Metabolic syndrome (MetS) refers to a cluster of risk factors that include central obesity, high LDL and low HDL cholesterol, hyperglycemia and/or insulin resistance, and hypertension. MetS serves as the major risk factor for cardio-metabolic diseases including atherosclerosis, type 2 diabetes mellitus, and nonalcoholic fatty liver disease 1(1). Although the relationship of between MetS and atherosclerosis is well established, the cellular and molecular basis that causes these diverse traits to cluster in a single disease entity is not known, although obesity-induced insulin resistance is hypothesized as a unifying factor. Diets high in sugar and fat can contribute to obesity, insulin resistance, and type 2 diabetes mellitus, but it is less clear how this diet may promote hypertension and its potentially serious sequelae of heart failure, stroke, and myocardial infraction.
Fibrotic remodeling of the vasculature may represent an initial step in the progression from diet to hypertension. Dysregulation of the transforming growth factor-beta (TGF-β) pathway by elevated glucose levels may be one mechanism that contributes to fibrotic remodeling 2. TGF-β signaling to Smad transcription factors is a master regulatory pathway for extracellular matrix proteins such as collagens 2. These matrix proteins are essential components that confer tissue structure and stability, and their upregulation is central to physiological wound healing, however, their aberrant or excessive deposition causes pathological fibrosis. Elevated glucose levels can induce TGF-β pro-fibrotic signaling through multiple mechanisms including production of reactive oxygen species and a shift in glycolytic metabolites (reviewed in 3).
The Hippo/YAP pathway also regulates wound healing and fibrosis 3. This pathway controls tissue and organ size by regulating proliferation and apoptosis, as well as cell fate and tissue regeneration. The Hippo pathway, named for the tissue overgrowth phenotypes that occur when key genes were deleted in Drosophila, consists of a kinase cascade that influences the nuclear localization of YAP and its paralogue, TAZ, transcriptional regulators that cooperate with TEAD family transcription factors to control gene expression. YAP phosphorylation leads to its cytosolic sequestration and degradation 3. Signals that activate the Hippo pathway inhibit YAP phosphorylation, allowing it to enter the nucleus. There are many upstream signals that can alter Hippo/YAP activity, including cell density, adhesion, and polarity, mechanical forces, and cellular and metabolic stresses. Aside from some G protein-coupled receptors4, the receptors that transduce these signals through the Hippo pathway are generally unknown, with no known ligands.
An intriguing study by Liu et al5 provides critical new insights into mechanisms by which a high-fat/high-sucrose (HF/HS) diet promotes vascular stiffness. Liu et al identify a novel connection between diet and arterial stiffness, mediated by YAP and TGFβ in smooth muscle cells (SMC)5. The authors noted that administering a HF/HS diet to young male mice for 8 weeks increased stiffness in the aorta and carotid artery as measured by changes in pulse wave velocity and circumferential cyclic strain. TGF-β signaling and target genes (including COL1A1, FN, and CTGF) were induced, and collagen deposition was enhanced in the vessel wall. These changes were preceded by a marked upregulation in both expression and nuclear localization of YAP that was evident after only two weeks of the HF/HS diet. The authors found that YAP was induced by HF/HS diet activation of the nutrient-sensing mTORC1 pathway which led to suppression of autophagy. mTORC1 hyperactivation through TSC depletion has been shown to enhance YAP expression by impairing YAP degradation6. This coupling between mTORC1, autophagy and YAP allows for coordination of YAP-dependent proliferative signaling with nutrient availability6.
The essential role for YAP as a mediator of HF/HS-induced vascular stiffening was revealed by generating mice with inducible smooth muscle-specific loss- or gain-of-function of YAP5. YAP gain of function worsened the fibrotic response induced by HF/HS diet, but did not exacerbate any other metabolic parameters. Liu et al identified a novel mechanism where YAP enhances the magnitude and duration of TGF-β signaling by stabilizing phosphorylation of nuclear Smad2 and Smad3. Using mass spectrometry on purified YAP and assays with recombinant proteins, the team discovered that the phosphatase PP1MB (protein phosphatase, Mg2+/Mn2+-dependent 1B) is a novel binding partner for YAP. The TGF-β pathway encompasses many levels of feedback regulation, including induction of its own negative regulators such as SMAD7, SMURFs7 and LMO78. PP1MB was identified as a novel negative feedback regulator, as TGF-β induced PPM1B nuclear translocation and subsequent Smad dephosphorylation. YAP interaction with PPM1B prevented the PPM1B polyubiquitination required for its nuclear translocation, thus allowing for prolonged Smad-dependent transcription of pro-fibrotic genes. By generating a series of mutant proteins, the authors identified the regions in both YAP and PPM1B required for their interaction. Interestingly, other studies have identified binding interactions between YAP and Smads9, 10, but this was not detected by Li et al5. Instead, mutations that specifically disrupt the YAP-PPM1B interaction prevented the YAP enhancement of TGF-β signaling. These data raise the possibility that specific inhibitors to disrupt YAP and PPM1B binding could potentially have therapeutic utility to target aberrant or pro-fibrotic TGF-β signaling. However, the kinetics of YAP activation in this study preceded fibrosis: YAP was upregulated after 2 weeks HF/HS diet, before the onset of arterial stiffness, but had returned to near baseline levels after 8 weeks, suggesting that targeting YAP may help prevent development of vascular stiffness, but may not have utility once this phenotype is established.
The identification of exciting new connections between diet, YAP, TGF-β, and stiffness5, suggests interesting new questions for future investigation. Understanding the long-term effects of HF/HS diet on YAP and TGF-β signaling beyond 8 weeks is important, along with determining whether intermittent exposures (modeling repeated cycles of weight gain and loss) could exacerbate YAP-amplified TGF-β signaling and worsen fibrosis. Separating the effects of sugar and fat, as well as determining whether depletion of either may have beneficial effects or promote reversibility would also be of interest, as would comparisons between aged and young mice, and males versus females. As YAP is ubiquitously expressed, whether the relationship between YAP, PPM1B, and TGF-β amplification is smooth muscle-specific or more broadly applicable remains to be determined. YAP and TEAD1 are important regulators of smooth muscle phenotype, promoting intimal hyperplasia following vascular injury in SMC11. Much of the elegant, detailed biochemistry studies were performed with overexpression approaches in HEK293 cells (Liu et al)5, so it is possible that some SMC-specific substrates or other interacting proteins may not be represented in this system. How YAP interactions with other proteins, or PP1MB interaction with other substrates may influence this feedback mechanism is not known. Future studies are poised to uncover many major questions linking MetS to vascular disease.
The conditions in the Liu et al study5 may reflect very early events in development of MetS and vascular pathology in young subjects that may precede development of overt hypertension. Not all parameters of MetS were present, but weight gain and impaired glucose tolerance were noted. Despite significant changes in stiffness in large conduit arteries after 2 months, neither the HF/HS diet nor transgenic overexpression of YAP in SMC caused changes in blood pressure. It is possible that compensatory mechanisms (e.g. renin-angiotensin system) may have been sufficient to maintain normal blood pressure, or that stiffness may not have affected peripheral resistance vessels at this relatively early time point. Dietary carbohydrate has been shown to activate the mTOR pathway in the liver and induce insulin resistance12. Over time, hyperinsulinemia may contribute to the progression of hypertension through multiple targets, including renal sodium retention, vascular SMC hypertrophy and vascular tone, alterations in nitric oxide and SMC calcium mobilization13, 14. These new studies reveal exciting connections between the nutrient-sensing mTORC1 pathway, the mechanosensing YAP pathway, and matrix-promoting TGF-β pathway that help link nutrient excess with the vascular sequelae of MetS and suggest that specific targeting of YAP/TGF-β interactions may have therapeutic possibilities for prevention of obesity-induced hypertension.
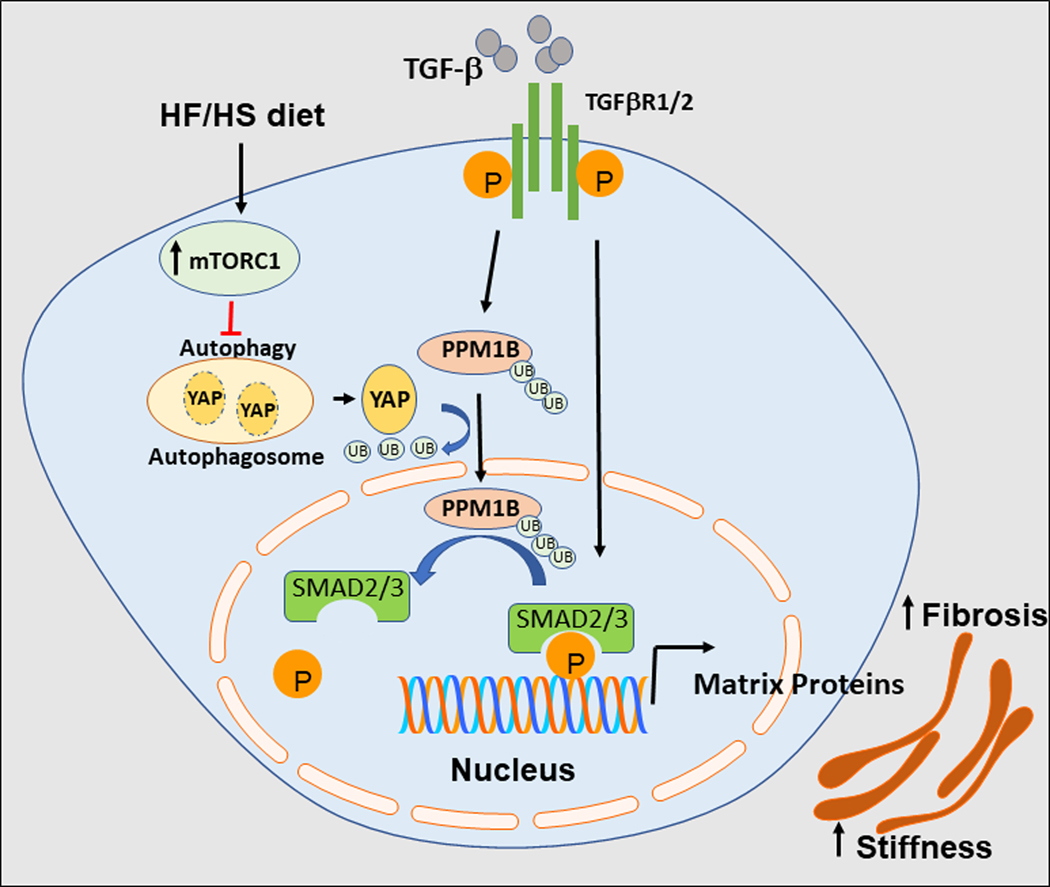
Figure:
Schematic of arterial stiffness induced by HF/HS diet. HF/HS diet increases the activation of mTORC1, suppressing autophagy-induced degradation of YAP in SMC. Increased YAP levels inhibit TGF-β-dependent PPM1B ubiquitination and translocation to the nucleus. Nuclear PPM1B dephosphorylates SMAD2/3, terminating TGF-β signaling. The HF/HS-induced cascade of events results in amplified TGF-β signaling, increasing extracellular matrix protein deposition and arterial stiffness.
Acknowledgments
Sources Of Funding
Supported by grants from the NIH to A.M. (Outstanding Investigator Award RHL135767A), J.H. (R01HL122815, R01HL150515, R01HL142818), and K.A.M. (R01HL142090, R01HL151222, R01HL146101.
Footnotes
Disclosures: None
References
- 1.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL and Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32. [DOI] [PubMed] [Google Scholar]
- 2.Tuleta I and Frangogiannis NG. Diabetic fibrosis. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dey A, Varelas X and Guan KL. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov. 2020;19:480–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo J and Yu FX. GPCR-Hippo Signaling in Cancer. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Li M, Lv X, Bao K, Tian XU, He L, Shi L, Zhu Y and Ai D. YAP targets the TGFb pathway to mediate high-fat/high-sucrose diet-induced arterial stiffness. . Circulation Research. 2022;In press. [DOI] [PubMed] [Google Scholar]
- 6.Liang N, Zhang C, Dill P, Panasyuk G, Pion D, Koka V, Gallazzini M, Olson EN, Lam H, Henske EP, et al. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med. 2014;211:2249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ceuninck van Capelle C, Spit M,Ten Dijke P. Current perspectives on inhibitory SMAD7 in health and disease. Crit Rev Biochem Mol Biol. 2020;55:691–715. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y, Ostriker AC, Jin Y, Hu H, Sizer AJ, Peng G, Morris AH, Ryu C, Herzog EL, Kyriakides T, et al. LMO7 Is a Negative Feedback Regulator of Transforming Growth Factor beta Signaling and Fibrosis. Circulation. 2019;139:679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piersma B, Bank RA and Boersema M. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front Med (Lausanne). 2015;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiemer SE, Szymaniak AD and Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor beta-induced tumorigenic phenotypes in breast cancer cells. The Journal of biological chemistry. 2014;289:13461–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osman I, He X, Liu J, Dong K, Wen T, Zhang F, Yu L, Hu G, Xin H, Zhang W and Zhou J. TEAD1 (TEA Domain Transcription Factor 1) Promotes Smooth Muscle Cell Proliferation Through Upregulating SLC1A5 (Solute Carrier Family 1 Member 5)-Mediated Glutamine Uptake. Circ Res. 2019;124:1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhat N, Narayanan A, Fathzadeh M, Kahn M, Zhang D, Goedeke L, Neogi A, Cardone RL, Kibbey RG, Fernandez-Hernando C, et al. Dyrk1b promotes hepatic lipogenesis by bypassing canonical insulin signaling and directly activating mTORC2 in mice. J Clin Invest. 2022;132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito F, Hori MT, Fittingoff M, Hino T and Tuck ML. Insulin attenuates agonist-mediated calcium mobilization in cultured rat vascular smooth muscle cells. J Clin Invest. 1993;92:1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N and Haring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12:721–737. [DOI] [PubMed] [Google Scholar]