Abstract
Although cancer-associated fibroblasts (CAFs) have gained increased attention for supporting cancer progression, current CAF-targeted therapeutic options are limited and failing in clinical trials. As the largest component of the tumor microenvironment (TME), CAFs alter the biochemical and physical structure of the TME, modulating cancer progression. Here, we review the role of CAFs in altering drug response, modifying the TME mechanics and the current models for studying CAFs. To provide new perspectives, we highlight key considerations of CAF activity and discuss emerging technologies that can better address CAFs; and therefore, increase the likelihood of therapeutic efficacy. We argue that CAFs are crucial components of the cancer drug discovery pipeline and incorporating these cells will improve drug discovery success rates.
Keywords: : biomaterials, cancer, cancer associated fibroblast, extracellular matrix, mechanobiology, microenvironment, tumor mechanics
Plain language summary
Recent advances in cancer research have improved our understanding of disease progression; however, the number of drugs failing in clinical trials remains high and therefore, present a critical challenge for cancer drug discovery. Although the interactions of the tissue surrounding the tumor, the tumor microenvironment, are now considered key targets for new interventions in cancer, the role of microenvironment is largely absent in drug discovery pipelines. Here we explore the role of the most prominent cell type in the tumor microenvironment, cancer-associated fibroblasts (CAFs), in altering cancer therapy response and ultimately patient outcome. To provide new perspectives for future studies, we draw attention to key complications of CAF biology and highlight emerging technologies that could be used to address this. We believe including CAFs in drug discovery, whether for targeting cancer cells or the microenvironment, will allow for a better understanding of therapeutic efficacy and ultimately improve clinical outcome.
Graphical abstract
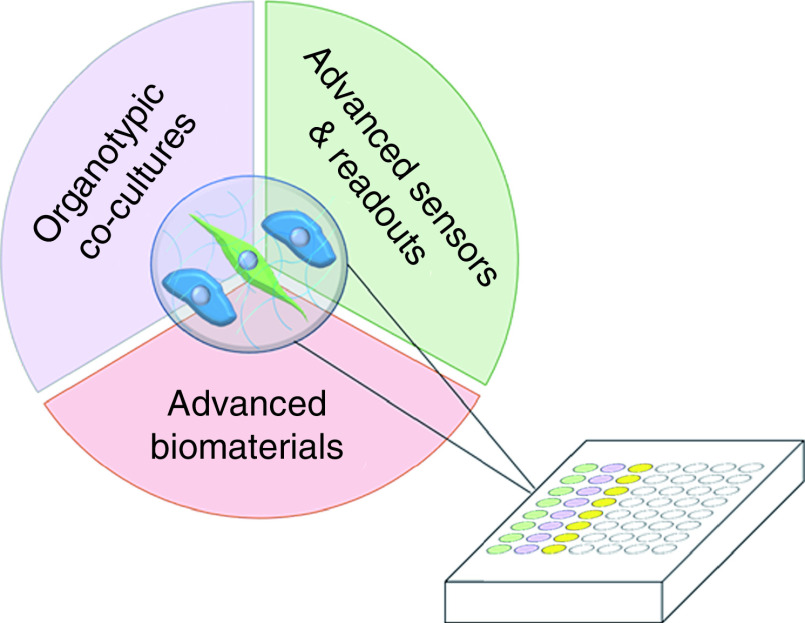
Incorporating emerging technologies in cancer drug discovery to improve translational impact.
The tumor microenvironment (TME) is a complex landscape, composed of cellular and non cellular components; it is comprised of tumoral cells, as well as fibroblasts, endothelial cells, various immune cells and non cellular matrix proteins and ligands, collectively referred to as stroma. Cancer associated fibroblasts (CAFs) are one of the most abundant stromal components of the TME and have been demonstrated to play a prominent role in cancer pathogenesis [1–4]. CAFs are a highly heterogenous ‘catch-all’ description for several subpopulations of activated fibroblasts that function differently depending on their numerous precursors (i.e., tissue-resident fibroblasts, trans-differentiated endothelial or epithelial cells or bone marrow-derived mesenchymal stem cells) [5–8], and on the local microenvironmental context (i.e., hypoxia or distance to tumor) [9–11]. For example, CAF subpopulations can undergo metabolic reprogramming to provide a supportive niche for adjacent cancer cells [12]. Moreover, recent single cell studies have demonstrated the broad heterogeneity of CAF within individual tumors in mice or humans [13–17]. The evolving nature of CAF subpopulation compositions makes CAFs difficult to study in culture, as conventional culture methods can select and modify the subpopulations, therefore changing their functional behavior.
Activation of CAFs depends on tumor induced signals, including TGF-β, to contract, remodel and secrete extracellular matrix (ECM) proteins, ultimately altering the TME [6]. Many studies have established a link between patient outcome and CAF number, complexity or function [18–20]. CAFs improve organoid and cancer cell growth in vitro [11], as well as enhance invasion and migration of associated cancer cells during metastatic disease progression [21,22]. While the mechanisms of these actions remain unclear, they are likely to require a multidisciplinary understanding of the cancer ecosystem, as CAFs direct remodeling and stiffening of the ECM, phenotypes which have been correlated with breast cancer aggression and therefore patient outcome [23]. Fibroblast heterogeneity also contributes to promoting an immunosuppressive microenvironment [8] as well as metastatic progression [24]. Hence, CAFs may serve as a viable target for anticancer therapies.
Although our knowledge of CAF complexity in the TME is still evolving [25], targeting CAF mediated ECM changes and associated downstream signaling have become increasingly appealing strategies to modulate CAF cancer cell communication. However, identifying such targets has not yet translated into clinical benefit. For example, inhibitors of the CAF dependent hedgehog pathway, IPI-926, failed to recapitulate the overall survival benefits shown in mouse model trials [26–28] and paradoxically decreased patient survival when added to the standard of care [29]. While the reasons for this failure remain unclear, this example highlights the complex roles of CAFs in both stabilizing and supporting the TME.
In this review, we outline the current understanding of CAF biology with specific emphasis on their role in modulating cancer cell drug response. We then discuss the limitations of current models, as well as the complications of studying CAFs in conventional model systems. We conclude by proposing that specific features arising from the relationship between CAFs and cancer cells should be included in the next generation of drug discovery platforms and suggest technological approaches currently being developed that may be of value in this area.
CAFs modulate cancer drug efficacy
Recent reviews have summarized the results of therapeutic strategies focused on modulating CAF behavior [25,30–34]. The general lack of success in this area suggests that we do not yet fully understand the role of CAFs in altering cancer responses, particularly to therapeutic strategies. It is therefore important to briefly review how CAFs are known to modulate cancer drug efficacy, as this will likely affect the design of drug screening platforms.
Collectively, CAF subpopulations modify therapeutic efficacy in several ways. First, CAFs are highly secretory cells, altering cancer cell phenotypes through paracrine cell-to-cell soluble signaling: modulating cancer cell stemness [35–37], increasing cancer cell epithelial to mesenchymal transition through TGF-β signaling [38], altering chemotherapeutic responsiveness [39–42], as well as immune evasion through production of chemokine CXCL12 [43–45] and TGF-β [46,47]. Further, a dense fibrotic stroma is also a common feature of immunotherapy resistant tumors, where signatures of TGF-β induced desmoplasia in the stroma is associated with restricted T-cell infiltration into the tumor [48]. Interestingly, dual targeting of TGF-β and immune checkpoint inhibitor, PD-1, is currently under clinical trial and showing some promise in improving the success of immune checkpoint therapies [49]. Second, existing therapies can often create fibrotic and tissue-stiffening side effects, which are thought to be mediated by CAFs. These fibrotic reactions are associated with overall worsened survival [23]. For example, the highly publicized B-Raf inhibitor used to treat advanced melanoma, activates stromal fibroblasts [50–52], while radiotherapy in general increases fibrosis [53]. Hence, CAF behavior may unintentionally be triggered by conventional therapies, in turn modulating the efficacy of said therapy. Taken together, these findings collectively suggest that CAFs are a crucial player in therapeutic response and ultimately in modulating patient outcome. Understanding the effect of therapeutic agents on CAF function and thus the TME, is evidently crucial for the development of new therapeutic strategies.
Current cancer models capturing CAF functionality
The biology of CAFs has been studied using a variety of strategies ranging from conventional 2D culture or histology sections, to mouse models and ex-vivo tissues slices [25]. Current consensus is that 3D models are essential for studying CAFs, as they support the formation of oxygen, nutrient and growth factor gradients similar to those that occur in vivo [54,55]. They also enable the formation of 3D spatial cellular organization, so that cells simulate bidirectional cell–cell and cell–ECM interactions critical for evaluating stroma-mediated effects on cancer development and progression [56]. Moreover, 2D tissue culture plastic presents mechanical and topographical cues that alter fibroblast behavior [57], and renders CAFs less secretory than in 3D [58]. Given this established knowledge, we will limit this section to 3D models that incorporate CAF activity for drug discovery.
Mouse models have been used extensively to build an understanding of CAF function in vivo. These models demonstrate that non-specific deletion of CAFs or fibrosis causes rapid tumor progression rather than suppression [4,59–61]. These findings outlined the foundation of future studies, promoting researchers to focus on altering CAF behavior rather than ablating CAFs altogether. Although useful, mouse models are far from a perfect system. In co-injection models, where human cancer samples are introduced into the mouse, host-derived fibroblasts will outgrow the co-injected CAFs, leading a study to focus on the interaction of mouse fibroblasts with foreign tissue [25]. With transgenic mouse models, activation of fibroblasts relies on a Cre-driver, yet no CAF-specific Cre driver exists, so intended CAF inhibition or ablation leads to off-target cellular effects. Moreover, they are extremely low-throughput and not a direct parallel to human disease progression.
Controlled in vitro studies of CAF biology are most commonly performed today using low throughput, 3D, collagen or Matrigel ECM scaffolds with tumor organotropic cultures. Mixing epithelial tumor organoids and fibroblast cells in 3D matrices supports CAF-induced improvements in organoid passaging capabilities and enhanced cellular growth through direct cell–cell contact [11,62–65], highlighting the symbiotic interactions between tumor cells and CAFs. Using an organotypic culture system where cells are seeded on top of a 3D matrix, CAFs have also been shown to enhance ECM remodeling in a manner that supports tumor cell invasion [21,22]. Moreover, conditioned media from CAF cultures can enhance tumor growth, invasion and resistance [41,66,67], without dynamic cell–cell interactions. The use of CAFs instead of normal fibroblasts in these systems is essential; in both premalignant and malignant mammary epithelial cells, CAFs promote epithelial to mesenchymal transition, while normal breast fibroblasts favour the maintenance of epithelial morphology and constrain metastasis [68], therefore altering therapeutic response.
While these organotypic approaches have defined key roles for CAFs in tumor biology, they are limited in throughput. To increase experimental throughput with minimal biological source material, microfluidic systems have been developed for CAF and cancer co-culture studies. These devices have been used to demonstrate increased cancer cell growth and invasion into physiologically-relevant matrices [69,70], as well as response to chemotherapy [71].
3D bioprinting is also gaining traction in studying CAF biology, as it allows the formation of precisely arranged cells within tissue-like structures, while simultaneously controlling the mechanical properties of the bio-printed ECM [72]. These specific capabilities allow the formation of realistic culture environments important for physiologically relevant CAF function. Printing lung cancer epithelial cells and CAFs in a physiologically relevant matrix stiffness [73], demonstrate that robust and manipulable in vitro models of human tumors can be bioprinted. Furthermore, Langer et al. successfully printed cancer cells, fibroblasts and epithelial cells, demonstrating that distinct microenvironments that differentially effect proliferation, ECM deposition and migration, can be recapitulated [74]. This demonstrates that these models can be used to interrogate complex tumor–stromal interactions in physiologically relevant and manipulable environment. However, the application of these as high-throughput screening methods, is limited by the availability of primary cells and cell detection methods (i.e., imaging techniques to decipher cell types).
These studies collectively demonstrate that the relationship between CAFs and cancer cells is both symbiotic and dynamic. While these studies have led to significant gains in the understanding of tumor biology, future drug discovery models need to better encompass the functionality of CAFs and their influence on tumor drug response. In this next section we aim to elucidate the key considerations for future drug studies.
Complications with CAFs: emerging questions for future studies
Given that CAFs play a complex role in tumor restraint and growth, drug discovery must incorporate CAFs. However, addressing the functionality of CAFs in such assays implies that a simple live/dead readout for drug screens is inadequate. Given the microenvironmental impacts outlined in the previous section, we believe that future cancer drug discovery must consider: the heterogeneity of CAF populations; their role in enhancing tumor invasion; and their contribution to dynamic tissue mechanics (Figure 1); all of which have been shown impact drug activity and response, and which cannot be easily recreated in standard drug screens. Here, we overview CAF functions that must be considered in future studies.
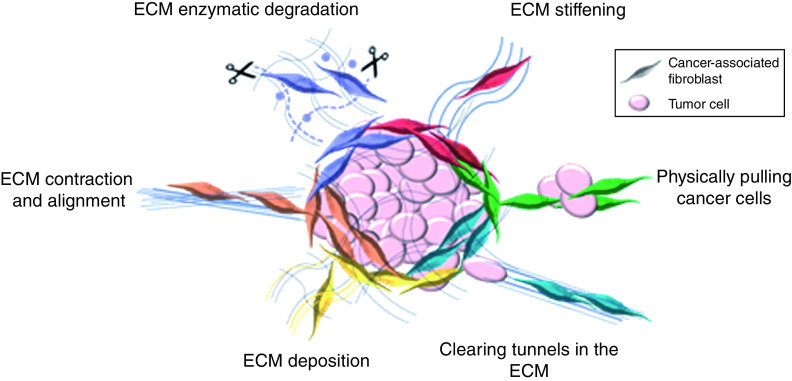
Figure 1. . The dynamic, functionality of cancer-associated fibroblasts impacts extracellular matrix remodeling and cancer cell invasion.
Cancer-associated fibroblasts (CAFs) are a highly heterogenous population of cells, with distinct key features that impact cancer progression. CAFs are responsible for dynamically modulating the ECM, though contraction and alignment (orange), ECM deposition (yellow), matrix stiffening (red) and enzymatic degradation (purple). CAFs also play a key role in modulating tumor invasion by clearing tunnels in the ECM (blue) or physically pulling cancer cells through cadherins junctions (green). ECM: Extracellular matrix.
First, sufficient evidence for the presence and impact of CAF heterogeneity now exists in both in vivo and in vitro models [5,7,8,75–77] to support considering this complication in drug discovery (Figure 1). It is likely that the subtypes of CAFs are plastic, with capacity to transition between CAF states; activated fibroblasts are known to exhibit multipotency [78–80], and CAFs are dependent on the microenvironment to influence their subtype [81]. Öhlund et al. demonstrated the conversion of CAF subtypes based on their proximity to tumor cells. Additionally, the heterogeneity of CAF populations is induced and stabilized by CAF signaling [82]. Current studies focusing on changes in CAF populations are limited, even though understanding the interconversion in response to therapies may be key for drug discovery.
Second, CAFs play a key functional role in tumor invasion by; secreting proteases that break down ECM to enable cancer cell motility [3,83,84], clearing tunnels in the ECM [22] and physically pulling cancer cells through heterotypic cell junctions (Figure 1) [85]. To understand drug efficacy on these CAF functional phenotypes, systems must track the movement of individual cells, a process that has been challenging to scale to high-throughput screens, while maintaining a suitable level of robustness. This is challenged by the fact that typical invasion assays follow cumbersome procedures [86,87] and have end point readouts with low signal to background ratio [88]. Moreover, they require precise, automated, multidimensional microscopy and analysis software, an expensive addition to these studies.
Finally, CAFs are mechanically competent cells that both respond to and change their physical environment, by remodeling the tumor ECM through contraction, secretion, crosslinking and aligning of the surrounding collagen and fibrillar proteins (Figure 1) [6]. Given the broadly-established impact of 3D tissue mechanics on biological function [89], this can significantly influence the direct response of cancer tumors to candidate therapeutics. Progressive deposition and remodeling of the ECM by CAFs is associated with disease transformation in human breast cancer [23] and in vitro analysis shows that changes in the ECM alter breast cancer aggression [90–94]. Moreover, remodeling also aligns, thickens and straightens ECM fibres, where signatures of this are an independent prognostic indicator of poor disease progression [95,96]. This remodeling, thickening and deposition of the ECM also contributes to an overall increase in tissue stiffening, shown in mouse models and human patient samples to foster tissue transformation and metastasis [23,97–99]. Although tissue stiffening is recognized as an important factor in cancer epithelial drug discovery [100–102] and promoting chemo resistance [103,104], current drug discovery models lack the dynamic interplay between CAFs and the ECM. In addition to stiffening the environment, aggressive tumors typically have dense and aligned ECM [23], providing highways for cells to invade and altering cancer cell signaling and behavior. We believe capturing these mechanical phenotypes arising from CAF inclusion is therefore critical to identifying better stromal targeted therapies and; thereby, improving patient outcome.
Moving forward: emerging technologies
Several emerging technologies from various fields might allow us to bridge the gaps between cancer drug discovery and patient benefit by incorporating CAFs. While there have been major advances in recent work aimed at targeting CAFs, the TME or cancer cells directly, implementing these approaches into next-generation high-throughput screening will improve overall drug efficacy. We highlight emerging strategies to improve the drug development process by: incorporating CAFs via high-throughput organoid co-cultures; conducting assays in matrices that consider realistic mechano- and biological elements; and integrating techniques designed to measure functional CAF behaviors in living cultures.
Organoid co-cultures
Patient cancer cell derived organoids have gained increased traction in drug discovery. While they have a 3D, functional ECM for the cancer cells to interact with, conventional cancer drug studies with organoids lack stromal CAFs. Since CAFs play a key role in reshaping the TME, we argue that the addition of CAFs to such organotypic cultures is essential. In fibrosis, by incorporating multiple cell types, a clear resemblance between the in vitro cultures and human disease pathophysiology is possible [105,106]. Similarly, in liver fibrosis in vitro, 3D multicellular tissues enable preclinical screening of antifibrotic drugs [107,108], further highlighting the importance of the microenvironment in drug screening. Multiple commercially available systems now exist for high-throughput multicellular, physiologically relevant in vitro assays. Some examples of these include; organ-on-a-chip systems for mechanically realistic lung–blood barriers [109]; 3D co-culture chips that support barrier integrity-, transport- and migration assays [110]; tissue culture force sensors to measure human heart health [111]; and bioreactors to model human pulmonary fibrosis [112]. The use of these assays would allow for the interrogation of complex biological questions involving cell–cell and cell–ECM interactions that would encompass the dynamic invasive and mechanical changes induced by CAFs.
Advanced biomaterials
Physical characteristics of the tissue ECM vary substantially in vivo, with changes in fiber length, thickness, density and organization. Given that these changes are induced largely by CAF remodeling, building models that recapitulate the mechanobiological elements of the surrounding TME will reduce the need for CAFs within the system. For example, the use of pre-aligned matrices [113] or fibroblast preconditioned matrices could be used for invasion assays incorporating the dense and aligned matrix highways seen in aggressive tumors [23]. Advanced biomaterial formulations that consider these factors may therefore capture the mechanical effect of CAFs, without the need to obtain and include live CAFs themselves. While this approach would not capture the dynamic interactions between CAFs and cancer cells, the ability to recreate this important phenotype may improve translational screening efficiency and translational realism while maintaining the assay robustness required for drug screening technologies.
Advanced biomaterials can be tailored de novo to present specific characteristics [114], or can be used in blended formulations to modify the properties of existing materials. For example, Matrigel is well-established in many organoid culture protocols, but is challenging to mechanically tune for specific applications. Interpenetrating polymer networks such as gelatin [115–117], hyaluronic acid [118–120] or alginate [102] may be used as a supporting network to modulate substrate stiffness to physiologically relevant levels, while avoiding any modifications to critically important ligand composition or density.
In addition to linear elastic modulations, physiological ECM also exhibits more complex material behaviors such as stress relaxation, or viscoelasticity, parameters proving to be critically important in designing matrices for drug discovery [89]. For example, it has been shown that use of soft substrates with stress relaxation in 3D, promotes cell spreading, fiber remodeling and focal adhesion formation [121–123], emphasizing the importance of incorporating physical cues from the ECM in regulating cellular phenotype and therefore drug response. Additionally, human breast tumor samples exhibit ECM plasticity [124], a permanent deformation of the ECM. Given that, fibroblasts can produce stresses large enough to permanently deform the biomaterials [125,126], incorporating these forces is critical to recapitulating the effect of CAF functionality, even in their absence.
The contribution of the ECM is more than just mechanical, and due to the diverse range of proteins, proteoglycans, growth factors and other enzymes, it presents a wide range of biological cues to the cells. The use of decellularized ECM (dECM) is well studied in idiopathic pulmonary fibrosis, where it activates myofibroblasts [127] and alters fibroblast gene expression [128]. By implementing this in gut models for intestinal fibrosis it increases the fidelity of disease modeling [129] and the throughput of drug screening [130]. However, the systematic use of dECM is not ideally suited for highly systematic drug screening processes in the context of cancer; biological material available for such assays is limited to the size of the excised tumor and tends to largely vary in composition from patient to patient. While it has proven reliable in other systems, the use of dECM may therefore only be relevant in the context of personalized cancer therapeutic screenings.
Advanced readouts
If CAFs primarily modulate tumor response via mechanical activity, studies to assess the extent to which CAFs remodel the ECM, exert mechanical forces and mechanically tune their surroundings will grow in importance. Emerging microscale-engineered technologies that allow quantitative measurements of mechanical changes in tissues, may prove an effective tool in understanding the changes made to the environment by CAFs, to better understand and ultimately simulate their activity. Several recently developed technologies can provide insight into fibroblast behavior at this extremely local length-scale. Asmani et al. developed a 3D fibrotic microtissue array, in which 3D-cultured fibroblasts remodel the surrounding matrix to deform micro-engineered pillars that anchor the matrix to the substrate. Analyzing the deformation of these pillars provides readouts of forces generated by the CAFS, and therefore enable quantification of fibrosis and drug efficacy testing [87]. This fundamental premise has recently been expanded toward developing dispersible microfabricated sensors that can be applied in a variety of culture contexts to quantify cell-generated mechanical forces [131,132], mechanical compressive forces [133], residual tissue elasticity [134] and other mechanical properties of tissues [135,136]. Reducing the size and accessibility of these sensors may hence prove quite valuable in understanding the CAF contributions to the surrounding matrix at the cellular level, to better understand tissue dynamics in response to therapy.
Conclusion
It is evident that tumors can no longer be viewed as static clumps of cancerous cells; the complex and dynamic interactions with the surrounding TME play a key role in altering cancer cell response to therapy and therefore patient outcome. Accumulating work suggests improved strategies could be possible by targeting CAFs; however, the disconnect between drug discovery and clinical benefit remains. Therefore, we believe carefully assessing the impact of cancer cell or TME targeted therapies on the mechanical and functional forces within the TME, prior to clinical translation, is critical for narrowing the translational gap. We propose the use of organotypic co-cultures, advanced biomaterials and various force sensors as technological advances that will be instrumental in improving the drug discovery pipeline. Accommodating CAFs and CAF modulated ECM in this process, has the potential for physiologically relevant discoveries, bridging the gap between bench-side and clinical benefits.
Future perspective
Unfortunately, nearly 25 years after the initial studies showing the physiological relevance of 3D culture systems [56,137,138], the vast majority of drug discovery pipelines are still in 2D. This is likely due to the ease of culture, growth and biochemical testing in 2D systems as well as the relative success of these technologies in identifying useful molecules. However, it would appear that the low-hanging fruit of easily identifiable therapeutic molecules have already been identified, as evidenced by the dwindling number of novel therapeutic discoveries, despite increases in economic resources allocated to this problem [139]. Furthermore, the poor clinical translatability for many seemingly-promising drugs suggests that 2D systems are no longer sufficient in this area, and that more complex 3D culture systems will be required to identify next-generation therapeutics. Moving forward, we believe advanced technologies will bridge this gap, in improving the physiological relevance of such assays, while also improving the ease of setting up, operating and analyzing information from 3D assays; such as those outlined in this review. Moreover, we anticipate the inclusion of immune cell populations will become more and more important, as numerous recent studies have demonstrated a key role for immune cells in tumor progression [23,44,48,49,140]. Given that there are small subsets of patients who respond to immune checkpoint inhibitors [141], perhaps other microenvironmental factors dictate patient responsiveness to checkpoint blockade. Recent findings are demonstrating that CAFs may be critically important in these microenvironmental feature sets that drive immune evasion [8,43,46,47,142–147], and so these advanced discovery systems may better pair patient populations with successful therapeutics. The consideration of immune cell infiltration in these future drug discovery models will be significant. In our view, the ongoing collaborations between engineers and biological scientists will be fundamental in the successful building and implementation of new models and technology for future drug discovery.
Executive summary.
Background
Cancer-associated fibroblasts (CAFs) are one of the most abundant stromal components of the tumor microenvironment, playing a prominent role in cancer pathogenesis.
Clinically targeting CAFs has become an increasingly appealing approach for cancer therapies, yet a disconnect between drug discovery and clinical benefit remains.
CAFs modulate cancer drug efficacy
CAFs modulate cancer drug efficacy through: secretions; extracellular matrix remodeling; and off-target activation by conventional chemotherapies.
Current models capturing CAF functionality
Current 3D models: mouse models; organotypic co-culture models; 3D bioprinting demonstrate that the relationship between CAFs and cancer cells is dynamic and that these functionalities are missing from current drug discovery.
Complications with CAFs: emerging questions for future studies
In order to improve cancer therapy efficacy, future drug discovery must capture: the heterogeneity of CAF populations; their role in enhancing tumor invasion; and their contribution to tissue mechanics.
Moving forward: emerging technologies
Several emerging technologies from various fields have the potential to improve the drug development by incorporating CAFs.
We propose the use of: high-throughput organoid co-cultures; conducting assays in matrices that consider realistic mechano- and biological elements; and integrating techniques designed to measure functional CAF behaviors in living cultures.
Conclusion
Given that the complex and dynamic interaction of the tumor and surrounding microenvironment play a key role in altering response to therapy, we believe that studying the impact of therapies on the mechanical and functional forces within the tumor microenvironment is critical for narrowing the translational gap.
Footnotes
Financial & competing interests disclosure
The authors gratefully acknowledge support from the NSERC post graduate scholarships program to G Brewer, the Canadian Cancer Society (Grant 706002), the Canadian Institutes of Health Research (Grant 01871-000) and the Canada Research Chairs in Advanced Cellular Microenvironments to C Moraes; and the Oncopole (265878), Tissue Bank Axis of the Réseau de Recherche en Cancer of the Fonds de Recherche du Québec-Santé and the Québec Breast Cancer Foundation and certified by Canadian Tumor Repository Network (CTRNet) to M Park. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell 144(5), 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Experimental Cell Res. 264(1), 169–184 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Finak G, Bertos N, Pepin F et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 14(5), 518 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Rhim AD, Oberstein PE, Thomas DH et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25(6), 735–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 211(8), 1503–1523 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16(9), 582–598 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 5(12), 1640–1646 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Costa A, Kieffer Y, Scholer-Dahirel A et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33(3), 463–479.e410 (2018). [DOI] [PubMed] [Google Scholar]; • Highlights the complexity and heterogeneity of cancer-associated fibroblasts (CAF) populations in vivo, outlining subpopulations across different subtypes of breast cancer. It underpins the need to expand our understanding of CAF populations for the purpose of clinical benefit.
- 9.Becker LM, O'connell JT, Vo AP et al. Epigenetic reprogramming of cancer-associated fibroblasts deregulates glucose metabolism and facilitates progression of breast cancer. Cell Rep. 31(9), 107701 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Giannakou A, Wyman S et al. Cancer-associated fibroblasts suppress SOX2-induced dysplasia in a lung squamous cancer coculture. Proc. Natl Acad. Sci. 115(50), E11671–E11680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Öhlund D, Handly-Santana A, Biffi G et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exper. Med. 214(3), 20162024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This seminal study demonstrates that CAF subtypes are microenvironmentally dependent, and recapitulates the heterogeneity of CAF populations seen in mouse and human pancreatic ductal adenocarcinoma.
- 12.Jia D, Park JH, Jung KH, Levine H, Kaipparettu BA. Elucidating the metabolic plasticity of cancer: mitochondrial reprogramming and hybrid metabolic states. Cells 7(3), 21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elyada E, Bolisetty M, Laise P et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9(8), 1102–1123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung HC, Cho EJ, Lee H et al. Integrated single-cell RNA sequencing analyses suggest developmental paths of cancer-associated fibroblasts with gene expression dynamics. Clin. Transl. Med. 11(7), e487 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieffer Y, Hocine HR, Gentric G et al. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. 10(9), 1330–1351 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Bartoschek M, Oskolkov N, Bocci M et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 9(1), 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Zhou L, Liu L et al. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 11(1), 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calon A, Lonardo E, Berenguer-Llergo A et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Gen. 47(4), 320 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Franco-Barraza J, Francescone R, Luong T et al. Matrix-regulated integrin αvβ5 maintains α5β1-dependent desmoplastic traits prognostic of neoplastic recurrence. Elife 6, e20600 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guinney J, Dienstmann R, Wang X et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21(11), 1350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvo F, Ege N, Grande-Garcia A et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15(6), 637–646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaggioli C, Hooper S, Hidalgo-Carcedo C et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392 (2007). [DOI] [PubMed] [Google Scholar]; • Highlights the role for CAFs in promoting tumor cell invasion.
- 23.Acerbi I, Cassereau L, Dean I et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 7(10), 1120–1134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Illustrates the correlation between CAF induced stromal stiffening and increased cancer aggression.
- 24.Pelon F, Bourachot B, Kieffer Y et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat. Commun. 11(1), 1–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahai E, Astsaturov I, Cukierman E et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldmann G, Habbe N, Dhara S et al. Hedgehog inhibition prolongs survival in a genetically engineered mouse model of pancreatic cancer. Gut 57(10), 1420–1430 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MJ, Hatton BA, Villavicencio EH et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc. Natl Acad. Sci. 109(20), 7859–7864 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olive KP, Jacobetz MA, Davidson CJ et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324(5933), 1457–1461 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madden JI. Infinity reports update from phase 2 study of saridegib plus gemcitabine in patients with metastatic pancreatic cancer. Infinity Pharmaceuticals (2012). http://phx.corporate-ir.net/phoenix.zhtml?c=121941&p=irol-newsArticle&ID=1653550
- 30.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 18(2), 99–115 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Lebleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis. Models Mech. 11(4), dmm029447 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaemmerer E, Loessner D, Avery VM. Addressing the tumour microenvironment in early drug discovery: a strategy to overcome drug resistance and identify novel targets for cancer therapy. Drug Discov. 26(3), 663–676 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Yang Q, Tan Y et al. Cancer-associated fibroblasts suppress cancer development: the other side of the coin. Front. Cell Dev. Biol. 9, 146 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T, Han C, Wang S et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 12(1), 1–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su S, Chen J, Yao H et al. CD10+ GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172(4), 841–856.e816 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Chen WJ, Ho CC, Chang YL et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat. Commun. 5(1), 1–17 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Vermeulen L, De Sousa E Melo F, Van Der Heijden M et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12(5), 468–476 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Yu Y, Xiao C, Tan L, Wang Q, Li X, Feng Y. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 110(3), 724–732 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ham IH, Oh HJ, Jin H et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 18(1), 68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiao Y, Zhang C, Li A et al. IL6 derived from cancer-associated fibroblasts promotes chemoresistance via CXCR7 in esophageal squamous cell carcinoma. Oncogene 37(7), 873–883 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Deng T, Liu R et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 19(1), 1–17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Zhu D, Chen F et al. SFRP2 augments WNT16B signaling to promote therapeutic resistance in the damaged tumor microenvironment. Oncogene 35(33), 4321–4334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Feig C, Jones JO, Kraman M et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. 110(50), 20212–20217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen IX, Chauhan VP, Posada J et al. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc. Natl Acad. Sci. 116(10), 4558–4566 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biasci D, Smoragiewicz M, Connell CM et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl Acad. Sci. 117(46), 28960–28970 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mariathasan S, Turley SJ, Nickles D et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554(7693), 544–548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tauriello DV, Palomo-Ponce S, Stork D et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554(7693), 538–543 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Gruosso T, Gigoux M, Manem VSK et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Investig. 129(4), 1785–1800 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lind H, Gameiro SR, Jochems C et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J. Immunother. Cancer 8(1), e000433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirata E, Girotti MR, Viros A et al. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin β1/FAK signaling. Cancer Cell 27(4), 574–588 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fedorenko IV, Wargo JA, Flaherty KT, Messina JL, Smalley KS. BRAF inhibition generates a host–tumor niche that mediates therapeutic escape. J. Investig. Dermatol. 135(12), 3115–3124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Straussman R, Morikawa T, Shee K et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487(7408), 500–504 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 141(11), 1985–1994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moraes C, Simon AB, Putnam AJ, Takayama S. Aqueous two-phase printing of cell-containing contractile collagen microgels. Biomaterials 34(37), 9623–9631 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langhans SA. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 9, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 294(5547), 1708–1712 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr. Biol. 4(4), 410–421 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Sung KE, Su X, Berthier E, Pehlke C, Friedl A, Beebe DJ. Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PloS ONE 8(10), e76373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Özdemir BC, Pentcheva-Hoang T, Carstens JL et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 28(6), 831–833 (2015). [DOI] [PubMed] [Google Scholar]; • Demonstrates the importance of CAFs in tumor progression, and outlines future studies focused on deactivating CAFs, rather than depleting them.
- 60.Zhang J, Chen L, Liu X, Kammertoens T, Blankenstein T, Qin Z. Fibroblast-specific protein 1/S100A4–positive cells prevent carcinoma through collagen production and encapsulation of carcinogens. Cancer Res. 73(9), 2770–2781 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Gerling M, Büller NV, Kirn LM et al. Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nat. Commun. 7(1), 1–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bachem MG, Schünemann M, Ramadani M et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128(4), 907–921 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Hwang RF, Moore T, Arumugam T et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 68(3), 918–926 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sousa CM, Biancur DE, Wang X et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536(7617), 479–483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vonlaufen A, Phillips PA, Xu Z et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 68(19), 7707–7710 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Shintani Y, Fujiwara A, Kimura T et al. IL-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J. Thorac. Oncol. 11(9), 1482–1492 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Awaji M, Futakuchi M, Heavican T, Iqbal J, Singh RK. Cancer-associated fibroblasts enhance survival and progression of the aggressive pancreatic tumor via FGF-2 and CXCL8. Cancer Microenviron. 12(1), 37–46 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dumont N, Liu B, Defilippis RA et al. Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia 15(3), 249–262 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu T, Lin B, Qin J. Carcinoma-associated fibroblasts promoted tumor spheroid invasion on a microfluidic 3D co-culture device. Lab Chip 10(13), 1671–1677 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Jeong SY, Lee JH, Shin Y, Chung S, Kuh HJ. Co-culture of tumor spheroids and fibroblasts in a collagen matrix-incorporated microfluidic chip mimics reciprocal activation in solid tumor microenvironment. PloS ONE 11(7), e0159013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ying L, Zhu Z, Xu Z et al. Cancer associated fibroblast-derived hepatocyte growth factor inhibits the paclitaxel-induced apoptosis of lung cancer A549 cells by up-regulating the PI3K/Akt and GRP78 signaling on a microfluidic platform. PloS ONE 10(6), e0129593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact. Mater. 3(2), 144–156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mondal A, Gebeyehu A, Miranda M et al. Characterization and printability of sodium alginate-gelatin hydrogel for bioprinting NSCLC co-culture. Sci. Rep. 9(1), 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Langer EM, Allen-Petersen BL, King SM et al. Modeling tumor phenotypes in vitro with three-dimensional bioprinting. Cell. Rep. 26(3), 608–623.e606 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bauer M, Su G, Casper C, He R, Rehrauer W, Friedl A. Heterogeneity of gene expression in stromal fibroblasts of human breast carcinomas and normal breast. Oncogene 29(12), 1732 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orimo A, Weinberg RA. Heterogeneity of stromal fibroblasts in tumor. Cancer Biol. Ther. 6(4), 618–619 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Hussain A, Voisin V, Poon S et al. Distinct fibroblast functional states drive clinical outcomes in ovarian cancer and are regulated by TCF21. J.Exp.Med. 217(8), e20191094 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyake T, Kalluri R. Cell plasticity helps hearts to repair. Nature 514(7524), 575–576 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Ubil E, Duan J, Pillai IC et al. Mesenchymal–endothelial transition contributes to cardiac neovascularization. Nature 514(7524), 585–590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lorenz K, Sicker M, Schmelzer E et al. Multilineage differentiation potential of human dermal skin-derived fibroblasts. Exp. Dermatol. 17(11), 925–932 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Elwakeel E, Brüggemann M, Fink AF et al. Phenotypic plasticity of fibroblasts during mammary carcinoma development. Int. J. Mol. Sci. 20(18), 4438 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Biffi G, Oni TE, Spielman B et al. IL1-Induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9(2), 282–301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J. Cell Biol. 139(7), 1861–1872 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120(3), 303–313 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Labernadie A, Kato T, Brugués A et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19(3), 224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Q, Chen C, Kapadia A et al. 3D models of epithelial-mesenchymal transition in breast cancer metastasis: high-throughput screening assay development, validation, and pilot screen. J. Biomol. Screen. 16(2), 141–154 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Adanja I, Debeir O, Mégalizzi V, Kiss R, Warzée N, Decaestecker C. Automated tracking of unmarked cells migrating in three-dimensional matrices applied to anti-cancer drug screening. Exp. Cell Res. 316(2), 181–193 (2010). [DOI] [PubMed] [Google Scholar]
- 88.Echeverria V, Meyvantsson I, Skoien A, Worzella T, Lamers C, Hayes S. An automated high-content assay for tumor cell migration through 3-dimensional matrices. J. Biomol. Screen. 15(9), 1144–1151 (2010). [DOI] [PubMed] [Google Scholar]
- 89.Ort C, Lee W, Kalashnikov N, Moraes C. Disentangling the fibrous microenvironment: designer culture models for improved drug discovery. Expert Opin.Drug Discov. 16(2), 159–171 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Jechlinger M, Podsypanina K, Varmus H. Regulation of transgenes in three-dimensional cultures of primary mouse mammary cells demonstrates oncogene dependence and identifies cells that survive deinduction. Genes Dev. 23(14), 1677–1688 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer 5(9), 675–688 (2005). [DOI] [PubMed] [Google Scholar]
- 92.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30(3), 256–268 (2003). [DOI] [PubMed] [Google Scholar]
- 93.Weaver VM, Petersen O, Wang F et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137(1), 231–245 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J. Cell Sci. 116(12), 2377–2388 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Provenzano PP, Inman DR, Eliceiri KW et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 6(1), 1–15 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4(1), 38 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This pioneering study demonstrates that stromal induced remodeling at the tumoral boundaries plays a prominent role in cancer cell invasion and; therefore, patient outcome.
- 97.Lopez JI, Kang I, You WK, Mcdonald DM, Weaver VM. In situ force mapping of mammary gland transformation. Integr. Biol. 3(9), 910–921 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levental KR, Yu H, Kass L et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139(5), 891–906 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pickup MW, Laklai H, Acerbi I et al. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-β–deficient mouse mammary carcinomas. Cancer Res. 73(17), 5336–5346 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Medina SH, Bush B, Cam M et al. Identification of a mechanogenetic link between substrate stiffness and chemotherapeutic response in breast cancer. Biomaterials 202, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shin JW, Mooney DJ. Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proc. Natl Acad. Sci. 113(43), 12126–12131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ort C, Chen Y, Ghagre A, Ehrlicher A, Moraes C. Bioprintable, stiffness-tunable collagen-alginate microgels for increased throughput 3D cell culture studies. ACS Biomater. Sci. Eng. 7(6), 2814–2822 (2021). [DOI] [PubMed] [Google Scholar]
- 103.Joyce MH, Lu C, James ER et al. Phenotypic basis for matrix stiffness-dependent chemoresistance of breast cancer cells to doxorubicin. Front. Oncol. 8, 337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rice A, Cortes E, Lachowski D et al. Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 6(7), e352–e352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sachs N, Papaspyropoulos A, Zomer-Van Ommen DD et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 38(4), e100300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strikoudis A, Cieślak A, Loffredo L et al. Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep. 27(12), 3709–3723.e3705 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Norona LM, Nguyen DG, Gerber DA, Presnell SC, Lecluyse EL. Editor's highlight: modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol. Sci. 154(2), 354–367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen DG, Funk J, Robbins JB et al. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PloS ONE 11(7), e0158674 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 328(5986), 1662–1668 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trietsch SJ, Israëls GD, Joore J, Hankemeier T, Vulto P. Microfluidic titer plate for stratified 3D cell culture. Lab Chip 13(18), 3548–3554 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Nunes SS, Miklas JW, Liu J et al. Biowire: a platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat. Methods 10(8), 781–787 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sundarakrishnan A, Zukas H, Coburn J et al. Bioengineered in vitro tissue model of fibroblast activation for modeling pulmonary fibrosis. ACS Biomater. Sci. Eng. 5(5), 2417–2429 (2019). [DOI] [PubMed] [Google Scholar]
- 113.Han W, Chen S, Yuan W et al. Oriented collagen fibers direct tumor cell intravasation. Proc. Natl Acad. Sci. 113(40), 11208–11213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature 462(7272), 433–441 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin CH, Su JJM, Lee SY, Lin YM. Stiffness modification of photopolymerizable gelatin-methacrylate hydrogels influences endothelial differentiation of human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 12(10), 2099–2111 (2018). [DOI] [PubMed] [Google Scholar]
- 116.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31(21), 5536–5544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xiao S, Zhao T, Wang J et al. Gelatin methacrylate (GelMA)-based hydrogels for cell transplantation: an effective strategy for tissue engineering. Stem Cell Rev. Rep. 15(5), 664–679 (2019). [DOI] [PubMed] [Google Scholar]
- 118.Gwon K, Kim E, Tae G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomaterialia 49, 284–295 (2017). [DOI] [PubMed] [Google Scholar]
- 119.Suo A, Xu W, Wang Y, Sun T, Ji L, Qian J. Dual-degradable and injectable hyaluronic acid hydrogel mimicking extracellular matrix for 3D culture of breast cancer MCF-7 cells. Carbohydr. Polym. 211, 336–348 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Shen YI, Abaci HE, Krupski Y, Weng LC, Burdick JA, Gerecht S. Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting. Biomater. Sci. 2(5), 655–665 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bauer A, Gu L, Kwee B et al. Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts. Acta Biomater. 62, 82–90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chaudhuri O, Gu L, Darnell M et al. Substrate stress relaxation regulates cell spreading. Nat. Commun. 6(1), 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lou J, Stowers R, Nam S, Xia Y, Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 154, 213–222 (2018). [DOI] [PubMed] [Google Scholar]
- 124.Wisdom KM, Adebowale K, Chang J et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 9(1), 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim J, Feng J, Jones CA et al. Stress-induced plasticity of dynamic collagen networks. Nat. Commun. 8(1), 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ban E, Franklin JM, Nam S et al. Mechanisms of plastic deformation in collagen networks induced by cellular forces. Biophys. J. 114(2), 450–461 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Booth AJ, Hadley R, Cornett AM et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Critt. Care Med. 186(9), 866–876 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Parker MW, Rossi D, Peterson M et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Investig. 124(4), 1622–1635 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Giuffrida P, Curti M, Al-Akkad W et al. Decellularized human gut as a natural 3D platform for research in intestinal fibrosis. Inflamm. Bowel Dis. 25(11), 1740–1750 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Li N, Sui Z, Liu Y, Wang D, Ge G, Yang L. A fast screening model for drug permeability assessment based on native small intestinal extracellular matrix. RSC Adv. 8(60), 34514–34524 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Campàs O, Mammoto T, Hasso S et al. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods 11(2), 183–189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee W, Kalashnikov N, Mok S et al. Dispersible hydrogel force sensors reveal patterns of solid mechanical stress in multicellular spheroid cultures. Nat. Commun. 10(1), 144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mohagheghian E, Luo J, Chen J et al. Quantifying compressive forces between living cell layers and within tissues using elastic round microgels. Nat. Commun. 9(1), 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mok S, Al Habyan S, Ledoux C et al. Mapping cellular-scale internal mechanics in 3D tissues with thermally responsive hydrogel probes. Nat. Commun. 11(1), 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Serwane F, Mongera A, Rowghanian P et al. In vivo quantification of spatially varying mechanical properties in developing tissues. Nat. Methods 14(2), 181–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Proestaki M, Ogren A, Burkel B, Notbohm J. Modulus of fibrous collagen at the length scale of a cell. Exp. Mech. 59(9), 1323–1334 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roskelley C, Bissell M. Dynamic reciprocity revisited: a continuous, bidirectional flow of information between cells and the extracellular matrix regulates mammary epithelial cell function. Biochem. Cell Biol. 73(7–8), 391–397 (1995). [DOI] [PubMed] [Google Scholar]
- 138.Friedl P, Bröcker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell. Mol. Life Sci. 57(1), 41–64 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chandrasekaran A, Abduljawad M, Moraes C. Have microfluidics delivered for drug discovery? 11(8), 745–748 (2016). [DOI] [PubMed] [Google Scholar]
- 140.Binnewies M, Roberts EW, Kersten K et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24(5), 541–550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. 19(5), 1021–1034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Monteran L, Erez N. The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front.Immunol. 10, 1835 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barrett R, Puré E. Cancer-associated fibroblasts: key determinants of tumor immunity and immunotherapy. Curr. Opin. Immunol. 64, 80–87 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maller O, Drain AP, Barrett AS et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mater. 20(4), 548–559 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suzuki J, Aokage K, Neri S et al. Relationship between podoplanin-expressing cancer-associated fibroblasts and the immune microenvironment of early lung squamous cell carcinoma. Lung Cancer 153, 1–10 (2021). [DOI] [PubMed] [Google Scholar]
- 146.Kato R, Haratani K, Hayashi H et al. Nintedanib promotes antitumour immunity and shows antitumour activity in combination with PD-1 blockade in mice: potential role of cancer-associated fibroblasts. Br. J. Cancer 124(5), 914–924 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ford K, Hanley CJ, Mellone M et al. NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Cancer Res. 80(9), 1846–1860 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]