Abstract
Pharmacovigilance improves patient safety by detecting and preventing adverse drug events. However, challenges exist that limit adverse drug event detection, resulting in many adverse drug events being underreported or inaccurately reported. One challenge includes having access to large data sets from various sources including electronic health records and wearable medical devices. Artificial intelligence, including machine learning methods, such as natural language processing and deep learning, can detect and extract information about adverse drug events, thus automating the pharmacovigilance process and improving the surveillance of known and documented adverse drug events. In addition, with the increased demand for telehealth services, for managing both acute and chronic diseases, artificial intelligence methods can play a role in detecting and preventing adverse drug events. In this review, we discuss two use cases of how artificial intelligence methods may be useful to improve the quality of pharmacovigilance and the role of artificial intelligence in telehealth practices.
Key Points
| Artificial intelligence has an important role in quickly and effectively detecting existing and new adverse drug events during post-marketing surveillance, using large datasets. |
| Artificial intelligence in telehealth can be used to improve pharmacovigilance by utilizing various sources of patient information such as electronic health records, health information technologies, and pharmacovigilance database systems, to detect and prevent medication-related problems. |
| Although artificial intelligence has a promising role in the field of pharmacovigilance and telehealth, there are still challenges including detecting undocumented or unknown adverse drug events, privacy concerns, and technical difficulties. |
Introduction
Pharmacovigilance is defined by the World Health Organization as “the science and activities relating to the detection, assessment, understanding and prevention of adverse effects (AEs) or any other medicine/vaccine related problem” [1]. It involves the surveillance of medications and characteristics of patients who receive them to determine what adverse drug events (ADEs) occur, and which patients are at risk. Adverse drug events, defined as any harm due to drug therapy, are common in all healthcare settings and cause significant morbidity and healthcare costs [2–6]. Rapid identification of ADEs is important to minimize the extent and duration of patient harm from drug therapy.
Pharmacovigilance activities are essential to improve patient safety, both to detect new AEs when drugs are used in large and diverse populations of patients that were not identified in premarketing clinical trials and to detect known AEs occurring in patients receiving care [7, 8]. Current systems used for pharmacovigilance are limited in their ability to efficiently identify ADEs. Many ADEs are unreported to spontaneous reporting systems such as the US Food and Drug Administration Adverse Event Reporting System, patient safety organizations, or health systems’ internal incident reporting systems; one systematic review showed that 94% of ADEs were underreported [9]. Methods to identify ADEs, through chart reviews, interviewing patients, or using rule-based trigger tools, are time consuming for individuals conducting interviews, reviewing charts, or addressing alerts. Signals for ADEs identified from spontaneous reporting systems have been combined with the use of population-based electronic health record (EHR) data to better identify potential new ADEs [10–12]. International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision billing codes from claims data have been used to identify ADEs in patient populations, but the methods are inconsistent, may lack sensitivity in identifying ADEs, and may not be used prospectively for patient care [13–16].
Implementation of artificial intelligence (AI) to identify and predict ADEs can greatly improve current methods of detection. Several of these types of automated models are currently being studied, including utilizing systems to detect outliers and triggers that could indicate potential ADEs [17]. Extensive research is also underway using AI, including machine learning (ML) methods (natural language processing [NLP] and deep learning with neural networks), to predict and identify ADEs with several potential benefits that have been proposed [18, 19]. Remote methods of patient care, such as telehealth visits and digital monitoring, offer an ideal opportunity to improve the detection of ADEs in patients during the long intervals that they are not being seen at a healthcare facility [20–22]. This paper reviews the existing use of AI for pharmacovigilance, and explores potential new applications for AI for pharmacovigilance in telehealth settings.
Role of Artificial Intelligence in Healthcare and Pharmacovigilance
Artificial intelligence is useful for processing vast quantities of data and assessing relationships [23]. Currently, many of the most successful AI applications in healthcare have focused on image interpretation to detect specific concerns, such as worrisome lesions in mammograms [24]. These algorithms can achieve levels of performance that equal or surpass that of expert humans for specific use cases. Digital pathology is another promising area that can apply ML [25].
Detection of AEs of drugs has parallels with radiology and pathology [26]. Although ADE information is not generally captured in images, it is buried in text and among other data such as laboratory results. Plain text searching has been demonstrated to be an effective approach for finding ADEs [27]. Techniques such as AI can be used to sift through very large quantities of EHR data to rapidly identify confluences of data that indicate events [28, 29]. We suggest two major ways in which AI and ML can improve understanding, assessment, and detection in the field of pharmacovigilance: first, processing existing data to develop more accurate estimates of currently documented ADEs and second, generating new data to measure actual rates of ADEs. The first use case leverages AI techniques, such as ML, to process the vast amount of unstructured text from various data sources such as case reports, EHRs including patient portals, registries, medical literature, insurance claims, internet searches, calls to poison control centers, and social media, to extract and interpret drug safety information [30]. These types of solutions could provide more timely and accurate surveillance of known ADEs and signal detection of previously unknown ADEs. Most of the AI and pharmacovigilance literature focuses on processing of AE case reports and signal detection to extract and evaluate the validity of potential ADEs. For example, previously unknown, and likely rare ADEs that may not have been detected in clinical trials, can be discovered using AI to mine scientific databases and patient-generated content (e.g., social media) for potential links between AEs and medication use to inform surveillance [31]. These new data sets can provide additional information outside of industry-reported pharmacovigilance. For example, the European Union’s Innovative Medicines Initiative WEB-RADR project, for example, demonstrates how social media can be used in pharmacovigilance for reporting, detecting signals, and evaluating signals [32, 33]. Social media can contribute to pharmacovigilance, but it will be difficult to implement multipurpose systems that will automatically detect ADEs, and with AI, predict ADEs.
In addition, EHR data can be used to support surveillance. This includes the relatively straightforward task of extracting ADEs reported by healthcare providers or patients using unstructured text in clinical notes or patient correspondence to allow for more comprehensive and timely estimates of documented ADEs [34]. However, most ADEs are not documented in the outpatient record [35]. Missed ADEs can potentially be detected based on reported symptoms, laboratory results, electrocardiograms, or imaging abnormalities documented in the EHR that may have been caused by medication use. In these cases, AI methods can be used to estimate the likelihood that the events are, in fact, ADEs rather than conditions or comorbidities unrelated to medication use.
The second use case leverages AI to facilitate the collection of new or additional information to support more comprehensive ADE assessment. In one study, eight times more ADEs were identified when patients were asked about them, compared with record review alone in the outpatient setting [36]. Artificial intelligence could be leveraged to define patient-level clinical phenotypes that are associated with specific ADEs and automate targeted follow-up with these patients regarding the occurrence of the ADEs of interest. In addition, any potential hidden patterns and missing patients who were not identified with current clinical definitions could be captured. Artificial intelligence can also identify the optimal times and frequencies to screen patients for specific ADEs to obtain more complete information [37]. Although collection and processing of additional data may introduce bias, if the same ADE is documented across multiple data sources or over time, AI techniques can be applied to minimize double counting to provide more accurate estimates.
The overarching goal of pharmacovigilance is prevention of AEs caused by medication use [7]. The knowledge generated through the understanding, assessment, and detection of ADEs can help inform population-level (e.g., guidelines) or patient-level (i.e., personalized) strategies to reduce the frequency and consequences of ADEs. Prevention lies at the heart of precision medicine, sometimes known as “personalized medicine,” which aims to tailor disease prevention and management by developing models that consider factors, such as patients’ genetic predispositions, environments, lifestyles, and health histories, to identify risk profiles and optimal therapies [38]. More accurate detection of ADEs in existing data sources, using AI coupled with patient-level risk factors, can lead to the creation and curation of large comprehensive datasets for the development of clinical decision support tools.
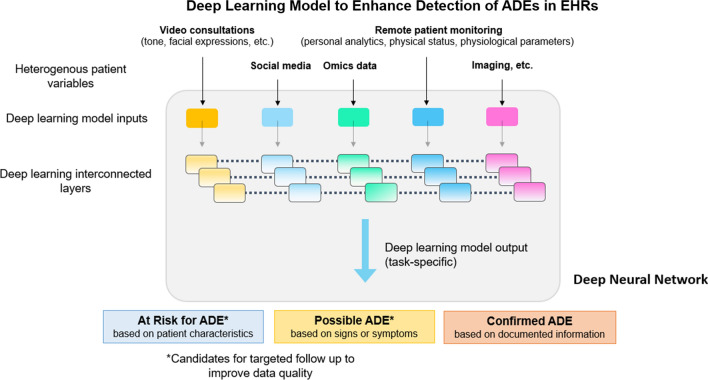
Machine learning methods have shown robust performance for disease risk predictions by learning from patients’ history (variables) and identifying hidden patterns among large study cohorts [39]. In most cases, supervised learning algorithms were implemented rather than unsupervised methods, in which the models were developed with known labels [40]. Similarly, tools can be developed and implemented at the point of care that leverage patient-specific information documented in the EHR for the prevention and early detection of ADEs. In this context, several ADEs have been associated with specific genetic mutations that can affect both pharmacodynamics and pharmacokinetics and predispose towards ADEs [41]. Artificial intelligence solutions may also provide insights for selecting alternative medications, optimizing dosage, or implementing strategies to manage anticipated AEs, and deep learning can play a significant role (Fig. 1) [42–50].
Fig. 1.
Advantages of deep learning for pharmacovigilance. The outcome of the model can be the high-risk patient population for future adverse drug events (ADEs) or specific types of ADEs, or patients’ responses to treatment. The data sources, both clinical and genetic variables, were shown to be able to contribute to the prediction performance, suggesting the advantages of integrating different types of input data for model development. Among different model types, the traditional regression model, given its better interpretability compared with more complicated machine learning approaches, can be limited by the number of input features. Different model types based on machine learning, including support vector machine and tree-based models, however, showed better predictive power in some recent studies [42, 43]. Deep learning, a subset of machine learning that refers to algorithms using complex neural networks with many hidden layers, was also applied for ADE prediction. In recent studies, deep learning-based algorithms showed superiority over other methods [44]. This is due to increasingly available large datasets and the ability to identify complex non-linear patterns using deep learning models. However, because of their complexity, the algorithms may be non-interpretable to the human brain and are considered black boxes [45, 46]. Factors other than genetic predispositions, such as age, polypharmacy, or environmental factors, can contribute to ADEs [47, 48]. Collecting a large amount of data from many sources may be beneficial for prevention, as it could fully exploit differences in characteristics between patients. However, not all machine learning methods are appropriate for processing potentially high-dimensional (e.g., genomic and phenotypic data, chemical information of drugs, clinical notes, environmental data) and heterogeneous datasets. Deep learning can transform the basic (raw input) representations of a patient at a higher level and can perform automatic feature extraction from big data containing incomplete and noisy information. Even though the lack of interpretability is still a major issue, deep learning can also be used to discover intricate patterns in large data sets [49, 50]. With these advantages, deep learning could help overcome some of the barriers responsible for underreporting in pharmacovigilance. EHRs electronic health records
‘Intelligent’ Information and Telehealth
Telehealth, often used interchangeably with telemedicine, is defined as the use of medical information exchanged between sites though electronic communication to improve health [51]. Telehealth is an umbrella term that includes services such as telepharmacy and telemonitoring. Telehealth has been around since the 1990s, but in recent years, there has been a growing demand for telemedicine. The COVID-19 pandemic was a huge inflection point in the adoption of telemedicine, especially as it required social distancing. In the USA, the number of patients who participated in telehealth visits increased by 57% within the first few months of the pandemic, and for patients with chronic diseases this figure was 77% [52]. With the increased development of home health settings, using health information technology, a large amount of data is transferred between hospitals and patient homes [53]. As a result, clinical assessment and evaluation can be made easier using telemonitoring and AI [54]. For example, chatbots can make history taking quicker and easier by using NLP to provide prompts and questions based on patient answers, such as self-reporting symptoms, and can provide possible diagnoses, including ADE detection, which can be coded and be applied to future patient visits [55]. Orbita, a Health Insurance Portability and Accountability Act-compliant conversational AI platform, has developed an AE detection module that uses deep learning and NLP, via a virtual assistant, to recognize and differentiate between different AEs based on the questions and phrases presented [56]. Once the AE is identified, the module will automatically transcribe and export the information to the pharmaceutical company and assist with US Food and Drug Administration (FDA) reporting.
Although many examples documenting AI applications in telehealth have been published, few have focused specifically on pharmacovigilance in telehealth. Some ongoing research studies are collecting data that could use AI to learn insights about pharmacovigilance using telehealth. For example, the All of Us Research Program, sponsored by the National Institutes of Health, is a large observational study recruiting at least 1 million patients from across the USA who are diverse in demographics and health status. This study is using various sources, including biosamples, EHR data, and mobile health device data, to build a diverse health database that is available to researchers [57]. Artificial intelligence can automate the process of identifying ADEs, using algorithms, in large pharmacovigilance database systems, such as VigiBase and EudraVigilance, a pharmacovigilance database to collect and analyze ADEs in Europe [58, 59]. Artificial intelligence could also potentially be used to improve existing pharmacovigilance efforts in telehealth settings that are using technology to detect ADEs in outpatients. One study showed success in identifying ADEs by utilizing automated phone calls to contact patients newly starting medications. After the automated screening, those whose responses to questions about symptoms could indicate ADEs were transferred to a pharmacist for triage. Use of AI to predict which patients to contact for this type of screening and the best time to contact them, coupled with additional technologies, such as EHR patient portals and texting to contact patients, could potentially increase the success and efficiency of this type of pharmacovigilance [60]. Artificial intelligence could also be used to improve results for programs that have incorporated telemedicine visits into comprehensive medication regimen reviews for long-term care patients that were found to prevent ADEs [61].
Health information technologies are also used for telemonitoring, mobile health applications, and wireless monitoring devices, which can have useful features including the collection of patient monitoring data, disease information, symptom diaries, medication logs and reminders, nutrition diaries, and communications among others. Wearable devices and mobile health applications measure personal analytics, physical status, and physiological parameters, and can help with medication scheduling. Some of the networked medical devices patients use include consumer products for health monitoring (e.g., Fitbit and Apple Watch), wearable external medical devices (e.g., portable insulin pumps), and internally embedded medical devices (e.g., pacemakers). Wearable devices can generate real-time dynamic data that providers can assess using software applications on computers, tablets, or smartphones [62]. The results are quickly available and allow providers to make appropriate adjustments more efficiently, rather than waiting for laboratory results. Research using the web and smartphone-based electronic health tools for medication monitoring has focused more on medication adherence and chronic disease monitoring and management, with fewer examples using these tools for the detection or prevention of ADEs [63]. However, the large amounts of data that are being collected by monitoring devices (such as glucose levels and blood pressure) could be used with AI to identify, predict, and prevent ADEs. Information collected from smartphone-based surveys and texts, such as the US Centers for Disease Control and Prevention’s “V-safe After Vaccination Health Checker” used for COVID-19 vaccinations could also benefit from AI to analyze AE information sent from millions of patients [64].
Machine learning can be very useful for data integration, processing and analyzing large data sets, and developing real-time models. This allows enhanced decision making by providing better information to clinicians at the point of care and minimizing the time it takes them to understand patient problems. For example, diagnosing heart failure involves analyzing the patient’s history, conducting a physical examination, and interpreting imaging and laboratory data. Artificial intelligence can leverage the data found in these resources. For example, one study showed the home use of smartphone-enabled technology to monitor the neonatal and infant cardiac heart rate, which was able to identify asymptomatic arrhythmias [65].
Patient status, including care quality, safety, and drug responses, can all be captured in the digital health format. Artificial intelligence methods can be used to identify missing information, compile follow-up questions, and log responses and attempts. For example, it has been proposed that using a random forest algorithm could potentially predict and detect ADEs during patient visits to the emergency room. The algorithm could identify older adults at a higher risk of ADEs by integrating validated decision criteria, such as the Beers Criteria and the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP), and identifying patients with polypharmacy [66]. It is important to note that these algorithms are proposed only and application may be difficult because of black-box outputs [67]. Another study applied deep learning to EHR data to predict which individuals are at higher risk of developing drug-induced QT prolongation [68]. Similar models can be applied within physician order entry systems to prevent drug-induced QT prolongation.
Pharmacovigilance is important during the drug development process, but is especially crucial during post-marketing surveillance, after the drug is approved and is being used by the public, when it can be done using “real-world evidence”. The World Health Organization notes that good pharmacovigilance identifies risks and risk factors in the shortest time possible to avoid or minimize harm [69]. Telemonitoring and video consults are useful for medication therapy management and chronic disease management, compared to only follow-up interventions via the phone. One review found that patients with chronic diseases who were managed through video conferencing compared to in-person visits or telephone visits had similar health outcomes [70]. Other studies have shown that telemedicine is beneficial for patients with chronic diseases such as hypertension, heart failure, diabetes mellitus, or chronic obstructive pulmonary disease [71–74]. Therefore, adherence to chronic disease management, which includes medication management, is essential to improve health outcomes and quality of life [75]. For example, a study by McFarland and colleagues included 103 veterans with uncontrolled type 2 diabetes at four primary care clinics. In this study, 36 patients worked with pharmacists who utilized Care Coordination Home Telehealth (CCHT) and 67 patients worked with pharmacists who did not utilize CCHT. Of the CCHT group, 69% met their hemoglobin A1c goal, with an average hemoglobin A1c of 6.9%. In the non-CCHT group, only 36% of the patients met their hemoglobin A1c goal, with an average hemoglobin A1c of 7.5%. This may have been because of more frequent contact with the pharmacist and more adjustments to their antihyperglycemic drugs [76].
Telepharmacy is an area of telehealth that can expand pharmacists’ and other providers’ interventions by preventing medication errors, minimizing prescribing errors, updating medication lists, monitoring drug therapy, assessing potential and/or active ADEs, and counseling patients [77]. Telepharmacy is important because about 74% of physician visits involve drug therapy [78]. Telepharmacy services allow easier access to pharmaceutical services and can reduce the number of potential ADEs, but the evidence is variable that it can accomplish this. One study found that pharmacists who used telemedicine services in three community hospitals reduced high-risk medication administration errors by 35% [22]. Another concluded that telepharmacy was as effective as in-person medication reviews to identify medication-related problems for patients in the inpatient setting [79]. Providers can educate patients about ADE-related symptoms to prevent ADEs or detect ADEs earlier.
In addition, pharmacoepidemiology databases are useful for detecting new ADEs and have years of clinical information from a large patient cohort. Spontaneous reporting systems, such as the US Food and Drug Administration Adverse Event Reporting System, can be useful because they allow rapid detection of potential ADEs and early detection of new ADEs. However, spontaneous reporting may not be the most effective method of detecting ADEs because of a lack of quality data and underreporting of ADEs. In addition to underreporting, there are existing pharmacovigilance data sources that are underutilized, such as poison control centers. In the USA, more than 200,000 medication errors are reported to poison control centers [80]. Artificial intelligence can extract ADE information from these calls and add it to databases. There are several organizations launching projects and systems to effectively collect and manage pharmacovigilance data. For example, VigiBase is a global pharmacovigilance database that, as of January 2022, has over 30 million ADE reports from about 130 countries [81].
Artificial intelligence can also reduce manual data entry and enable prioritization of cases in large datasets, including assessment of type or severity of ADE; ML can also be used in this setting to detect previously unrecognized patterns. Electronic health records represent an increasingly critical data source because they enable real-time surveillance with fewer errors compared with reporting systems. Electronic health records contain information about patient problem lists, admission notes, outpatient office visits, clinical history, symptoms, medications, laboratory results, and discharge summaries. Utilizing AI and telemedicine allows a thorough assessment of potential and active ADEs. Yang and colleagues utilized an NLP system (MADEx) to detect medications, ADEs, and their relationships from clinical notes [82]. Natural language processing systems can extract comprehensive clinical information and structured medication information that can allow for a more thorough assessment of potential ADEs [83, 84]. One study automatically detected adverse drug reactions (ADRs) in patient reports using unstructured data, from a knowledge database, to structure free text data and a ML model to learn ADR coding. Of the 1061 ADRs, their system correctly detected 703 ADRs and incorrectly detected 190 ADRs [85]. Combining EHR data with spontaneous reports has shown to improve ADE detection [86]. Social media, including social networks and health forums, have also become resources for early detection of ADEs [87, 88].
Discussion
Drug safety remains a major concern in both the pre-marketing and post-marketing settings. Current pharmacovigilance methods in the post-marketing setting rely heavily on spontaneous reporting for detecting and analyzing ADEs, which can introduce biases. Artificial intelligence methods have great potential to make pharmacovigilance more efficient and effective. This can be demonstrated in the two use cases in AI that were discussed in this paper. The first use case utilizes AI techniques, including NLP and deep learning, to process documented ADEs, from various data sources, especially EHRs. Thus, it is important for individuals participating in the pharmacovigilance process to have more access to these tools and data. The second use case utilizes AI to collect new or additional information to measure actual rates of ADEs. Specifically, AI tools can be developed for automated targeted follow-up of patients at higher risk of ADEs at optimal times and frequencies to obtain more comprehensive pharmacovigilance data. Such datasets, with more accurate and complete documentation of ADEs, can be leveraged to develop clinical decision support tools to prevent or mitigate the AEs caused by medications and optimize treatment outcomes.
These efforts will be guided by support and actionable changes from the FDA. The FDA has developed an action plan for an AI-based software for medical devices [89]. It is important for the FDA to incorporate these efforts in pharmacovigilance and ADE detection and prevention, especially during telehealth visits. In addition, the use of AI and wearable technologies in telemedicine have played a big role in pharmacovigilance. Artificial intelligence and remote monitoring of patients, using real-time data, can minimize patient harm and improve patient outcomes.
Limitations
Although the use of AI in pharmacovigilance and telemedicine can be useful, it also raises ethical, security, regulatory, and privacy concerns. Clearly, an overriding concern is protecting patient data and personal information [90]. Security threats can compromise confidentiality, integrity, and availability of data [91]. A remaining concern is who will be allowed to access and make decisions about the data. Telemedicine services will usually require wireless network connections that collect and transmit information from patients to telemedicine systems, allowing providers to access that data. To transmit and store data, users rely on a wireless connection that can face multiple system attacks. The data are transmitted to third parties using a wireless connection, which creates privacy issues in the communication process. The telemedicine service system can be exposed to security threats and man-in-the-middle attacks [92].
Scope of practice and reimbursements are other concerns regarding providing expanded telehealth services in the USA. Telehealth may be for applicable for certain diseases such as mental health issues. Telehealth visits may not be reimbursed adequately by health insurers, and state regulations may not allow providers to conduct telehealth visits for patients who live in a state in which they are not licensed to practice. In addition, there are data available about cost savings regarding ADEs [93]. Artificial intelligence has the potential to reduce costs by automating the manual and repetitive tasks of processing ADE cases, thus allowing more time and resources to be allocated for other tasks.
Wearable technology can predict events before they occur, using sensors to collect data and generating personal metrics. The metrics generated can be sent to companies for analysis but do raise concerns regarding user privacy. There are several regulatory authorities, such as the FDA, and privacy policies in place to assist developers in creating legally compliant health applications that follow high standards to protect the user [94]. Institutions should focus on minimizing threats and vulnerable areas that can be attacked. Health Insurance Portability and Accountability Act-compliant systems that have standards for securing protected health information should be implemented. Patient information should be protected using authentication and data encryption [95]. It is important to use secure logins that use multi-factor authentication and security questions, for both patient identification and provider identification. In addition, periodic security assessments should be completed to prevent accidental disclosure of health data and fraud. Employee and end-user training should occur to recognize these threats as well. In addition to privacy concerns, another limitation to consider is patient access to internet services, smartphones, or monitoring devices. Some individuals may not have access to these technologies or devices, which could lead to inequities in treatment and outcomes [96].
Although these technologies may be useful, there may be system errors that occur. For example, NLP tools may omit or miss drug-ADE relationships because of inaccurate word choice, incorrect inference, or reasoning [97]. There are also difficulties surrounding the detection of undocumented and unknown ADEs, including rare events, and the potential burden of false-positive ADE alerts [98]. In addition, ML models can generate black-box algorithms that are difficult to understand and explain predictions at the human level [99]. Other considerations include maintaining data integrity, by minimizing the use of biased datasets and optimizing EHR data [100]. Inaccurate predictions may occur because of gaps in the information or clinical data manipulation. The datasets used to train algorithms may reinforce existing biases, may lack data from underrepresented populations, or may not ensure algorithm fairness [101]. Technical difficulties may also occur. For example, failures in telecommunication links may occur. It is important to have an existing IT department, but also a support specialist on the telemedicine team. It will be essential to ensure that these models are optimized before they are integrated into routine patient care. Last, AI and telemedicine can impact patient-provider relationships and limit the ability to perform comprehensive medical examinations [102].
Conclusions
As the volume and complexity of clinical data continue to grow, it is critical to understand how AI can be integrated into clinical practice, whether in person or virtually. This will help clinicians enhance the clinical decision-making process and provide personalized patient care. Artificial intelligence and telemedicine are useful technology-driven approaches that have the potential to reduce emergency department visits and hospitalizations, improve health outcomes, and increase healthcare quality. As the evidence for the use of AI for pharmacovigilance, in general and particularly telehealth, is limited, more evaluations are required to understand how it can be beneficial, and to identify the best directions for expanded implementation.
Declarations
Funding
No funding was received to assist with the preparation of this article.
Conflict of interest
David W. Bates reports grants and personal fees from EarlySense, personal fees from CDI Negev, equity from ValeraHealth, equity from Clew, equity from MDClone, personal fees and equity from AESOP, personal fees and equity from Feelbetter, and grants from IBM Watson Health, outside the submitted work. All other authors have no conflicts of interest to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Not applicable.
Author contributions
All authors contributed to drafting the manuscript. HE and DWB made revisions to the manuscript draft. All authors read and approved the final version of the manuscript.
References
- 1.World Health Organization. Regulation and prequalification: what is pharmacovigilance? Available from: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance. Accessed 28 Sep 2021.
- 2.Agency for Healthcare Research and Quality (AHRQ) Patient Safety Network. Medication errors and adverse drug events. Available from: https://psnet.ahrq.gov/primer/medication-errors-and-adverse-drug-event. Accessed 29 Sep 2021.
- 3.Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington DC: National Academy Press, Institute of Medicine of the National Academy of Sciences; 1999. [PubMed] [Google Scholar]
- 4.Weiss AJ, Freeman WJ, Heslin KC, Barrett ML. HCUP statistical brief #234. Rockville: AHRQ; 2018. [Google Scholar]
- 5.Panagioti M, Khan K, Keers N, et al. Prevalence, severity, and nature of preventable patient harm across medical care settings: systematic review and meta-analysis. BMJ. 2019;366:l4185. doi: 10.1136/bmj.l4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe JH, McInnis T, Hirsch JD. Cost of prescription drug-related morbidity and mortality. Ann Pharmacother. 2018;52(9):829–837. doi: 10.1177/1060028018765159. [DOI] [PubMed] [Google Scholar]
- 7.Beninger P. Pharmacovigilance: an overview. Clin Ther. 2018;40(12):1991–2004. doi: 10.1016/j.clinthera.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Liu F, Jagannatha A, Yu H. Towards drug safety surveillance and pharmacovigilance: current progress in detecting medication and adverse drug events from electronic health records. Drug Saf. 2019;42:95–97. doi: 10.1007/s40264-018-0766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 10.Welch HK, Kellum JA, Kane-Gill SL. Drug-associated acute kidney injury identified in the United States Food and Drug Administration Adverse Event Reporting System Database. Pharmacotherapy. 2018;38(8):785–793. doi: 10.1002/phar.2152. [DOI] [PubMed] [Google Scholar]
- 11.Mohamoud M, Horgan C, Eworuke E, et al. Complementary use of US FDA’s adverse event reporting system and sentinel system to characterize direct oral anticoagulants associated cutaneous small vessel vasculitis. Pharmacotherapy. 2020;40(11):1099–1107. doi: 10.1002/phar.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Ryan PB, Wei Y, Friedman CF. A method to combine signals from spontaneous reporting systems and observational healthcare data to detect adverse drug reactions. Drug Saf. 2015;38(10):895–908. doi: 10.1007/s40264-015-0314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennessy S, Leonard CE, Freeman CP, et al. Validation of diagnostic codes for outpatient-originating sudden cardiac death and ventricular arrhythmia in Medicaid and Medicare claims data. Pharmacoepidemiol Drug Saf. 2010;19(6):555–562. doi: 10.1002/pds.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jinjuvadia K, Kwan W, Fontana RJ. Searching for a needle in a haystack: use of ICD-9-CM codes in drug-induced liver injury. Am J Gastroenterol. 2007;102(11):2437–2443. doi: 10.1111/j.1572-0241.2007.01456.x. [DOI] [PubMed] [Google Scholar]
- 15.Hohl CM, Karpov A, Reddekopp L, Doyle-Waters M, Stausberg J. ICD-10 codes used to identify adverse drug events in administrative data: a systematic review. J Am Med Inform Assoc. 2014;21(4):757. doi: 10.1136/amiajnl-2013-002116corr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuklik N, Stausberg J, Jöckel KH. Adverse drug events in German hospital routine data: a validation of International Classification of Diseases, 10th Revision (ICD-10) diagnostic codes. PLoS ONE. 2017;12(11):e0187510. doi: 10.1371/journal.pone.0187510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segal G, Segev A, Brom A, et al. Reducing drug prescription errors and adverse drug events by application of a probabilistic, machine-learning based clinical decision support system in an inpatient setting. J Am Med Inform Assoc. 2019;26(12):1560–1565. doi: 10.1093/jamia/ocz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JC. Evaluation of a novel system to enhance clinician’s recognition of preadmission adverse drug reactions. Appl Clin Inform. 2018;9:313–325. doi: 10.1055/s-0038-1646963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates DW, Levine D, Syrowatka A, et al. The potential of artificial intelligence to improve patient safety: a scoping review. NPG Digit Med. 2021;4:54. doi: 10.1038/s41746-021-00423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho JM, Tung J, Maitland J, et al. GeriMedRisk, a telemedicine geriatric pharmacology consultation service to address adverse drug events in long-term care: a stepped-wedge cluster randomized feasibility trial protocol (ISRCTN17219647) Pilot Feasibility Stud. 2018;4:116. doi: 10.1186/s40814-018-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surapat B, Sungkanuparph S, Kirdlarp S, Lekpittaya N, Chunnguleum K. Role of clinical pharmacists in telemonitoring for patients with coronavirus disease 2019 (COVID-19) J Clin Pharm Ther. 2021;46(1):236–239. doi: 10.1111/jcpt.13293. [DOI] [PubMed] [Google Scholar]
- 22.Schneider PJ. Evaluating the impact of telepharmacy. Am J Health Syst Pharm. 2013;70(23):2130–2135. doi: 10.2146/ajhp130138. [DOI] [PubMed] [Google Scholar]
- 23.Miotto R, Wang F, Wang S, Jiang X, Dudley JT. Deep learning for healthcare: review, opportunities and challenges. Brief Bioinform. 2018;19(6):1236–1246. doi: 10.1093/bib/bbx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribli D, Horváth A, Unger Z, Pollner P, Csabai I. Detecting and classifying lesions in mammograms with deep learning. Sci Rep. 2018;8(1):4165. doi: 10.1038/s41598-018-22437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jahn SW, Plass M, Moinfar F. Digital pathology: advantages, limitations and emerging perspectives. J Clin Med. 2020;9(11):3697. doi: 10.3390/jcm9113697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho TB, Le L, Thai DT, Taewijit S. Data-driven approach to detect and predict adverse drug reactions. Curr Pharm Des. 2016;22(23):3498–3526. doi: 10.2174/1381612822666160509125047. [DOI] [PubMed] [Google Scholar]
- 27.Honigman B, Lee J, Rothschild J, Light P, Pulling RM, Yu T, Bates DW. Using computerized data to identify adverse drug events in outpatients. J Am Med Inform Assoc. 2001;8(3):254–266. doi: 10.1136/jamia.2001.0080254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang F, Jiang Y, Zhi H, et al. intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230–243. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo-Ciganic WH, Huang JL, Zhang HH, et al. Using machine learning to predict risk of incident opioid use disorder among fee-for-service Medicare beneficiaries: a prognostic study. PLoS ONE. 2020;15:e0235981. doi: 10.1371/journal.pone.0235981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spasic I, Nenadic G. Clinical text data in machine learning: systematic review. JMIR Med Inform. 2020;8(3):e17984. doi: 10.2196/17984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Q, Ji Z, Li Z, et al. A study of deep learning approaches for medication and adverse drug event extraction from clinical text. J Am Med Inform Assoc. 2020;27(1):13–21. doi: 10.1093/jamia/ocz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Stekelenborg J, Ellenius J, Maskel S. Recommendations for the use of social media in pharmacovigilance: lessons from IMI WEB-RADR. Drug Saf. 2019;42:1393–1407. doi: 10.1007/s40264-019-00858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gattepaille LM, Vidlin SH, Bergvall T, Pierce CE, Ellenius J. Prospective evaluation of adverse event recognition systems in Twitter: results from the Web-RADR Project. Drug Saf. 2020;43:797–808. doi: 10.1007/s40264-020-00942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wunnava S, Qin X, Kakar T, Sen C, Rundensteiner EA, Kong X. Adverse drug event detection from electronic health records using hierarchical recurrent neural networks with dual-level embedding. Drug Saf. 2019;42(1):113–122. doi: 10.1007/s40264-018-0765-9. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi TK, Seger AC, Overhage JM, et al. Outpatient adverse drug events identified by screening electronic health records. J Patient Saf. 2010;6(2):91–96. doi: 10.1097/PTS.0b013e3181dcae06. [DOI] [PubMed] [Google Scholar]
- 36.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348(16):1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 37.Galeano D, Li S, Gerstein M, Paccanaro A. Predicting the frequencies of drug side effects. Nat Commun. 2020;11(1):4575. doi: 10.1038/s41467-020-18305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boccia S, Pastorino R, Ricciardi W, et al. How to integrate personalized medicine into prevention? Recommendations from the Personalized Prevention of Chronic Diseases (PRECeDI) Consortium. Public Health Genomics. 2019;22(5–6):208–214. doi: 10.1159/000504652. [DOI] [PubMed] [Google Scholar]
- 39.Du AX, Emam S, Gniadecki R. Review of machine learning in predicting dermatological outcomes. Front Med. 2020;7:266. doi: 10.3389/fmed.2020.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uddin S, Khan A, Hossain ME, Moni MA. Comparing different supervised machine learning algorithms for disease prediction. BMC Med Inform Decis Mak. 2019;19(1):281. doi: 10.1186/s12911-019-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer UA. Pharmacogenetics and adverse drug reactions. Lancet. 2000;356(9242):1667–1671. doi: 10.1016/S0140-6736(00)03167-6. [DOI] [PubMed] [Google Scholar]
- 42.Song D, Chen Y, Min Q, et al. Similarity-based machine learning support vector machine predictor of drug-drug interactions with improved accuracies. J Clin Pharm Ther. 2019;44(2):268–275. doi: 10.1111/jcpt.12786. [DOI] [PubMed] [Google Scholar]
- 43.McMaster C, Liew D, Keith C, Aminian P, Frauman A. A machine-learning algorithm to optimise automated adverse drug reaction detection from clinical coding. Drug Saf. 2019;42(6):721–725. doi: 10.1007/s40264-018-00794-y. [DOI] [PubMed] [Google Scholar]
- 44.Rabhi S, Jakubowicz J, Metzger MH. Deep learning versus conventional machine learning for detection of healthcare-associated infections in French clinical narratives. Methods Inf Med. 2019;58(1):31–41. doi: 10.1055/s-0039-1677692. [DOI] [PubMed] [Google Scholar]
- 45.Molnar C. Chapter 2: Interpretability. In: Interpretable machine learning. A guide for making black box models explainable. Leanpub; 2019. p. 15–33.
- 46.Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719–731. doi: 10.1038/s41551-018-0305-z. [DOI] [PubMed] [Google Scholar]
- 47.Abe J, Umetsu R, Uranishi H, et al. Analysis of polypharmacy effects in older patients using Japanese Adverse Drug Event Report database. PLoS ONE. 2017;12(12):e0190102. doi: 10.1371/journal.pone.0190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao PJ, Mao CT, Chen TL, Deng ST, Hsu KH. Factors associated with adverse drug reaction occurrence and prognosis, and their economic impacts in older inpatients in Taiwan: a nested case–control study. BMJ Open. 2019;9(5):e026771. doi: 10.1136/bmjopen-2018-026771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 50.Lee CY, Chen YP. Machine learning on adverse drug reactions for pharmacovigilance. Drug Discov Today. 2019;7:1332–1343. doi: 10.1016/j.drudis.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 51.NEJM Catalyst. What is telehealth? Published February 1, 2018. Available from: https://catalyst.nejm.org/what-is-telehealth/. Accessed 28 Sep 2021.
- 52.Doximity. 2020 state of telemedicine report. Published September 2020. Available from: https://c8y.doxcdn.com/image/upload/Press%20Blog/Research%20Reports/2020-state-telemedicine-report.pdf. Accessed 22 Aug 2021.
- 53.Pew Research Center. Mobile fact sheet. Available from: https://www.pewinternet.org/fact-sheet/mobile/. Accessed 22 Aug 2021.
- 54.Andrès E, Meyer L, Zulfiqar AA, et al. Telemonitoring in diabetes: evolution of concepts and technologies, with a focus on results of the more recent studies. J Med Life. 2019;3:203–214. doi: 10.25122/jml-2019-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greene A, Greene CC, Greene C. Artificial intelligence, chatbots, and the future of medicine. Lancet Oncol. 2019;20(4):481–482. doi: 10.1016/S1470-2045(19)30142-1. [DOI] [PubMed] [Google Scholar]
- 56.Rogers B. Engaging healthcare providers in pharmacovigilance with Orbita’s new adverse event detection module. Orbita. Published October 29, 2020. Available from: https://blog.orbita.ai/engaging-healthcare-providers-in-pharmacovigilance-with-orbita-new-adverse-event-detection-module. Accessed 22 Aug 2021.
- 57.National Institutes of Health. Data sources. Available from: https://www.researchallofus.org/data-tools/data-sources/. Accessed 5 Oct 2021.
- 58.Zhao Y, Lu H, Thai S, Li X, Hui J, Tang H, Zhai S, Sun L, Wang T. Development and validation of an algorithm to identify drug-induced anaphylaxis in the Beijing Pharmacovigilance Database. Int J Clin Pharm. 2018;40(4):862–869. doi: 10.1007/s11096-018-0594-z. [DOI] [PubMed] [Google Scholar]
- 59.EMA. EudraVigilance. Available from: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance. Accessed 30 Nov 2021.
- 60.Schiff GD, Klinger E, Salazar A, et al. Screening for adverse drug events: a randomized trial of automated calls coupled with phone-based pharmacist counseling. J Gen Intern Med. 2019;34(2):285–292. doi: 10.1007/s11606-018-4672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kane-Gill SL, Wong A, Culley CM, et al. Transforming the medication regimen review process using telemedicine to prevent adverse events. J Am Geriatr Soc. 2021;69(2):530–538. doi: 10.1111/jgs.16946. [DOI] [PubMed] [Google Scholar]
- 62.Wilson LS, Maeder AJ. Recent directions in telemedicine: review of trends in research and practice. Healthc Inform Res. 2015;21(4):213–222. doi: 10.4258/hir.2015.21.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lancaster K, Abuzour A, Khaira M, et al. The use and effects of electronic health tools for patient self-monitoring and reporting of outcomes following medication use: systematic review. J Med Internet Res. 2018;20(12):e294. doi: 10.2196/jmir.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. COVID-19. Updated on September 2, 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html. Accessed 5 Oct 2021.
- 65.Young ML, Flores L. Asymptomatic idiopathic Belhassen ventricular tachycardia in a neonate detected using ‘Smart Sock’ wearable smartphone-enabled cardiac monitoring. Am J Case Rep. 2020;21:e921092. doi: 10.12659/AJCR.921092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ouchi K, Lindvall C, Chai PR, Boyer EW. Machine learning to predict, detect, and intervene older adults vulnerable for adverse drug events in the emergency department. J Med Toxicol. 2018;14(3):248–252. doi: 10.1007/s13181-018-0667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldstein A, Kapelner A, Bleich J, Pitkin E. Peeking inside the black box: visualizing statistical learning with plots of individual conditional expectation. J Comput Graph Stat. 2015;24(1):44–65. doi: 10.1080/10618600.2014.907095. [DOI] [Google Scholar]
- 68.Simon ST, Mandair D, Tiwari P, Rosenberg MA. Prediction of drug-induced long QT syndrome using machine learning applied to harmonized electronic health record data. J Cardiovasc Pharmacol Ther. 2021;26(4):335–340. doi: 10.1177/1074248421995348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.World Health Organization (WHO). The safety of medicines in public health programmes. 2013. Available from: https://www.who.int/hiv/pub/pharmacovigilance/safety/en/. Accessed 23 Aug 2021.
- 70.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 71.Verberk WJ, Kessels AGH, Thien T. Telecare is a valuable tool for hypertension management, a systematic review and meta-analysis. Blood Press Monit. 2011;16(3):149–155. doi: 10.1097/MBP.0b013e328346e092. [DOI] [PubMed] [Google Scholar]
- 72.Steventon A, Bardsley M, Billings J, et al. Whole System Demonstrator Evaluation Team Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomized trial. BMJ. 2012;344:e3874. doi: 10.1136/bmj.e3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakamura N, Koga T, Iseki H. A meta-analysis of remote patient monitoring for chronic heart failure patients. J Telemed Telecare. 2014;20(1):11–17. doi: 10.1177/1357633X13517352. [DOI] [PubMed] [Google Scholar]
- 74.Kamei T, Yamamoto Y, Kajii F, Nakayama Y, Kawakami C. Systematic review and meta-analysis of studies involving telehome monitoring-based telenursing for patients with chronic obstructive pulmonary disease. Jpn J Nurs Sci. 2013;10(2):180–192. doi: 10.1111/j.1742-7924.2012.00228.x. [DOI] [PubMed] [Google Scholar]
- 75.Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015;(9):CD002098. [DOI] [PMC free article] [PubMed]
- 76.McFarland M, Davis K, Wallace J, et al. Use of home telehealth monitoring with active medication therapy management by clinical pharmacists in veterans with poorly controlled type 2 diabetes mellitus. Pharmacotherapy. 2012;32(5):420–426. doi: 10.1002/j.1875-9114.2011.01038.x. [DOI] [PubMed] [Google Scholar]
- 77.Keeys C, Kalejaiye B, Skinner M, et al. Pharmacist-managed inpatient discharge medication reconciliation: a combined onsite and telepharmacy model. Am J Health Syst Pharm. 2014;71(24):2159–2166. doi: 10.2146/ajhp130650. [DOI] [PubMed] [Google Scholar]
- 78.Centers for Disease Control and Prevention. National Center for Health Statistics. Available from: https://www.cdc.gov/nchs/fastats/drug-use-therapeutic.htm. Accessed 5 Oct 2021.
- 79.Poulson LK, Nissen L, Coombes I. Pharmaceutical review using telemedicine: a before and after feasibility study. J Telemed Telecare. 2010;16(2):95–99. doi: 10.1258/jtt.2009.090716. [DOI] [PubMed] [Google Scholar]
- 80.Chyka PA, McCommon SW. Reporting of adverse drug reactions by poison control centres in the US. Drug Saf. 2000;23(1):87–93. doi: 10.2165/00002018-200023010-00006. [DOI] [PubMed] [Google Scholar]
- 81.World Health Organization. The WHO programme for international drug monitoring. Geneva: WHO; 2018. Available from: http://www.who.int/medicines/regulation/medicines-safety/about/drugmonitoring_prog/en/. Accessed 30 Nov 2021.
- 82.Yang X, Bian J, Gong Y, Hogan WR, Wu Y. MADEx: a system for detecting medications, adverse drug events, and their relations from clinical notes. Drug Saf. 2019;42(1):123–133. doi: 10.1007/s40264-018-0761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu H, Stenner SP, Doan S, Johnson KB, Waitman LR, Denny JC. MedEx: a medication information extraction system for clinical narratives. J Am Med Inform Assoc. 2010;17:19–24. doi: 10.1197/jamia.M3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meystre SM, Haug PJ. Randomized controlled trial of an automated problem list with improved sensitivity. Int J Med Inform. 2008;77:602–612. doi: 10.1016/j.ijmedinf.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Létinier L, Jouganous J, Benkebil M, et al. Artificial intelligence for unstructured healthcare data: application to coding of patient reporting of adverse drug reactions. Clin Pharmacol Ther. 2021;110(2):392–400. doi: 10.1002/cpt.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harpaz R, Vilar S, Dumouchel W, et al. Combing signals from spontaneous reports and electronic health records for detection of adverse drug reactions. J Am Med Inform Assoc. 2013;20(3):413–419. doi: 10.1136/amiajnl-2012-000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leaman R, Wojtulewicz L, Sullivan R, Skariah A, Yang J, Gonzalez G. Towards internet-age pharmacovigilance: extracting adverse drug reactions from user posts to health-related social networks. In: BioNLP’10 Proceedings of the 2010 Workshop on Biomedical in Processing; 2010; Uppsala. p. 117–25.
- 88.Wang X, Hripcsak G, Markatou M, Friedman C. Active computerized pharmacovigilance using natural language processing, statistics, and electronic health records: a feasibility study. J Am Med Inform Assoc. 2009;16(3):328–337. doi: 10.1197/jamia.M3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.US Food and Drug Administration. Artificial intelligence/machine learning (AI/ML)-based software as a medical device (SaMD) action plan. Published January 2021. Available from: https://www.fda.gov/media/145022/download. Accessed 7 Oct 2021.
- 90.Itnoline. Available from: https://www.itnonline.com/article/radiologytechnology-Trends-watch-2020. Accessed 5 Oct 2021.
- 91.Chaet D, Clearfield R, Sabin JE, Skimming K, Council on Ethical and Judicial Affairs American Medical Association Ethical practice in telehealth and telemedicine. J Gen Intern Med. 2017;32(10):1136–1140. doi: 10.1007/s11606-017-4082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Camara C, Peris-Lopez P, Tapiador JE. Security and privacy issues in implantable medical devices: a comprehensive survey. J Biomed Inf. 2015;55:272–289. doi: 10.1016/j.jbi.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Rozenblum R, Rodriguez-Monguio R, Volk LA, et al. Using a machine learning system to identify and prevent medication prescribing errors: a clinical and cost analysis evaluation. Jt Comm J Qual Patient Saf. 2020;46:3–10. doi: 10.1016/j.jcjq.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 94.Parker L, Karliychuk T, Gillies D, Mintzes B, Raven M, Grundy Q. A health app developer's guide to law and policy: a multi-sector policy analysis. BMC Med Inform Decis Mak. 2017;17(1):141. doi: 10.1186/s12911-017-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rohloff K, Polyakov Y. Presented at 17th International Conference on E-health Networking, Application & Services (HealthCom), October 2015, Boston, MA.
- 96.McComas T, Yang YT. Legal and ethical considerations for home-based telemedicine. Int J Telemed Clin Pract. 2015;1(1):32–46. doi: 10.1504/IJTMCP.2015.069471. [DOI] [Google Scholar]
- 97.Henry S, Buchan K, Filannino M, Stubbs A, Uzuner O. 2018 n2c2 shared task on adverse drug events and medication extraction in electronic health records. J Am Med Inform Assoc. 2020;27(1):3–12. doi: 10.1093/jamia/ocz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.González-Rubio F, CalderónLarrañaga A, Poblador-Plou B, et al. Underreporting of recognized adverse drug reactions by primary care physicians: an exploratory study. Pharmacoepidemiol Drug Saf. 2011;20(12):87–94. doi: 10.1002/pds.2172. [DOI] [PubMed] [Google Scholar]
- 99.Angelino E, Larus-Stone N, Alabi D, Seltzer M, Rudin C. Certifably optimal rule lists for categorical data. J Mach Learn Res. 2018;234(18):1–78. [Google Scholar]
- 100.Richardson JP, Smith C, Curtis S, et al. Patient apprehensions about the use of artificial intelligence in healthcare. NPJ Digit Med. 2021;4(1):140. doi: 10.1038/s41746-021-00509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matheny ME, Whicher D, Israni ST. Artificial intelligence in health care: a report from the national academy of medicine. JAMA. 2020;323(6):509–510. doi: 10.1001/jama.2019.21579. [DOI] [PubMed] [Google Scholar]
- 102.Verghese A, Shah NH, Harrington RA. What this computer needs is a physician: humanism and artificial intelligence. JAMA. 2018;319(1):19–20. doi: 10.1001/jama.2017.19198. [DOI] [PubMed] [Google Scholar]