Abstract
Epstein-Barr virus (EBV) infects 95% of adults worldwide, causes infectious mononucleosis, is etiologically linked to multiple sclerosis and is associated with 200 000 cases of cancer each year. EBV manipulates host epigenetic pathways to switch between a series of latency programs and to reactivate from latency in order to colonize the memory B-cell compartment for lifelong infection and to ultimately spread to new hosts. Here, we review recent advances in the understanding of epigenetic mechanisms that control EBV latency and lytic gene expression in EBV-transformed B and epithelial cells. We highlight newly appreciated roles of DNA methylation epigenetic machinery, host histone chaperones, the Hippo pathway, m6A RNA modification and nonsense mediated decay in control of the EBV lifecycle.
Introduction
The EBV lifecycle begins with epigenetically naïve virion. The encapsidated linear, double-stranded EBV DNA lacks methylation, histones or other epigenetic marks [1-4]. Upon delivery to target cell nuclei, nucleo-somal packaging limits expression of the ~80 lytic EBV genes. Similarly, lack of EBV genomic DNA methylation further limits activity of any leaky immediate early lytic cycle activator BZLF1 (also called Zebra or ZTA), which preferentially induces early gene targets from methylated response elements [4,5].
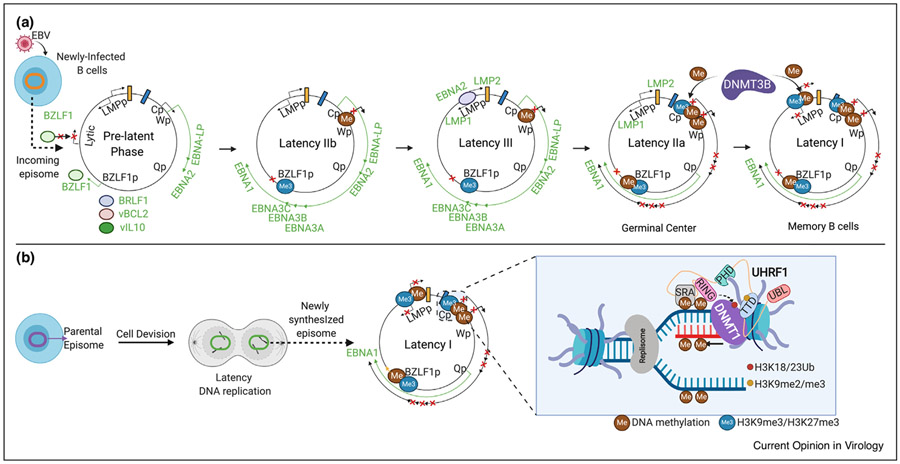
According to the germinal center model, EBV then utilizes a series of latency programs, in which different combinations of Epstein-Barr nuclear antigens (EBNA), latent membrane proteins (LMP), and non-coding RNAs (ncRNA) are expressed [6]. The EBV pre-latency program is observed in newly infected B-cells, in which the viral W promoter (Wp) drives EBNA2 and EBNA-LP expression, together with EBV-encoded BCL2 homolog BHRF1 and the viral IL-10 homolog BCRF1 (Figure 1a). EBNA2 highly induces expression of the proto-oncogene MYC and multiple other host targets [7••,8••,9,10], which together with EBNA2 serve key roles in B-cell metabolism remodeling. Recent RNAseq and proteomic analyses highlight extensive B-cell remodeling even by day 2 post-infection [7••,8••,11•].
Figure 1. Model of DNA methylation epigentic roles in EBV genome regulation.
(a) The EBV genomic W promoter (Wp) drives the prelatency program in newly infected B-cells, where low CpG methylation levels limit early gene activation by leaky BZLF1. Nonetheless, leaky expression of several lytic genes, including BRLF1, viral IL10 (vIL10/BCRF1) and viral BCL2 (vBCL2/BHRF1) transiently occurs as latency is established. Next, Cp drives the latency IIb program, comprised of six EBNAs. In Latency III, Cp drives expression of six EBNAs, and EBNA2 activates the LMPp to induce LMP1/2A expression. As infected cell enter germinal centers, LMP1/2A remain expressed in the latency IIa program, but Cp methylation suppresses expression of all EBNA except for EBNA1, whose expresion is driven by Qp. We speculate that DNMT3B, which is induced in germinal center B-cells, may initiate Cp methylation. In latency I, DNA methylation and histone polycomb epigenetic marks silence LMPp, leaving Qp-driven EBNA1 as the only EBV-encoded protein expressed. (b) EBV genomic CpG DNA methylation marks are propagated in latency I by UHRF1 and DNMT1. The UHRF1 SRA domain reads hemimethylated CpG marks, stimulating its RING domain to write H3K18/23 ubiquitin marks that recruit and activate DNMT1.
Epigenetic mechanisms then switch the EBV program to Latency IIb, where the viral C promoter (Cp) drives expression of six EBNAs to support Burkitt-like hyper-proliferation [6]. Latency IIb is observed in certain HIV-associated diffuse large B-cell lymphomas [7••,8••,12]. EBNA2 is particularly important for EBV-driven B-cell transformation over the first week of B-cell transformation in vitro [13••]. Yet in humanized mouse models, EBNA2-deleted EBV could still cause lymphomas in humanized mice, suggesting additional microenvironment roles in B-cell transformation [14••].
EBNA2 transactivates the LMP promoters (LMPp) to induce the EBV latency III program, comprised of six EBNA, two LMP and ncRNA (Figure 1a). LMP1 and LMP2A mimic aspects of CD40 and B-cell receptor signaling, respectively [6,15••]. As B-cells enter germinal centers, epigenetic mechanisms switch the EBV program to latency IIa, comprised of EBNA1, LMP1 and LMP2A and non-coding RNA. Latency IIa is observed in EBV+ Hodgkin lymphoma Reed-Sternberg tumor cells [6,15••]. Upon reaching the memory B-cell compartment, epigenetic factors further restrict EBV genome expression in the latency I program, where EBNA1 is the only EBV-encoded protein expressed and is driven by the Q promoter (Qp) (Figure 1a) [16]. Burkitt lymphoma exploits latency I to evade anti-EBV responses [6].
Here, we review recent advances in understanding epigenetic contributions to the EBV lifecycle.
DNA methylation suppresses latency III oncoprotein expression in Burkitt cells
A CpG hypermethylation phenotype (CIMP) is observed in EBV+ cancers with restricted forms of latency (latency IIa or I). EBV+ gastric carcinoma is the human tumor with the highest level of host genomic CpG methylation [17]. EBV infection of telomerase-immortalized oral keratinocytes also causes CIMP, with generation of >13 000 CpG methylated host genome sites, a number of which persist even if the viral genome is lost [18]. EBV genomes also exhibit CIMP in these tumors, the presence of which is being harnessed in novel diagnostic approaches for EBV+ malignancies [19•].
Accumulating evidence suggests that CpG methylation represses EBV latent and lytic gene expression in latency I. Treatment with the hypomethylation agent 5-azaciti-dine diminishes Cp methylation and upregulates latency III and lytic antigens in vitro [18,20-22]. A low dose of the hypomethylation agent decitabine can even sensitize Burkitt cells to killing by HLA-matched CD8+cytotoxic T lymphocytes (CTL) specific for EBNA3A, EBNA3C or LMP1 epitopes [23••]. In vivo, low dose decitabine hypo-methylates Cp and LMPp to induce sustained latency III antigen expression in a Burkitt xenograft model [23••], where adoptively transferred EBNA3C-specific T-cells can then infiltrate the tumor [23••]. This xenograft result is notable, given considerable interest in the use of latency III epitope-specific, HLA-matchedcytotoxic T-cells to treat post-transplant lymphoproliferative disease, including off-the-shelf banks of third party T-cells [24,25].
EBV episomes are copied by host machinery and must be epigenetically reprogrammed with each cell cycle to maintain EBV latency I. CRISPR/Cas9 analysis highlighted the host enzymes URHF1 and DNMT1 as necessary for the propagation of Cp, LMPp and BZLF1 promoter methylation marks and for latency I [26••]. Mechanistically, UHRF1 has multiple reader and writer domains, including a tandem tudor domain that reads repressive histone H3 di/tri-methylated lysine 9 (H3K9me2/me3) marks present at heterochromatin sites, a SRA domain that reads hemi-methylated DNA and a RING domain (Figure 1b). When UHRF1 recognizes the combination of H3K9me2/me3 and hemi-methylated DNA marks, the RING domain then deposits monoubiquitin marks on histone 3 lysines 18 and 23 (K18/K23). These serve to recruit and activate DNMT1, which then copies epigenetic methylation marks from the parent to the daughter (newly copied) DNA strand. All UHRF1 epigentic reader and writer domains are necessary for latency III silencing [26••], suggesting that both histone and DNA methylation are required for maintenance of latency I in Burkitt cells.
EBV induces UHRF1 and DNMT1 expression upon B-cell infection [26••], raising the question of how cells can then progress to latency III with lower DNA methylation levels. Notably, EBNA2, RBP-Jκ, and EBF1 upregulate ten-eleven translocation 2 (TET2), a tumor suppressor commonly altered in hematological malignancies that oxidizes 5-methylcytosine (5mC) to 5-hydroxymethylcy-tosine (5hmC) [27,28]. EBNA2, EBF1, RBP-Jκ and TET2 co-occupy multiple genomic regions in EBV-transformed lymphoblastoid cell lines (LCL) [27,28]. TET2 knockdown diminishes LCL 5hmC, increases 5mC levels, which results in the repression of latency III and the de-repression of particular lytic genes [27,28]. Interestingly, 5hmC marks accumulate during epithelial cell differentiation and increase immediate-early gene RTA-meidated target gene activitation [29]. By contrast, EBV+ nasopharyngeal carcinoma have low levels of 5hmC-modified DNA, which may therefore serve to repress early gene expression, given that RTA preferentially acts at 5hmC sites [29]. Likely by epigenetic mechanisms, latent EBV infection also restrains differentiation-induced pathways in normal oral keratinocytes, even in the absence of lytic gene induction [30•].
How then might restricted forms of EBV latency arise in vivo? It is notable that ~30% of the DNA methylome is modified during human B-cell development [31]. Whereas TET2 levels diminish in germinal center B-cells [27,28,32], key DNA methylation writers are instead induced as B-cells enter germinal centers [26••]. These include the de novo methyltransferase DNMT3B, which is a major initiator of 5mc marks, UHRF1 and DNMT1. Although EBV strongly induces the initiator methyltransferase DNMT3A in newly infected B-cells, DNMT3A levels are diminished in germinal centers [33]. Dynamic UHRF1, DNMT1 and DNMT3B expression may therefore provide a mechanism to restrict latency III as EBV+ cells enter germinal centers. We speculate that DNMT3B, which is also robustly expressed in human tonsil germinal center B-cells [33], initiates latency III silencing at Cp [26••]. In support, ectopic DNMT3B expression silences LCL latency III antigen expression and increases 5mC levels at EBV latency and lytic promoters[26••]. Cp may therefore have evolved to be targeted by DNMT3B, in order to link B-cell differentiation state to pre-specified changes in the EBV latency genome program [26••]. Notably, DNMT3B deficiency results in immunodeficiency, centromeric instability, and facial anomalies (ICF) syndrome, a component of which can be severe mononucleosis [34]. Further studies are required to identify whether altered EBV latency gene expression in ICF patient germinal center B-cells contributes to this clinical observation.
Histone chaperone roles in establishment and maintenance of EBV latency
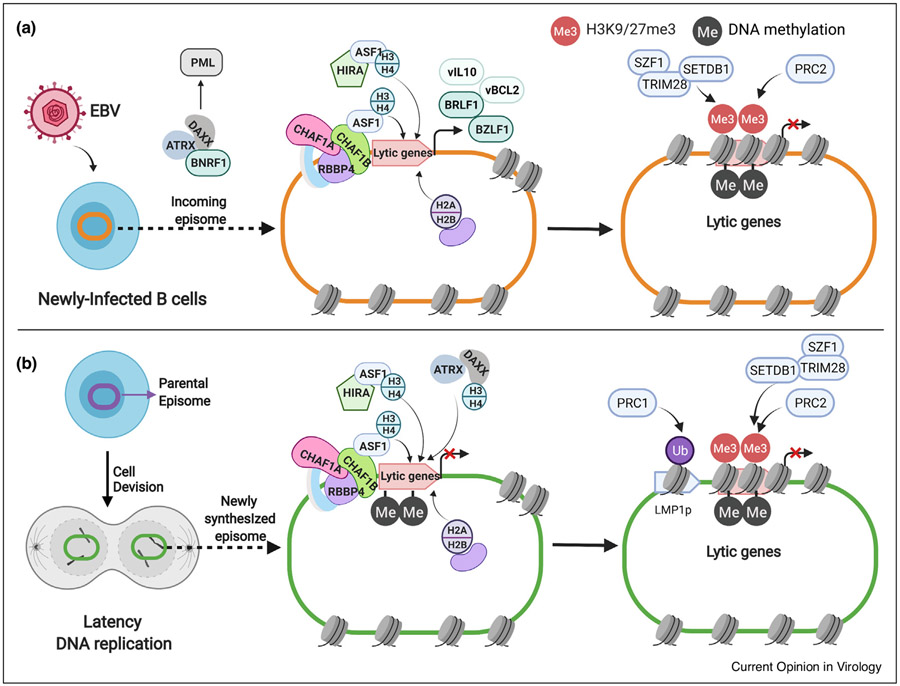
Histone octamers, comprised of two copies of histone 2A (H2A), H2B, H3 and H4, are loaded onto incoming and newly replicated EBV genomes to exert key epigenetic roles. Underscoring the importance of histone composition, the EBV major tegument protein BNRF1 subverts the ATRX/DAXX histone chaperone complex over the first 4–7 days post-infection to prevent loading of repressive H3.3 histones onto nascent EBV genomes [35]. Thus, BNRF1 subverts H3.3-induced heterochromatin formation and EBV genomic transcription silencing, thereby enabling EBV to subvert an intrinsic immune defense against foreign nuclear DNA in newly infected cells. Mechanistically, BNRF1 forms a ternary complex with DAXX, H3.3 and H4, disrupts DAXX binding to ATRX and relocalizes DAXX to promyelocytic leukemia (PML) nuclear bodies [36] (Figure 2). Interestingly, BNRF1 also induces centrosome amplification, though this does not appear to be linked to its effects on ATRX/DAXX [37].
Figure 2. Schematic model of histone loaders’ functions on EBV lytic/latency switch.
(a) In newly infected B cells, the chaperones CAF1 and HIRA load H3/H4 histones onto incoming episomes to form histone octamers together with H2A/B and thereby limit EBV lytic gene expression. This likely occurs together with the histone chaperone ASF1. Additionally, EBV tegument protein BNRF1 perturbs ATRX/DAXX complex assembly and routes DAXX to PML bodies in order to suppress loading of suppressive H3.3 histones onto nascent viral genomes. CAF1 is comprised of CHAF1A, CHAF1B and RBBP4 subunits. . Polycomb repressive complex 2 (PRC2) deposits histone trimethyl marks that further contribute to latency gene silencing. (b) In latency I, CAF1, HIRA and DAXX/ATRX have non-redundant roles in histone H3/4 loading, which are each important for maintenance of repressive histone methylation marks and latencymaintenance. Polycomb repressive complexes (PRC) have additional roles in latency gene silencing, including PRC complex 1 (PRC1) monoubiquitination of the LMP1 promoter LMP1p. Likewise, SZF1 and KAP1 promote H3K9me3 deposition. Me, DNA methylation; Me3, H3K9me3/H3K27me3. Ub, H2A lysine 119 monoubiquitin.
Recent work has highlighted additional chromatin roles in EBV lytic gene silencing [38•]. The histone chaperone chromatin assembly factor 1 (CAF1), comprised of CHAF1A/B and RBBP4 subunits, loads histones H3 and H4 onto newly synthesized or repaired DNA and is strongly EBV-upregulated in newly infected B-cells [38•] (Figure 2). CAF1 knockout induces lytic gene expression and release of infectious virion [38•,39••]. Similarly, CAF1 perturbation impairs latency establishment in newly infected Burkitt cells. Notably, the DNA synthesis-independent histone 3.3 loader HIRA also has non-redundant Burkitt cell latency maintenance roles, as does ATRX/DAXX which is not targeted by BNRF1 in latency I [38•].
The EBV-encoded BALF5 DNA polymerase produces lytic genomes in nuclear membrane-less structures termed viral replication compartments. EBV lytic genomes evade histone loading, at least in part due to exclusion of histones from replication compartments [1]. This may serve to facilitate viral DNA packaging into capsids and perhaps to evade repressive histone effects on late genes, whose expression is dependent on newly replicated lytic cycle DNA [40]. BZLF1 causes rapid and widespread host chromatin remodeling, characterized by broadly decreased inter-chromatin connections and chromatin accessibility [41••]. Such drastic remodeling may support EBV replication compartment formation, which fill 30% of nuclear volume, itself expanded by 50% [42••].
Chromosome conformation capture approaches identify that latent EBV episomes associate in Burkitt cells with host chromatin gene poor regions that have AT-rich flanking regions, EBNA1 binding sites and repressive H3K9me3 marks [43,44•]. Episomes redistribute to euchromatin regions upon lytic reactivation. Likewise, histone activation markers such as H3K27ac and H3K4me1 are enriched in host genome tethering sites in latency III LCLs, where EBV episomes are often found near promoters [45•].
Epigenetic histone modification roles in EBV gene regulation
In addition to the above UHRF1 roles in histone ubiqui-tylation and cross-talk with H3K9me2/3, CRISPR also highlighted polycomb repressive complex 1 (PRC1) regulation of Burkitt cell LMP promoter activity. Knockout of PRC1 subunits reduces LMPp histone 2 lysine 119 monoubiquitin (H2AK119Ub1) repressive marks, de-repressing LMP1/2A even in the absence of EBNA2 expression [26••] (Figure 2b). These data suggest that whereas DNA methylation is critical for Cp silencing, both DNA methylation and H2 ubiqutination are each important for LMPp repression, with relevance to the programing of latency IIa. Furthermore, histone 3 lysine 27 trimethyl (H3K27me3) marks deposited on EBV genomes in latently infected B-cells repress EBV immediate early lytic gene expression [46,47] (Figure 2). Likewise, the KRAB-domain containing zinc finger protein SZF1 and TRIM28/KAP1 recruit histone modifying proteins, using distinct DNA motifs at host versus EBV DNA sites, resulting in the deposition of silencing H3K9me3 marks [48•].
EBNA1 covalently attaches to the EBV latency origin of replication
EBNA1 promotes EBV genome replication and episome partitioning to daughter cells in latency. The EBNA1 C-terminus binds the EBV latency origin of plasmid replication (oriP) in a DNA sequence specific manner. Recent work highlights that EBNA1 also establishes a cell cycle-dependent covalent bond with oriP DNA [49••]. EBNA1 tyrosine 518 (Y518) crosslinkage is required for replication fork termination at oriP and resembles single-strand endonuclease activity found at host replication-termination site DNA structures. Point mutation of Y518 to phenylalanine impairs DNA crosslinking, episome replication termination and maintenance [49••]. It will be of interest to determine whether other gamma-herpesviruses utilize similar chemistry for episome maintenance in latency.
Host genome-wide EBNA1 targets have recently been identified by chromatin immunoprecipitation and deep sequencing (ChIP-seq), including EBNA1-occupied regions conserved across B and epithelial cell lines [50,51]. Circular chromosome conformation capture sequencing (4C-seq) analysis further revealed contacts made between EBV episomes and host chromosome regions. Episomal/human genome docking sites preferentially overlap with EBNA1 binding sites, are enriched for occupancy by the transcription factors EBF1 and RBP-Jκ and also for H3K9me3 marks and AT-rich flanking sequences [44•]. EBV docking sites vary with EBV latency programs and cell types [44•]. EBNA1 bound to enhancers of multiple purine metabolism genes, including adenosine deaminase [52].
Higher order DNA structure regulates EBV gene expression
Host factors that control long range DNA interactions alter EBV genomic loop structures to regulate latency and lytic gene expression. These include cohesins and CTCF, which stabilize DNA loops and which also have independent epigenetic roles in establishing chromatin territories. ChIP-seq studies have highlighted multiple EBV genome-wide cohesin and CTCF binding sites, several of which have been characterized in detail [53•,54]. CTCF occupancy enables the oriP enhancer to interact with Cp in latency III or Qp in latency I in order to upregulate their activity [55]. Thus, EBV genomic CTCF motifs at Cp and Qp are critical for loop formation in latency III versus I, respectively [55,56]. Interestingly, the incompletely characterized EBV BHLF1 gene was also recently implicated in sustaining latency III, possibly through a highly expressed ncRNA [57•]. It will be of interest to determine whether BHLF1 contributes to oriP looping. CTCF also acts as a chromatin insulator at Qp to prevent epigenetic silencing [58]. Furthermore, Poly (ADP-ribose) polymerase (PARP1) and DNA methylation post-transcriptionally regulate CTCF activity. PARP1 co-occupies key EBV genomic locations with CTCF and stabilizes its DNA occupancy to restrict EBV lytic reactivation [59].
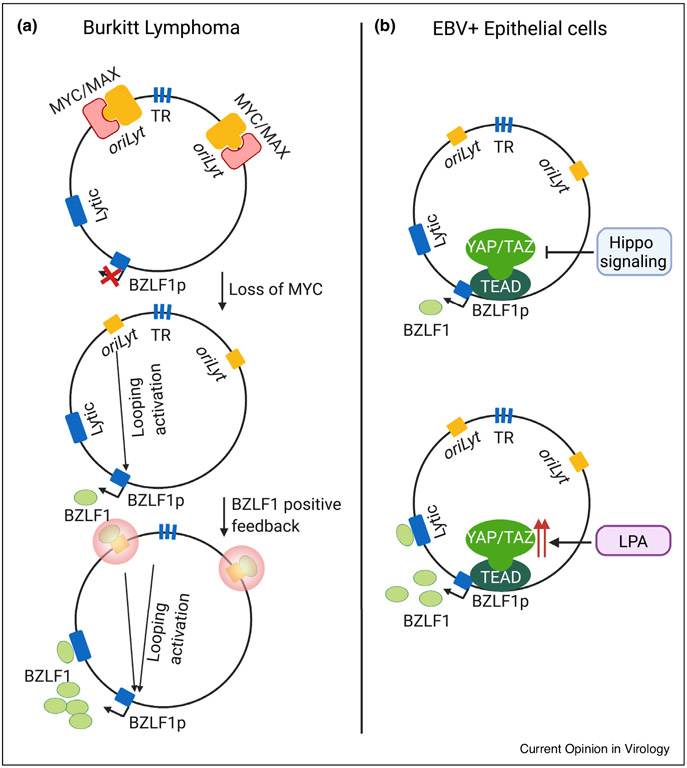
CRISPR and chemical genetic analysis highlighted that MYC abundance is a critical signal that regulates EBV genomic three-dimensional architecture to control the viral lytic switch in Burkitt cells [39••] (Figure 3a). Cohesins, the histone loader FACT and the transcription regulators STAGA and mediator were also identified as important for maintenance of Burkitt EBV latency, at least in part through obligatory roles in support of MYC transcription. Mechanistically, MYC binds to EBV genomic origin of lytic replication (oriLyt) sites to prevent their looping to the immediate early BZLF1 promoter region. This may serve to initiate immediate early BZLF1 expression, which can then bind to and strengthen the oriLyt enhancer (Figure 3a). EBV genomic terminal repeat regions also loop to oriLyt. FACT inhibition by the small molecule curaxin CBL0137 induces EBV lytic antigens in Burkitt xenografts, suggesting a novel potential approach for lytic induction therapy [39••]. Likewise, polo-like kinase 1 was found to regulate MYC and trigger EBV lytic reactivation [60•].
Figure 3. Schematic models of EBV lytic reactivation in B and epithelial cells.
(a) MYC/MAX heterodimers bind to oriLyt regions in Burkitt latency I. MYC loss triggers DNA looping, which juxtaposes oriLyt and terminal repeat (TR) regions with the BZLF1promoter region. Induced BZLF1 protein binds to oriLyt response elements, converting it into a robust enhancer that stimulates high level BZLF1 expression and drives early lytic gene expression. (b)Hippo signaling inhibits YAP and TAZ, which are highly expressed in epithelial cells. Upon Hippo inhibition, newly activated YAP and TAZ, together with TEAD transcription factors, bind to and coactivate the immediate early BRLF1 gene. Thus, the YAP/TAZ activator lysophosphatidic acid (LPA) induces epithelial cell lytic reactivation.
The transcriptional repressor BLIMP1 inhibits MYC expression in plasma cell differentiation, suggesting that EBV may exploit this mechanism to trigger lytic reactivation in response to B-cell differentiation [39••]. BLIMP1 also has effects at the BZLF1 promoter [61]. Further emphasizing the multiple epigenetic connections between MYC and the EBV genome, MYC overexpression in newly infected B-cells suppresses LMP1 expression [62]. Interestingly, EBF1 promotes transformation of LMP1+ cells by inhibiting plasma cell differentiation, a role physiologically phenocopied by EBNA3A in settings of high MYC expression [63••]. By contrast, CRISPR analyses highlighted obligatory EBNA3C roles in suppression of the tumor suppressor p16INK4A and revealed a new mechanism by which EBNA3C disrupts the activity of the host transcription repressor CtBP to upregulate other host targets [64••]. Recent studies highlighted that LMP2A instead counteracts MYC-induced apoptosis to promote B-cell growth and survival, as well as by inhibiting tumor suppressor RB1 function to promote cell cycle [15••]. Multiple EBNA oncoproteins further subvert the Rb-E2F-HDAC complex to induce key targets, including KLF14 [65•].
At least partially distinct mechanisms control the epithelial cell EBV lytic switch. Differentiation-dependent KLF4 and BLIMP1 expression induce EBV lytic reactivation in stratified squamous epithelial cells through immediate early gene activation [61]. Hippo pathway effectors YAP and TAZ also have key epithelial cell lytic reactivation roles, which together with TEAD family transcription factors activate the BZLF1 promoter (Figure 3b) [66••]. Notably, YAP, TAZ and TEAD proteins are more highly expressed in epithelial than in B-cell lines, likely contributing cell type specificity.
mRNA decay pathways control EBV latency
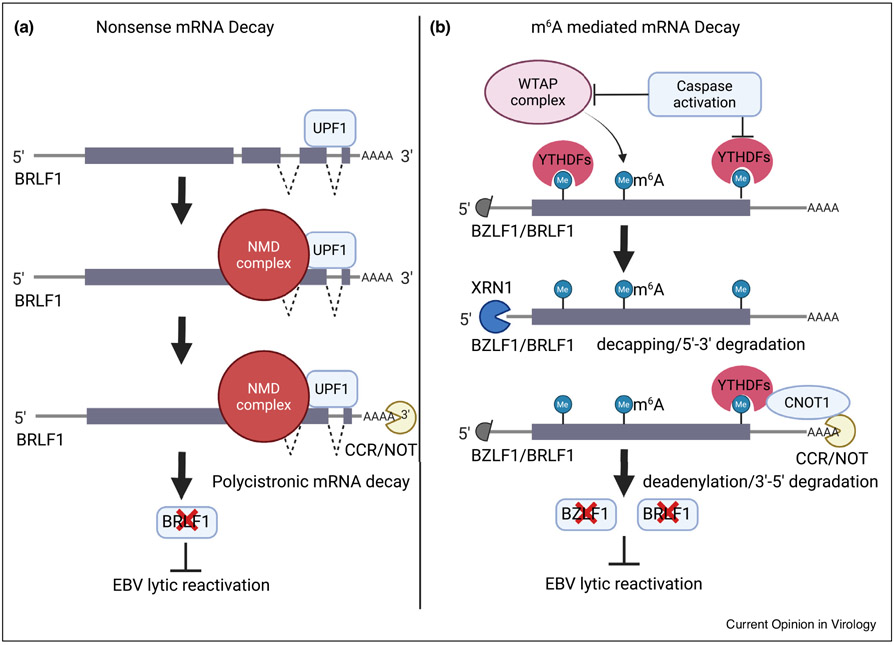
Nonsense-mediated decay (NMD) is a translation-coupled RNA degradation pathway that removes transcripts with premature stop codons or that have unusually long (i.e. >1 kb) 3′ untranslated regions (UTR). Recent studies highlight NMD roles in latency maintenance of EBV and of the related γ-herpesvirus Kaposi’s Sarcoma Associated Herpesvirus (KSHV) [67••,68••]. NMD machinery recognizes EBV and KSHV polycistronic transcripts encoding the immediate early genes, in a manner dependent on their extended 3′ UTRs [68••] (Figure 4a). NMD also links the host unfolded protein response pathway to Rta mRNA transcription in KSHV-infected cells [67••]. Knockdown or chemical inhibition of NMD machinery induces lytic reactivation in EBV and KSHV-infected cells, providing an interesting target for γ-herpesvirus lytic induction therapy [67••,68••].
Figure 4. Schematic models of RNA decay pathway functions in EBV lytic reactivation.
(a) Nonsense mediated decay (NMD) component UPF1 binds to the elongated 3′UTR of a polycistronic message encoding BRLF1. NMD complexes destabilize the message to suppress EBV lytic reactivation. (b) The WTAP complex deposits m6A marks on EBV immediate early transcripts, which recruits YTHDF reader proteins and activates the CNOT1 RNA decay pathway. This serves to limit the accumulation of immediate early transcripts in latency. Particular lytic reactivation stimuli activate caspases, which then degrade YTHDF2 and thereby stabilize the BZLF1 and BRFL1 messages.
RNA methylation at the N6 position of adenosine (m6A) also regulates EBV gene expression [69••,70••,71••]. mRNA profiling identified widespread m6A modification in EBV-infected cells, including of latency and lytic transcripts. Mechanistically, EBNA3C upregulates m6A methyltransferase METTL14 expression to promote m6A deposition [69••]. Interestingly, a m6A mediated mRNA degradation pathway has recently been connected to EBV lytic reactivation [70••,71••]. BZLF1 mRNA m6A modifications are recognized by YTHDF1, which then recruits RNA decay machinery to downmodulate BZLF1 levels and suppress lytic reactivation in nasopharyngeal carcinoma cells [70••]. Caspase activity is also implicated in promoting EBV and KSHV lytic reactivation [72,73], and the m6A reader YTHDF2 was recently found to be a caspase target. Thus, caspase cleavage of YTHFD2 and also of multiple m6A pathway components results in the stabilization of BZLF1 transcripts to support lytic reactivation [72] (Figure 4b). Caspase activation by the NLRP3 inflammasome also promotes EBV lytic reactivation through KAP1 degradation [74••].
Concluding remarks
Much remains to be learned about epigenetic mechanisms that regulate EBV/host interactions. We look forward to ongoing discoveries of EBV non-coding RNA epigenetic roles in EBV lifecycle regulation, such as the recent finding that EBV-encoded RNA EBER2 accelerates transformed B cell growth by upregulating expression of the cell cycle regulatory UCHL1 deubiquitinase [75]. Likewise, metabolic remodeling is a hallmark of cancer, and we anticipate that many layers of crosstalk exist between epigenetic and metabolic pathways that control the EBV lifecycle. Indeed, recent work highlights that EBNA2, MYC and LMP1 temporally control the expression of the lactate transporters MCT1 and 4 to support Warburg metabolism at distinct stages of B-cell transformation [76••]. Metabolic pathways also support interplay between EBNA and LMP oncoproteins [77•]. We anticipate that organoids and humanized mice will contribute new layers of understanding of how cellular microenvironments shape the EBV-infected cell epigenetic landscape.
Acknowledgements
RG is supported by a Lymphoma Research Foundation fellowship. BEG is supported by a Burroughs Wellcome Career Award in Medical Sciences, by N.I.H. R01 AI137337, AI164709 and CA228700. We apologize to colleagues whose work we could not cite due to space constraints. The authors declare no conflict of interest. The graphic illustrations were made with Biorender.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Chiu YF, Sugden AU, Sugden B: Epstein-Barr viral productive amplification reprograms nuclear architecture, DNA replication, and histone deposition. Cell Host Microbe 2013, 14:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalla M, Gobel C, Hammerschmidt W: The lytic phase of epstein-barr virus requires a viral genome with 5-methylcytosine residues in CpG sites. J Virol 2012, 86:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalla M, Schmeinck A, Bergbauer M, Pich D, Hammerschmidt W: AP-1 homolog BZLF1 of Epstein-Barr virus has two essential functions dependent on the epigenetic state of the viral genome. Proc Natl Acad Sci U S A 2010, 107:850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhende PM, Seaman WT, Delecluse HJ, Kenney SC: The EBV lytic switch protein, Z, preferentially binds to and activates the methylated viral genome. Nat Genet 2004, 36:1099–1104. [DOI] [PubMed] [Google Scholar]
- 5.Bergbauer M, Kalla M, Schmeinck A, Gobel C, Rothbauer U, Eck S, Benet-Pages A, Strom TM, Hammerschmidt W: CpG-methylation regulates a class of Epstein-Barr virus promoters. PLoS Pathog 2010, 6:e1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gewurz BE, Longnecker RM, Cohen JI: Epstein-barr virus. edn 7. Fields Virology. Wolters Kluwer Health Adis (ESP); 2021. [Google Scholar]
- 7. ••. Mrozek-Gorska P, Buschle A, Pich D, Schwarzmayr T, Fechtner R, Scialdone A, Hammerschmidt W: Epstein-Barr virus reprograms human B lymphocytes immediately in the prelatent phase of infection. Proc Natl Acad Sci U S A 2019, 116:16046–16055 RNAseq was used to construct temporal profiles of primary human B-cells infected by EBV over the first seven days of infection, revealing waves of cellular gene activation and metabolic pathway remodeling.
- 8. ••. Wang LW, Shen H, Nobre L, Ersing I, Paulo JA, Trudeau S, Wang Z, Smith NA, Ma Y, Reinstadler B et al. : Epstein-Barr-Virus-Induced One-Carbon Metabolism Drives B Cell Transformation. Cell Metab 2019, 30:539–555 Utilizing a temporal proteomic map of primary human B-cell transformation, this study identifies EBV-mediated metabolism remodeling and highlighted EBV induction of mitochondrial one carbon metabolism in support of purine nucleotide synthesis and redox defense.
- 9.Alfieri C, Birkenbach M, Kieff E: Early events in Epstein-Barr virus infection of human B lymphocytes. Virology 1991, 181:595–608. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser C, Laux G, Eick D, Jochner N, Bornkamm GW, Kempkes B: The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J Virol 1999, 73:4481–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. •. Wang C, Li D, Zhang L, Jiang S, Liang J, Narita Y, Hou I, Zhong Q, Zheng Z, Xiao H et al. : RNA sequencing analyses of gene expression during Epstein-Barr virus infection of primary B lymphocytes. J Virol 2019, 93 Uses RNAseq to create temporal maps of host and viral transcriptomes over the first 28 days of EBV-driven primary human B-cell immortalizational in vitro.
- 12.Nikitin PA, Yan CM, Forte E, Bocedi A, Tourigny JP, White RE, Allday MJ, Patel A, Dave SS, Kim W et al. : An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe 2010, 8:510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. ••. Pich D, Mrozek-Gorska P, Bouvet M, Sugimoto A, Akidil E, Grundhoff A, Hamperl S, Ling PD, Hammerschmidt W: First days in the life of naive human B lymphocytes infected with Epstein-Barr virus. mBio 2019, 10 EBV genome recombineering is used to highlight that EBNA2 implements a temporal program and is the only EBV-encoded factor essential for primary human B-cell outgrowth over the first eight days of infection.
- 14. ••. Li C, Romero-Masters JC, Huebner S, Ohashi M, Hayes M, Bristol JA, Nelson SE, Eichelberg MR, Van Sciver N, Ranheim EA et al. : EBNA2-deleted Epstein-Barr virus (EBV) isolate, P3HR1, causes Hodgkin-like lymphomas and diffuse large B cell lymphomas with type II and Wp-restricted latency types in humanized mice. PLoS Pathog 2020, 16:e1008590. This study shows that P3HR-1, an EBV strain that lacks EBNA2 and much of EBNA-LP, can cause lymphomas in humanized mice with restricted forms of viral latency that resemble Hodgkin lymphoma Reed-Sternberg cells or EBV+ diffuse lareg B-cell lymphoma, implicating the tumor microenvironment in EBV epigenome programing in vivo.
- 15. ••. Fish K, Comoglio F, Shaffer AL 3rd, Ji Y, Pan KT, Scheich S, Oellerich A, Doebele C, Ikeda M, Schaller SJ et al. : Rewiring of B cell receptor signaling by Epstein-Barr virus LMP2A. Proc Natl Acad Sci U S A 2020, 117:26318–26327 This study uses phosphoproteomics and transcriptome profiling to crosscompare LMP2A and B-cell receptor signaling, highlighting key similarities as well as significant differences, including differential transcriptional output as well as the ability of LMP2A to counteract MYC-induced apoptosis.
- 16.Westhoff Smith D, Sugden B: Potential cellular functions of Epstein-Barr Nuclear Antigen 1 (EBNA1) of Epstein-Barr virus. Viruses 2013, 5:226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Cancer Genome Atlas Research Network: Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birdwell CE, Queen KJ, Kilgore PC, Rollyson P, Trutschl M, Cvek U, Scott RS: Genome-wide DNA methylation as an epigenetic consequence of Epstein-Barr virus infection of immortalized keratinocytes. J Virol 2014, 88:11442–11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. •. Shamay M, Kanakry JA, Low JSW, Horowitz NA, Journo G, Ahuja A, Eran Y, Barzilai E, Dann EJ, Stone J et al. : CpG methylation in cell-free Epstein-Barr virus DNA in patients with EBV-Hodgkin lymphoma. Blood Adv 2020, 4:1624–1627 Develops a new diagnostic technique to quantitate EBV genomic methyl CpG DNA levels in peripheral blood samples as a biomarker of EBV+ B-cell lymphomas with restricted forms of EBV latency characterized by CpG hypermethylation.
- 20.Masucci MG, Contreras-Salazar B, Ragnar E, Falk K, Minarovits J, Ernberg I, Klein G: 5-Azacytidine up regulates the expression of Epstein-Barr virus nuclear antigen 2 (EBNA-2) through EBNA-6 and latent membrane protein in the Burkitt’s lymphoma line rael. J Virol 1989, 63:3135–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson KD, Hayward SD, Ling PD, Samid D, Ambinder RF: Transcriptional activation of the Epstein-Barr virus latency C promoter after 5-azacytidine treatment: evidence that demethylation at a single CpG site is crucial. Mol Cell Biol 1995, 15:6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Su X, Choi GC, Cao Y, Ambinder RF, Tao Q: Methylation profiling of Epstein-Barr virus immediate-early gene promoters, BZLF1 and BRLF1 in tumors of epithelial, NK- and B-cell origins. BMC Cancer 2012, 12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ••. Dalton T, Doubrovina E, Pankov D, Reynolds R, Scholze H,Selvakumar A, Vizconde T, Savalia B, Dyomin V, Weigel C et al. : Epigenetic reprogramming sensitizes immunologically silent EBV+ lymphomas to virus-directed immunotherapy. Blood 2020, 135:1870–1881 This study demonstrates that low doses of the hypomethylation agent decitabine stabily de-repress latency III antigen expression in EBV+ Burkitt cells, sensitizing them to adoptive CD8+ T-cell immunotherapy in a murine xenograft model.
- 24.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, Bollard CM, Liu H, Wu MF, Rochester RJ et al. : Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010, 115:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prockop S, Doubrovina E, Suser S, Heller G, Barker J, Dahi P, Perales MA, Papadopoulos E, Sauter C, Castro-Malaspina H et al. : Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J Clin Invest 2020, 130:733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ••. Guo R, Zhang Y, Teng M, Jiang C, Schineller M, Zhao B,Doench JG, O’Reilly RJ, Cesarman E, Giulino-Roth L et al. : DNA methylation enzymes and PRC1 restrict B-cell Epstein-Barr virus oncoprotein expression. Nat Microbiol 2020, 5:1051–1063 This study used a human genome-wide CRISPR/Cas9 screen to identify that the epigenetic enzymes UHRF1 and DNMT1 have critical roles in the propagation of EBV genomic CpG methylation marks necessary for maintenance of latency I, and identified polycomb repressive complex I as having additional roles in LMP promoter regulation in Burkitt B-cells.
- 27.Wille CK, Li Y, Rui L, Johannsen EC, Kenney SC, Longnecker RM:Restricted TET2 expression in germinal center type B cells promotes stringent Epstein-Barr virus latency. J Virol 2017, 91:e01987–01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu F, Wiedmer A, Martin KA, Wickramasinghe P, Kossenkov AV, Lieberman PM: Coordinate regulation of TET2 and EBNA2 controls the DNA methylation state of latent Epstein-Barr virus. J Virol 2017, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wille CK, Nawandar DM, Henning AN, Ma S, Oetting KM, Lee D, Lambert P, Johannsen EC, Kenney SC: 5-hydroxymethylation of the EBV genome regulates the latent to lytic switch. Proc Natl Acad Sci U S A 2015, 112:E7257–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. •. Eichelberg MR, Welch R, Guidry JT, Ali A, Ohashi M, Makielski KR, McChesney K, Van Sciver N, Lambert PF, Keles S et al. : Epstein-Barr virus infection promotes epithelial cell growth by attenuating differentiation-dependent exit from the cell cycle. mBio 2019, 10 Using a telomerase immortalized normal oral keratinocyte EBV infection model, this study identified that EBV impairs differentiation-induced changes in host gene expression, in a manner that is not dependent on lytic gene expression.
- 31.Kulis M, Merkel A, Heath S, Queiros AC, Schuyler RP,Castellano G, Beekman R, Raineri E, Esteve A, Clot G et al. : Whole-genome fingerprint of the DNA methylome during human B cell differentiation. Nat Genet 2015, 47:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoeler K, Aufschnaiter A, Messner S, Derudder E, Herzog S, Villunger A, Rajewsky K, Labi V: TET enzymes control antibody production and shape the mutational landscape in germinal centre B cells. FEBS J 2019, 286:3566–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai AY, Mav D, Shah R, Grimm SA, Phadke D, Hatzi K, Melnick A, Geigerman C, Sobol SE, Jaye DL et al. : DNA methylation profiling in human B cells reveals immune regulatory elements and epigenetic plasticity at Alu elements during B-cell activation. Genome Res 2013, 23:2030–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterlin D, Velasco G, Moshous D, Touzot F, Mahlaoui N, Fischer A, Suarez F, Francastel C, Picard C: Genetic, cellular and clinical features of ICF syndrome: a French national survey. J Clin Immunol 2016, 36:149–159. [DOI] [PubMed] [Google Scholar]
- 35.Tsai K, Chan L, Gibeault R, Conn K, Dheekollu J, Domsic J, Marmorstein R, Schang LM, Lieberman PM: Viral reprogramming of the Daxx histone H3.3 chaperone during early Epstein-Barr virus infection. J Virol 2014, 88:14350–14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang H, Deng Z, Vladimirova O, Wiedmer A, Lu F, Lieberman PM, Patel DJ: Structural basis underlying viral hijacking of a histone chaperone complex. Nat Commun 2016, 7:12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shumilov A, Tsai MH, Schlosser YT, Kratz AS, Bernhardt K, Fink S, Mizani T, Lin X, Jauch A, Mautner J et al. : Epstein-Barr virus particles induce centrosome amplification and chromosomal instability. Nat Commun 2017, 8:14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. •. Zhang Y, Jiang C, Trudeau SJ, Narita Y, Zhao B, Teng M, Guo R,Gewurz BE, Damania B: Histone loaders CAF1 and HIRA restrict Epstein-Barr virus B-cell lytic reactivation. mBio 2020, 11:e01063–01020 This study reveals that type I and II EBV strains coopt multiple cellular histone loaders to establish and maintain latency via nonredundant roles.
- 39. ••. Guo R, Jiang C, Zhang Y, Govande A, Trudeau SJ, Chen F, Fry CJ,Puri R, Wolinsky E, Schineller M et al. : MYC controls the Epstein-Barr virus lytic switch. Mol Cell 2020, 78:653–669.e8 Human genome-wide CRISPR/Cas9 analysis reveals that a network of host factors centered on MYC suppress the EBV lytic switch in Burkitt B-cells. It highlights that EBV senses MYC abundance to maintain latency, and undergoes higher order changes in viral 3D genome structure in response to MYC depletion, which occurs in plasma cell differentiation, a known lytic induction trigger.
- 40.Li D, Fu W, Swaminathan S: Continuous DNA replication is required for late gene transcription and maintenance of replication compartments in gammaherpesviruses. PLoS Pathog 2018, 14:e1007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ••. Buschle A, Mrozek-Gorska P, Cernilogar FM, Ettinger A, Pich D,Krebs S, Mocanu B, Blum H, Schotta G, Straub T et al. : Epstein-Barr virus inactivates the transcriptome and disrupts the chromatin architecture of its host cell in the first phase of lytic reactivation. Nucleic Acids Res 2021, 49:3217–3241 This study uses ChIP-seq and ATAC-seq to reveals identify host genome-wide BZLF1 binding sites with different sequence motifs and higlights that EBV lytic induction rapidly causes global alterations in host chromatin architecture, with widespread reduction of host transcripts.
- 42. ••. Nagaraju T, Sugden AU, Sugden B: Four-dimensional analyses show that replication compartments are clonal factories in which Epstein-Barr viral DNA amplification is coordinated. Proc Natl Acad Sci U S A 2019, 116:24630–24638 Uses single cell, computational and population approaches to identify that EBV lytic replication compartments are initially seeded by single viral genomes, which are massively amplified over the next 14 hours to occupy up to 30% of nuclear volume, which itself expands by 50% with lytic reactivation.
- 43.Moquin SA, Thomas S, Whalen S, Warburton A, Fernandez SG, McBride AA, Pollard KS, Miranda JL: The Epstein-Barr virus episome maneuvers between nuclear chromatin compartments during reactivation. J Virol 2018, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. •. Kim KD, Tanizawa H, De Leo A, Vladimirova O, Kossenkov A, Lu F,Showe LC, Noma KI, Lieberman PM: Epigenetic specifications of host chromosome docking sites for latent Epstein-Barr virus. Nat Commun 2020, 11:877. This study uses circular chromosome conformation capture (4C) to characterize EBV episomal host genomic docking sites in Burkitt B-cells, which are found to be enriched for sequence-specific transcription factor EBF1 and RBP-Jκ motifs, for repressive H3K9me3 histone marks, and for AT-rich flanking sequences.
- 45. •. Wang L, Laing J, Yan B, Zhou H, Ke L, Wang C, Narita Y, Zhang Z,Olson MR, Afzali B et al. : Epstein-Barr virus episome physically interacts with active regions of the host genome in lymphoblastoid cells. J Virol 2020, 94 Using high resolution Hi-C maps, this study identifies contacts between EBV and human genomes, highlighting epigenetic features of contact regions, including enrichment for active lCl chromatin regions.
- 46.Woellmer A, Arteaga-Salas JM, Hammerschmidt W: BZLF1 governs CpG-methylated chromatin of Epstein-Barr virus reversing epigenetic repression. PLoS Pathog 2012, 8:e1002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murata T, Kondo Y, Sugimoto A, Kawashima D, Saito S,Isomura H, Kanda T, Tsurumi T: Epigenetic histone modification of Epstein-Barr virus BZLF1 promoter during latency and reactivation in Raji cells. J Virol 2012, 86:4752–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. •. Burton EM, Akinyemi IA, Frey TR, Xu H, Li X, Su LJ, Zhi J,McIntosh MT, Bhaduri-McIntosh S: A heterochromatin inducing protein differentially recognizes self versus foreign genomes. PLoS Pathog 2021, 17:e1009447. This study reveals that SZF1 and TRIM28/KAP1 recruit histone modifying proteins to host and EBV genomic sites via distinct DNA motifs, resulting in the deposition of silencing H3K9me3 marks.
- 49. ••. Dheekollu J, Wiedmer A, Ayyanathan K, Deakyne JS, Messick TE, Lieberman PM: Cell-cycle-dependent EBNA1-DNA crosslinking promotes replication termination at oriP and viral episome maintenance. Cell 2021, 184:643–654.e13 This study reveals that EBNA1 forms cell-cycle dependent covalent DNA crosslinkages with the EBV origen of plasmid replication, oriP. EBNA1 tyrosine 518 is necessary for this DNA crosslinking and for EBV episome maintenance, revealing a novel mechanism of EBV genome regulation.
- 50.Lu F, Wikramasinghe P, Norseen J, Tsai K, Wang P, Showe L, Davuluri RV, Lieberman PM: Genome-wide analysis of host-chromosome binding sites for Epstein-Barr Virus Nuclear Antigen 1 (EBNA1). Virol J 2010, 7:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tempera I, De Leo A, Kossenkov AV, Cesaroni M, Song H, Dawany N, Showe L, Lu F, Wikramasinghe P, Lieberman PM:Identification of MEF2B, EBF1, and IL6R as direct gene targets of Epstein-Barr virus (EBV) nuclear antigen 1 critical for EBV-infected B-lymphocyte survival. J Virol 2016, 90:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamontagne RJ, Soldan SS, Su C, Wiedmer A, Won KJ, Lu F, Goldman AR, Wickramasinghe J, Tang HY, Speicher DW et al. : A multi-omics approach to Epstein-Barr virus immortalization of B-cells reveals EBNA1 chromatin pioneering activities targeting nucleotide metabolism. PLoS Pathog 2021, 17:e1009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salamon D, Banati F, Koroknai A, Ravasz M, Szenthe K, Bathori Z, Bakos A, Niller HH, Wolf H, Minarovits J: Binding of CCCTC-binding factor in vivo to the region located between Rep* and the C promoter of Epstein-Barr virus is unaffected by CpG methylation and does not correlate with Cp activity. J Gen Virol 2009, 90:1183–1189. [DOI] [PubMed] [Google Scholar]
- 54.Arvey A, Tempera I, Tsai K, Chen HS, Tikhmyanova N, Klichinsky M, Leslie C, Lieberman PM: An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe 2012, 12:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tempera I, Klichinsky M, Lieberman PM: EBV latency types adopt alternative chromatin conformations. PLoS Pathog 2011, 7:e1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hughes DJ, Marendy EM, Dickerson CA, Yetming KD, Sample CE, Sample JT: Contributions of CTCF and DNA methyltransferases DNMT1 and DNMT3B to Epstein-Barr virus restricted latency. J Virol 2012, 86:1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. •. Yetming KD, Lupey-Green LN, Biryukov S, Hughes DJ, Marendy EM, Miranda JL, Sample JT: The BHLF1 locus of Epstein-Barr virus contributes to viral latency and B-cell immortalization. J Virol 2020, 94 This study utilizes BAC recomineering to reveal that highly expressed EBV BHLF1 has unexpected roles in support of latency III, possibly by acting as a long non-coding RNA.
- 58.Tempera I, Wiedmer A, Dheekollu J, Lieberman PM: CTCF prevents the epigenetic drift of EBV latency promoter Qp. PLoS Pathog 2010, 6:e1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lupey-Green LN, Caruso LB, Madzo J, Martin KA, Tan Y, Hulse M, Tempera I: PARP1 stabilizes CTCF binding and chromatin structure to maintain Epstein-Barr virus latency type. J Virol 2018, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. •. Biswas A, Zhou D, Fiches GN, Wu Z, Liu X, Ma Q, Zhao W, Zhu J, Santoso NG: Inhibition of polo-like kinase 1 (PLK1) facilitates reactivation of gamma-herpesviruses and their elimination. PLoS Pathog 2021, 17:e1009764. Identifies that PLK1 regulates MYC to support maintenance of KSHV and EBV latency.
- 61.Reusch JA, Nawandar DM, Wright KL, Kenney SC, Mertz JE:Cellular differentiation regulator BLIMP1 induces Epstein-Barr virus lytic reactivation in epithelial and B cells by activating transcription from both the R and Z promoters. J Virol 2015, 89:1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price AM, Messinger JE, Luftig MA: c-Myc represses transcription of Epstein-Barr virus latent membrane protein 1 early after primary B cell infection. J Virol 2018, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. ••. Sommermann T, Yasuda T, Ronen J, Wirtz T, Weber T, Sack U, Caeser R, Zhang J, Li X, Chu VT et al. : Functional interplay of Epstein-Barr virus oncoproteins in a mouse model of B cell lymphomagenesis. Proc Natl Acad Sci U S A 2020, 117:14421–14432 Identifies secondary EBF1 mutations that arise in murine B-cell lymphomas driven by conditional LMP1 expression in germinal center B-cells. Mechanistically, aberrant EBF1 activation inhibits LMP1-driven plasma cell differentiation, an effect phenocopied by EBNA3A.
- 64. ••. Ohashi M, Hayes M, McChesney K, Johannsen E: Epstein-Barr virus nuclear antigen 3C (EBNA3C) interacts with the metabolism sensing C-terminal binding protein (CtBP) repressor to upregulate host genes. PLoS Pathog 2021, 17:e1009419. This study reveals obligatory EBNA3C roles in suppressing tumor suppressor p16INK4A and a novel mechanism by which EBNA3C disrupts host transcription repressor CtBP activity to upregulate other host targets.
- 65. •. Pei Y, Wong JH, Jha HC, Tian T, Wei Z, Robertson ES: Epstein-Barr virus facilitates expression of KLF14 by regulating the cooperative binding of the E2F-Rb-HDAC complex in latent infection. J Virol 2020, 94 Uses next-generation sequencing to characterize profiles of E2F, Rb and HDAC host transcription factors in LCLs, highlighting that E2F-Rb-HDAC complexes are associated with superenhancers via long-range regulatory regions, and that this comlex is subverted to facilitate KLF14 expression in LCLs.
- 66. ••. Van Sciver N, Ohashi M, Pauly NP, Bristol JA, Nelson SE, Johannsen EC, Kenney SC: Hippo signaling effectors YAP and TAZ induce Epstein-Barr Virus (EBV) lytic reactivation through TEADs in epithelial cells. PLoS Pathog 2021, 17:e1009783. Identifies that Hippo effectors YAP and TAZ have key roles in EBV epithelial cell reactivation, by co-activating immediate early BRLF1 expression together with cellular TEAD transcription factors.
- 67. ••. Zhao Y, Ye X, Shehata M, Dunker W, Xie Z, Karijolich J: The RNA quality control pathway nonsense-mediated mRNA decay targets cellular and viral RNAs to restrict KSHV. Nat Commun 2020, 11:3345. This study uses high-throughput transcriptomic analysis to identify that NMD restricts KSHV immediate early RTA expression to suppress lytic reactivation in primary effusion lymphoma cells. It also reveals an NMD-regulated link between RTA and the unfolded protein response.
- 68. ••. van Gent M, Reich A, Velu SE, Gack MU: Nonsense-mediated decay controls the reactivation of the oncogenic herpesviruses EBV and KSHV. PLoS Biol 2021, 19:e3001097. This study reveals that the cellular NMD pathway restricts EBV and KSHV lytic reactivation via suppressing immediate early gene RTA mRNA levels. Mechanistically, NMD pathway components bind to and destabilize polycistronic RTA transcripts with elongated 3’ UTR. A small molecule NMD inhibitor was found to induce lytic reactivation.
- 69. ••. Lang F, Singh RK, Pei Y, Zhang S, Sun K, Robertson ES: EBV epitranscriptome reprogramming by METTL14 is critical for viral-associated tumorigenesis. PLoS Pathog 2019, 15:e1007796. This study was the first to identify widespread m6A modification on EBV latent and lytic transcripts. It identifies that EBNA3C upregulates METTL14, a component of the WTAP m6A methyltransferase, to increase m6A modification of EBV transcripts.
- 70. ••. Xia TL, Li X, Wang X, Zhu YJ, Zhang H, Cheng W, Chen ML, Ye Y,Li Y, Zhang A et al. : N(6)-methyladenosine-binding protein YTHDF1 suppresses EBV replication and promotes EBV RNA decay. EMBO Rep 2021, 22:e50128. Identifies that nasopharyngeal carcinoma cells exhibit m6A modification of EBV transcripts, which recruit the reader YTHDF1 and limit lytic reactivation by destabilizing immediate early BZLF1 and BRLF1 mRNAs.
- 71. ••. Zhang K, Zhang Y, Maharjan Y, Sugiokto FG, Wan J, Li R: Caspases switch off the m(6)A RNA modification pathway to foster the replication of a ubiquitous human tumor virus. mBio 2021,12:e0170621. This paper reveals that activated caspases cleave m6A writers and readers to upregulate abundances of m6A-modified EBV immediate early gene transcripts and support lytic reactivation.
- 72.Zhang K, Lv DW, Li R: B cell receptor activation and chemical induction trigger caspase-mediated cleavage of PIAS1 to facilitate epstein-barr virus reactivation. Cell Rep 2017, 21:3445–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Leo A, Chen H-S, Hu C-CA, Lieberman PM: Deregulation of KSHV latency conformation by ER-stress and caspase-dependent RAD21-cleavage. PLoS Pathog 2017, 13:e1006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. ••. Burton EM, Goldbach-Mansky R, Bhaduri-McIntosh S: A promiscuous inflammasome sparks replication of a common tumor virus. Proc Natl Acad Sci U S A 2020, 117:1722–1730 This study reveals that caspase activation by the NLRP3 inflammasome supports EBV lytic reactivation through degradation of the heterochromatin-inducing epigenetic repressor KAP1/TRIM28.
- 75.Li Z, Baccianti F, Delecluse S, Tsai MH, Shumilov A, Cheng X, Ma S, Hoffmann I, Poirey R, Delecluse HJ: The Epstein-Barr virus noncoding RNA EBER2 transactivates the UCHL1 deubiquitinase to accelerate cell growth. Proc Natl Acad Sci U S A 2021, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. ••. Bonglack EN, Messinger JE, Cable JM, Ch’ng J, Parnell KM, Reinoso-Vizcaino NM, Barry AP, Russell VS, Dave SS, Christofk HR et al. : Monocarboxylate transporter antagonism reveals metabolic vulnerabilities of viral-driven lymphomas. Proc Natl Acad Sci U S A 2021, 118 This study identified that EBNA2 and LMP1 induce the lactate transporters MCT1 and MCT4 at distinct stages of B-cell proliferation to support Warburg metabolism and glutathione levels. Dual MCT1/4 inhibition was revealed to be a novel therapeutic target that sensitizes LCLs to electron transport chain complex I inhibition.
- 77. •. Wang LW, Wang Z, Ersing I, Nobre L, Guo R, Jiang S, Trudeau S,Zhao B, Weekes MP, Gewurz BE: Epstein-Barr virus subverts mevalonate and fatty acid pathways to promote infected B-cell proliferation and survival. PLoS Pathog 2019, 15:e1008030. Identifies that EBV highly induces mevalonate metabolism to generate geranylgeranyl pyrophosphate, which activates the EBNA3-induced small G protein Rab13 to support LMP1 and 2A trafficking and target gene regulation.