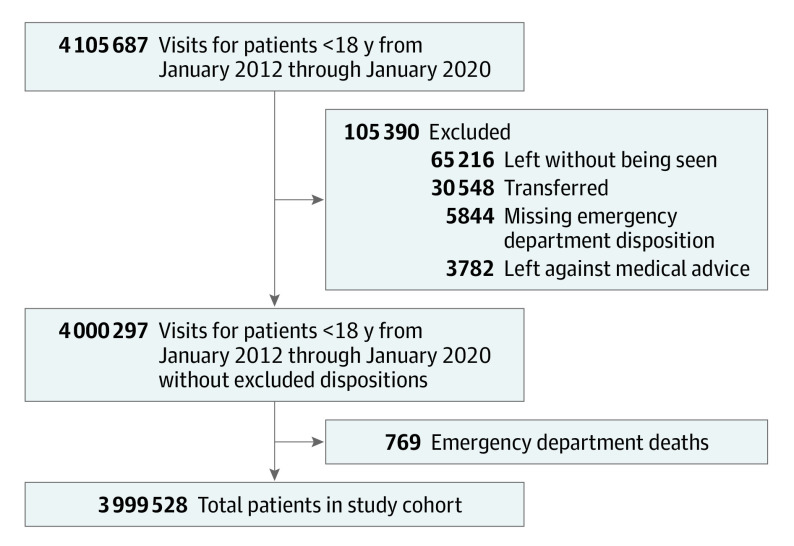
There were 3 999 528 ED visits (female, 47.3%) meeting inclusion criteria during the study period, and 1114 in-hospital deaths (0.03%). The STARD diagram detailing the formation of the study cohort is shown in Figure 1. The median (IQR) ED length of stay was 2.7 (1.7-4.1) hours. The median (IQR) age was 4.9 (1.7-10.4) years in survivors and 4.2 (0.8-11.6) years in nonsurvivors. Compared with survivors, a higher proportion of nonsurvivors had an underlying complex chronic condition (929 of 1114 [84.1%] vs 256 647 of 3 998 414 [6.5%]; P < .001) (Table 1).
Figure 1. STARD Diagram for Study Cohort.
Table 1. Characteristics of Survivors vs Nonsurvivors.
| Variable | No. (%) | P value | |
|---|---|---|---|
| Survivors | Non-survivors | ||
| No. | 3 998 414 | 1114 | NA |
| Age, median (IQR), y | 4.9 (1.7-10.4) | 4.2 (0.8-11.6) | <.001a |
| Sex | |||
| Female | 1 890 363 (47.3) | 514 (46.1) | .45b |
| Male | 2 108 051 (52.7) | 600 (53.9) | .45 |
| Race and ethnicityc | |||
| Non-Hispanic | <.001b | ||
| Black | 1 602 247 (40.8) | 320 (29.9) | |
| White | 1 184 438 (30.1) | 422 (39.5) | |
| Hispanic | 813 973 (20.7) | 190 (17.8) | |
| Otherd | 328 369 (8.4) | 137 (12.8) | |
| Presence of a complex chronic condition | 256 647 (6.5) | 929 (84.1) | <.001b |
| In ED | |||
| Mechanical ventilation | 12 028 (0.3) | 686 (61.6) | <.001b |
| Vasoactive infusion | 1038 (0.03) | 143 (12.8) | <.001b |
| Highest pSOFA score during ED visit, median (IQR) | 0 (0-0) | 3.0 (0-5.0) | <.001a |
| pSOFA score ≥2 during ED visit | 125 530 (3.1) | 720 (64.6) | <.001b |
| Hospital LOS, median (IQR), h | 40.0 (19.5-84.2) | 106.8 (36.4-337.6) | <.001a |
Abbreviations: ED, emergency department; LOS, length of stay; NA, not applicable; pSOFA, Pediatric Sequential Organ Failure Assessment Score.
χ2.
Kruskal-Wallis test.
Practice at each site was to record self-reported race and ethnicity in the electronic health record.
Other category includes American Indian/Eskimo/Alaska native, Asian, Indian, Native Hawaiian/Pacific Islander, multiple races, patient declined, missing, and other.
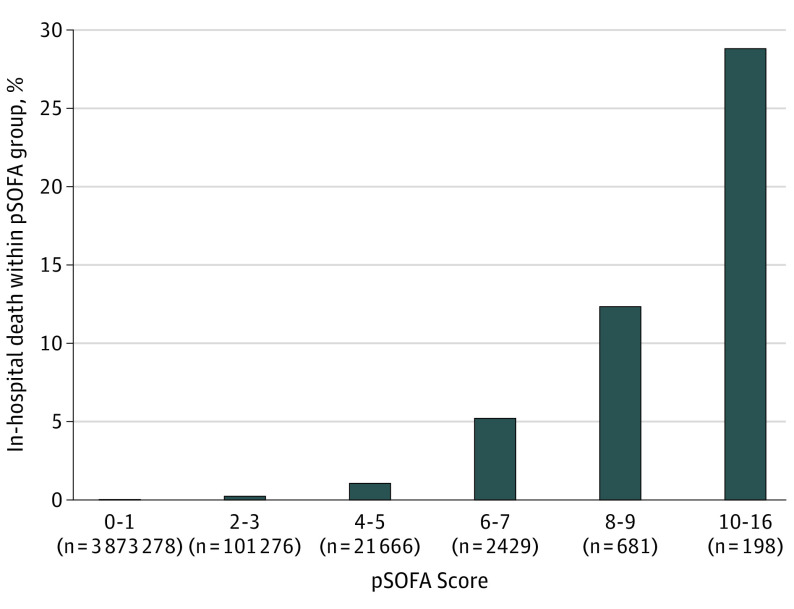
Of all ED visits, 3 681 858 (92.1%) had a maximum pSOFA score of 0, 191 420 (4.8%) had a pSOFA score of 1, and 126 250 (3.2%) had a pSOFA score of 2 or more. A very small proportion (0.003%) had a pSOFA score more than 10, with a maximum score of 16. The contribution of each organ system to pSOFA points for patients with a pSOFA score of 2 or more is shown in eTable 3 in Supplement 1. The missingness of values contributing to pSOFA scores is shown eTable 4 in Supplement 1. Nonsurvivors had a higher median (IQR) maximum pSOFA score during the ED stay compared with survivors (3.0 [IQR, 0-5.0] vs 0 [IQR, 0-0]; P < .001). The in-hospital mortality rate increased with increasing pSOFA score (Figure 2). The AUC for pSOFA scores to discriminate in-hospital mortality from survival across all ED visits was 0.81 (95% CI, 0.79-0.82). A cut point of pSOFA scores of 2 or more yielded sensitivity of 0.65 (95% CI, 0.62-0.67), specificity of 0.97 (95% CI, 0.97-0.97), PPV 0.006 (95% CI, 0.005-0.006), and NPV 1.00 (95% CI, 1.00-1.00) (Table 2). Test characteristics for each cut point of pSOFA scores and the AUCs are shown in eTable 5 in Supplement 1. Secondary analyses where ED deaths were included are shown in eTable 6 in Supplement 1. pSOFA scores of 2 or higher in this population had lower sensitivity of 0.45 (0.43-0.47) and similar specificity of 0.97 (0.97-0.97), PPV of 0.007 (0.006-0.007), and NPV of 1.00 (1.00-1.00) to the main analyses.
Figure 2. Proportion of In-Hospital Death Within Each Group of Pediatric Sequential Organ Failure Assessment (pSOFA) Scores.
Table 2. Test Characteristics of Pediatric Sequential Organ Failure Assessment Score of 2 or More as Predictor of In-Hospital Mortality for All Emergency Department Patients.
| Test characteristic | Estimate (95% CI) |
|---|---|
| Sensitivity | 0.65 (0.62-0.67) |
| Specificity | 0.97 (0.97-0.97) |
| Predictive value | |
| Positive | 0.006 (0.005-0.006) |
| Negative | 1.00 (1.00-1.00) |
| Likelihood ratio | |
| Positive | 20.59 (19.68-21.51) |
| Negative | 0.37 (0.34-0.39) |
| AUC | 0.81 (0.79-0.82) |
Abbreviation: AUC, area under the receiver operating characteristic curve.
There were 642 868 ED visits (16.1%) that met criteria for suspected infection. Of these, 42 992 (6.7%) met criteria for sepsis (pSOFA ≥2), and 374 (0.1%) met criteria for septic shock (pSOFA ≥2; plus vasoactive medication and lactate >18.0 mg/dL) in the ED. Patients with sepsis and septic shock were more likely to have an underlying complex chronic condition than those without sepsis (sepsis, 23 339 of 42 992 [55.2%]; septic shock, 265 of 374 [72.8%]; suspected infection alone, 68 228 of 599 502 [11.5%]) (P < .001). The proportion of visits with each type of complex chronic condition is shown in eTable 8 in Supplement 1. There were 162 (<0.1%) in-hospital deaths among children with suspected infection, 369 (0.9%) in those with sepsis, and 30 (8.0%) in those with septic shock (P < .001) (Table 3). The pSOFA score had similar discrimination for in-hospital death in the subset with suspected infection (AUC, 0.82; 95% CI, 0.80-0.84) as in the entire cohort. At a cut point of pSOFA scores of 2 or more in the subset with suspected infection, sensitivity was 0.71 (95% CI, 0.67-0.75), specificity was 0.93 (95% CI, 0.93-0.93), PPV was 0.009 (95% CI, 0.008-0.01), and NPV was 1.00 (95% CI, 1.0-1.00) for hospital mortality (eTable 9 in Supplement 1). Of the patients with in-hospital death, 399 (35.8%) met criteria for sepsis or septic shock during their ED stay.
Table 3. Assessment of Sepsis-3 Definitions in Children in the Emergency Departmenta.
| Variable | No. (%) | P value | ||
|---|---|---|---|---|
| Suspected infection | Sepsis | Septic shock | ||
| No. | 599 502 | 42 992 | 374 | NA |
| Age, median (IQR), y | 5.6 (2.0-10.3) | 3.6 (0.9-9.7) | 8.8 (3.9-13.9) | <.001b |
| Female | 342 329 (57.1) | 19 624 (45.6) | 167 (44.7) | <.001c |
| Male | 257 173 (42.9) | 23 368 (54.4) | 207 (55.3) | <.001 |
| Complex chronic condition | 68 228 (11.5) | 23 339 (55.2) | 265 (72.8) | <.001c |
| Mechanical ventilation | 3206 (0.5) | 3690 (8.6) | 143 (38.2) | <.001c |
| Vasoactive infusion | 2 (0) | 521 (1.2) | 374 (100) | <.001c |
| Outcomes | ||||
| In-hospital death | 162 (0.03) | 369 (0.9) | 30 (8.0) | <.001 |
| Hospital LOS, median (IQR), h | 44.4 (24.3-85.8) | 79.6 (43.1-163.5) | 167.6 (93.1-291.0) | <.001 |
Abbreviations: LOS, length of stay; NA, not applicable.
Suspected infection is defined as any infectious testing sent in the emergency department. Sepsis is suspected infection plus a pediatric Sequential Organ Failure Assessment Score of 2 or more. Septic shock is sepsis with vasoactive medication and lactate level >2.0 mg/dL.
Kruskal-Wallis test.
χ2.
Given the high proportion of missing data for ED serum lactate (missing in 580 318 of 599 502 [96.8%] in the suspected infection subgroup), we conducted a sensitivity analysis for the septic shock population exclusive of the lactate component of the definition. Using this modified definition, 523 patients (0.08%) had septic shock with 42 deaths (8.0%) (eTable 7 in Supplement 1).
To determine if the test characteristics of pSOFA scores improved when we considered only deaths that occurred early in the hospitalization, we conducted a sensitivity analysis considering the outcome of death at 48 hours and 1 week after admission in the suspected infection subpopulation. pSOFA score discrimination was slightly better for death at these earlier time points compared with death at any point during the hospitalization, with AUCs ranging from 0.84 (95% CI, 0.82-0.87) to 0.85 (95% CI, 0.81-0.88), but this was not statistically significantly different from our primary analyses. Details of this analysis are shown in eTable 9 in Supplement 1.
Discussion
To our knowledge, we report here the first multicenter assessment and validation of the pSOFA score for children outside of the ICU setting, using electronic health data from a centralized database to determine pSOFA score and evaluate Sepsis-3 criteria for suspected infection, sepsis, and septic shock. We found that increasing ED pSOFA scores are associated with increasing in-hospital mortality, and that ED patients identified with suspected infection, sepsis, and septic shock have sequentially increasing rates of in-hospital mortality.
In an undifferentiated pediatric ED population, pSOFA scores of 2 or more had good discrimination for in-hospital mortality, with an AUC curve of 0.81 (95% CI, 0.79-0.82), and similar discrimination in the subset of ED patients with suspected infection, defined as children who had any infectious testing during their ED stay. In the suspected infection subset, 6.7% of children had sepsis as defined by Sepsis-3, and 0.1% had septic shock.
The acuity spectrum of disease in our ED population was strikingly different from previously published ICU populations. More than 90% of patients had a pSOFA score of 0, compared with an ICU-based study11 where only 12% had a score of 0. In addition, death following hospitalization in our study population of children seeking care at an ED was rare, with a prevalence in this cohort of 0.03%, in contrast to mortality in a published pediatric ICU cohort of 2.6%.12 Mortality rate in visits identified as sepsis was 0.9% and in visits with septic shock was 8.0%, contrasting with published pediatric ICU mortality rates in these populations of 12% and 32%, respectively, and is consistent with published overall hospital mortality estimates of 9%.12 Although we do not have data on which deaths in the suspected infection population were sepsis attributable, we do know that deaths in this subgroup occurred subsequent to infectious testing being performed. Despite these population differences, increasing mortality with increasing pSOFA scores and sequential definitions of sepsis-related illness demonstrate face validity of pSOFA scores in an ED population.
Interestingly, a pSOFA score of 2 or more had limited sensitivity in predicting in-hospital mortality risk in both the whole ED population (65%) and in the suspected infection subset (71%). We performed a sensitivity analysis to evaluate whether the performance of the score would improve when only deaths early in the hospitalization were considered in the outcome, hypothesizing that an ED-based pSOFA score would be more likely to be associated with early events. The sensitivity of pSOFA scores of 2 or more was 75% (95% CI, 0.70-0.80) to 76% (95% CI, 0.68-0.82) in these earlier time frames, an increase that was not statistically significantly different from that of any hospital death. Therefore, deaths remote in time from the ED visit do not fully account for the low sensitivity of ED-based pSOFA scores to predict hospital death; thus a significant proportion of patients who had in-hospital death did not have organ dysfunction in the ED as defined by pSOFA. One possible explanation for this is that laboratory tests defining organ dysfunction in the pSOFA scores were not sent during the ED time frame and were therefore missed. Alternatively, the sickest patients may be transferred out of the ED faster, such that their organ dysfunction is not captured prior to ICU admission. Although we did not cut the ED visit data at a specific time point, it is notable that the 75th percentile for ED length of stay was 4.1 hours. A third potential explanation may be that pSOFA measures of organ dysfunction are not present in the first hours of emergency care, underscoring the need for further research to identify early ED sepsis predictors and risk factors for in-hospital death. The most common contributor to pSOFA score points in this population was for respiratory dysfunction, and of note, we considered supplemental oxygen via face mask or nasal cannula as Spo2 of 21%, so children receiving oxygen in this manner would not receive pSOFA score points for respiratory dysfunction.
One potential use for pSOFA scores in an ED population would be as an overall severity of illness score, identifying children at risk of prolonged hospital stay or death. In a pediatric ICU population, pSOFA had excellent discrimination for in-hospital mortality and it performed as well or better than other illness severity scores, such as pediatric logistic organ dysfunction, pediatric logistic organ dysfunction 2, and pediatric multiple organ dysfunction score.12 Although these severity of illness scores are commonly used in the pediatric ICU, there have been few attempts to develop risk stratification scores in the pediatric ED.17 The Pediatric Risk of Hospital Admission score was derived and validated to identify children at high risk of requiring hospital admission.18 However, this score did not evaluate risk of severe long-term clinical outcomes, such as mortality. Others have evaluated the performance of Pediatric Early Warning Score in children with intended hospital admission from the ED,19,20 but this did not include patients who were discharged.
Our study was intended to assess the utility of the pSOFA score in the ED, where most children receive no laboratory testing based on clinical assessment. In this context, it is only the more ill children who have laboratory results that drive higher pSOFA scores. The lack of tests are an important indication of clinical state. Attempting to impute the missing laboratory results would be a major deviation from actual ED care for children, be dependent on tests from children that were importantly clinically different, and would not accurately reflect how pSOFA scores would be applied in an ED setting. Treating missing laboratory values as normal for sepsis severity scores is a clinically based precedent that has been applied in prior applications of SOFA, both in Sepsis-3 and in the initial article describing the derivation of pSOFA.7,8,12
While the AUC of the pSOFA score of 2 or more for hospital mortality may indicate promising utility of this tool to identify the highest-risk patients at the conclusion of ED care, it is important to note that some factors that contribute to the pSOFA score are actions taken by ED clinicians, such as being treated with mechanical ventilation or vasopressors. Because these patients’ pSOFA scores are contingent on critical illness treatments initiated by emergency physicians, and are also contingent on laboratory testing that is often not indicated in the ED setting, this score does not solve the important clinical dilemma of identifying, and potentially preventing, critical illness before it occurs. Our study does suggest that a tool derived specifically for ED use that uses variables known in the ED may be necessary. In that vein, work by several groups including our own is ongoing to develop an ED-specific score using machine learning and artificial intelligence methods.21,22,23
Limitations
This study has several limitations. First, we were unable to trend pSOFA scores over time. Second, because our operational definition of suspected infection was based on sending laboratory testing in the ED, it is possible that children with infections that did not require testing or those not suspected by ED clinicians could have been missed by this definition, thus potentially underestimating the prevalence of sepsis. Conversely, patients who were critically ill and rapidly transported to the ICU may have had missing laboratory tests defining organ dysfunction, as discussed above. However, in sensitivity analyses with and without lactate as a component of septic shock, hospital mortality was similar. Additionally, these data were derived largely from academic medical centers and their associated satellite EDs, so generalizability outside of this population may be limited.
Conclusions
In conclusion, we have performed a large, multicenter evaluation of the pSOFA score in the ED setting demonstrating increased risk of in-hospital death with increasing pSOFA values, and identifying a population of children with sepsis and septic shock using Sepsis-3 definitions that have associated increased risk of in-hospital mortality and prolonged hospital stays. Our findings, however, also indicate that pSOFA is not adequately sensitive to function as a screening tool in the ED. Future efforts will incorporate inpatient data to evaluate the predictive ability of serial pSOFA values. Additional work is also needed to fully allow for discrimination and prediction of sepsis in pediatric ED patients.
eTable 1. List of laboratory testing used to define suspected infection
eTable 2. Adapted definitions of pSOFA elements based on emergency department data
eTable 3. Contributions to pSOFA points by element
eTable 4. Presence of each pSOFA element in survivors and non survivors
eTable 5. Area under the curve analysis for each pSOFA cutpoint truncated at 13
eTable 6. Test characteristics of pSOFA ≥2 as predictor of in-hospital mortality for all ED patients when ED deaths are included
eTable 7. Sensitivity analysis where septic shock is defined as suspected infection + pSOFA ≥2 + vasoactive medication, removing the requirement for lactate testing.
eTable 8. Distribution of CCC in study population. Visits may have more than one CCC
eTable 9. a. Timing of death in suspected infection cohort, b. test characteristics of pSOFA ≥2 as predictor of in-hospital mortality among patients with suspected infection
Nonauthor contributors
References
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med. 2013;14(7):686-693. doi: 10.1097/PCC.0b013e3182917fad [DOI] [PubMed] [Google Scholar]
- 2.Carlton EF, Barbaro RP, Iwashyna TJ, Prescott HC. Cost of pediatric severe sepsis hospitalizations. JAMA Pediatr. 2019;173(10):986-987. doi: 10.1001/jamapediatrics.2019.2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balamuth F, Weiss SL, Fitzgerald JC, et al. Protocolized treatment is associated with decreased organ dysfunction in pediatric severe sepsis. Pediatr Crit Care Med. 2016;17(9):817-822. doi: 10.1097/PCC.0000000000000858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42(11):2409-2417. doi: 10.1097/CCM.0000000000000509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul R, Melendez E, Stack A, Capraro A, Monuteaux M, Neuman MI. Improving adherence to PALS septic shock guidelines. Pediatrics. 2014;133(5):e1358-e1366. doi: 10.1542/peds.2013-3871 [DOI] [PubMed] [Google Scholar]
- 6.Cruz AT, Perry AM, Williams EA, Graf JM, Wuestner ER, Patel B. Implementation of goal-directed therapy for children with suspected sepsis in the emergency department. Pediatrics. 2011;127(3):e758-e766. doi: 10.1542/peds.2010-2895 [DOI] [PubMed] [Google Scholar]
- 7.Singer M, Deutschman CS, Seymour C, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774. doi: 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deutschman CS. Sepsis-3:of love and bliss. Crit Care Med. 2017;45(4):739-740. doi: 10.1097/CCM.0000000000002248 [DOI] [PubMed] [Google Scholar]
- 10.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 11.Schlapbach LJ, Straney L, Bellomo R, MacLaren G, Pilcher D. Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and pSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med. 2018;44(2):179-188. doi: 10.1007/s00134-017-5021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171(10):e172352. doi: 10.1001/jamapediatrics.2017.2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlapbach LJ, Kissoon N. Defining pediatric sepsis. JAMA Pediatr. 2018;172(4):312-314. doi: 10.1001/jamapediatrics.2017.5208 [DOI] [PubMed] [Google Scholar]
- 14.Deakyne Davies SJ, Grundmeier RW, Campos DA, et al. ; Pediatric Emergency Care Applied Research Network . The Pediatric Emergency Care Applied Research Network Registry: a multicenter electronic health record registry of pediatric emergency care. Appl Clin Inform. 2018;9(2):366-376. doi: 10.1055/s-0038-1651496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ying G-S, Maguire MG, Glynn RJ, Rosner B. Calculating sensitivity, specificity, and predictive values for correlated eye data. Invest Ophthalmol Vis Sci. 2020;61(11):29. doi: 10.1167/iovs.61.11.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiger N, Maconochie I, Oostenbrink R, Moll HA. Validity of different pediatric early warning scores in the emergency department. Pediatrics. 2013;132(4):e841-e850. doi: 10.1542/peds.2012-3594 [DOI] [PubMed] [Google Scholar]
- 18.Chamberlain JM, Patel KM, Pollack MM. The Pediatric Risk of Hospital Admission score: a second-generation severity-of-illness score for pediatric emergency patients. Pediatrics. 2005;115(2):388-395. doi: 10.1542/peds.2004-0586 [DOI] [PubMed] [Google Scholar]
- 19.Gold DL, Mihalov LK, Cohen DM. Evaluating the Pediatric Early Warning Score (PEWS) system for admitted patients in the pediatric emergency department. Acad Emerg Med. 2014;21(11):1249-1256. doi: 10.1111/acem.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breslin K, Marx J, Hoffman H, McBeth R, Pavuluri P. Pediatric early warning score at time of emergency department disposition is associated with level of care. Pediatr Emerg Care. 2014;30(2):97-103. doi: 10.1097/PEC.0000000000000063 [DOI] [PubMed] [Google Scholar]
- 21.Scott HF, Colborn KL, Sevick CJ, et al. Development and validation of a predictive model of the risk of pediatric septic shock using data known at the time of hospital arrival. J Pediatr. 2020;217:145-151.e6. doi: 10.1016/j.jpeds.2019.09.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehwerhemuepha L, Heyming T, Marano R, et al. Development and validation of an early warning tool for sepsis and decompensation in children during emergency department triage. Sci Rep. 2021;11:8578. doi: 10.1038/s41598-021-87595-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon K, Schlapbach LJ, Akech S, et al. Pediatric sepsis definition—a systematic review protocol by the Pediatric Sepsis Definition Taskforce. Crit Care Explor. 2020;2(6):e0123. doi: 10.1097/CCE.0000000000000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. List of laboratory testing used to define suspected infection
eTable 2. Adapted definitions of pSOFA elements based on emergency department data
eTable 3. Contributions to pSOFA points by element
eTable 4. Presence of each pSOFA element in survivors and non survivors
eTable 5. Area under the curve analysis for each pSOFA cutpoint truncated at 13
eTable 6. Test characteristics of pSOFA ≥2 as predictor of in-hospital mortality for all ED patients when ED deaths are included
eTable 7. Sensitivity analysis where septic shock is defined as suspected infection + pSOFA ≥2 + vasoactive medication, removing the requirement for lactate testing.
eTable 8. Distribution of CCC in study population. Visits may have more than one CCC
eTable 9. a. Timing of death in suspected infection cohort, b. test characteristics of pSOFA ≥2 as predictor of in-hospital mortality among patients with suspected infection
Nonauthor contributors