Abstract
Introduction
MVC-COV1901 is a protein subunit COVID-19 vaccine based on the stable prefusion spike protein S-2P adjuvanted with CpG 1018 and aluminum hydroxide. Interim results of a phase 2 clinical trial demonstrated favorable safety profile and immunogenicity and the vaccine has been authorized for use in Taiwan. However, waning antibody levels after immunization and variants of concern (VoC) could negatively impact vaccine-induced neutralization of virus. In this extension to the phase 1 clinical study we investigated a three-dose regimen of MVC-COV1901 for durability of antibody levels and virus neutralization capacity, including neutralization of the Omicron variant.
Methods
Forty-five healthy adults from 20 to 49 years of age were divided into three groups of 15 participants receiving two doses of either low dose (LD), medium dose (MD), or high dose (HD) of MVC-COV1901. Six months after the second dose (day 209), a third MD dose of MVC-COV1901 was administered to the LD and MD groups and a HD dose was given to the HD group. Safety was followed for up to 28 days after the booster dose by monitoring incidences of adverse events (AE). Immunogenicity and antibody persistence for up to 6 months after the booster dose were assessed by neutralizing assay with the wild-type (Wuhan) SARS-CoV-2 virus. To examine the immunogenicity of booster dose against variants, neutralizing assays were carried out with the Alpha, Beta, and Delta variant viruses and the Omicron variant pseudovirus using samples from 4 weeks after the booster dose.
Results
Adverse reactions after the booster dose were mostly mild and comparable to that of the first two doses. Compared to day 209, neutralizing antibodies were increased by 10.3–28.9 times at 4 weeks after the booster. During the 6-month follow-up after the booster, the rate of decline of neutralizing antibody level was much less than that after the second dose. Three doses of MVC-COV1901 also improved antibody-mediated neutralization of Alpha, Beta, and Delta variants as well as the Omicron variant pseudovirus.
Conclusion
Our data showed increased persistence of neutralizing antibodies and enhancement of immunogenicity against VoCs offered after a third dose of MVC-COV1901.
Trial Registration
ClinicalTrials.gov identifier NCT04487210.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00652-6.
Keywords: Booster vaccination, COVID-19, MVC-COV1901, SARS-CoV-2, SARS-CoV-2 vaccine
Key Summary Points
| Why carry out this study? |
| Antibody levels against SARS-CoV-2 wane over time after vaccination and booster doses are required to maintain high levels of neutralizing antibodies. |
| In the absence of variant-specific vaccines, administration of booster doses are currently the only means to improve immunogenicity against variants of concern. |
| This study investigated the durability and neutralizing capability of antibodies against variants of concern after three doses of MVC-COV1901. |
| What has been learned from this study? |
| Antibody levels persisted after each dose of vaccine but waned over time. |
| Neutralizing antibody rate of decay after the third dose is slower than that of the second dose. |
| Third dose of MVC-COV1901 improved neutralizing capacity against variants of concern including Delta and Omicron variants. |
| Three doses of MVC-COV1901 may be effective in combating the current dominating variants of concern. |
Introduction
The ongoing COVID-19 pandemic caused by SARS-CoV-2 virus has claimed nearly 6 million lives and infected over 440 million people worldwide as of March 2022 [1]. As the virus continued to mutate, surging waves of COVID-19 infections by variants of concern (VoCs) such as Delta and Omicron in individuals that completed primary vaccination series against COVID-19 have prompted countries to implement booster doses to improve immunity against the VoCs [2]. The boosting regimen was also justified on the basis that antibody-mediated immunity likely diminishes over time and because it has been directly observed to wane by 6 months after the last vaccination in the case of BNT162b2 [3]. Boosting with same type of vaccine (homologous) or different type of vaccine (heterologous) to the primary series of vaccination has been effective in enhancing immunogenicity against VoCs [4, 5]. MVC-COV1901 is a protein subunit COVID-19 vaccine based on the stable prefusion spike protein S-2P adjuvanted with CpG 1018 and aluminum hydroxide. The phase 1 clinical trial of MVC-COV1901 was a dose-escalation trial with three dose levels administered to groups of 15 subjects and where the middle dose (MD) of 15 μg S-2P was chosen as the standard dose that received emergency use authorization (EUA) in Taiwan [6, 7]. The current study is an extension of the MVC-COV1901 phase 1 study to compare antibody levels after primary vaccination with levels achieved by a booster vaccination 6 months after the second dose. We also assessed immunogenicity and reactogenicity after the booster dose. Moreover, to explore the neutralizing capability of the booster dose against the VoCs, we have performed virus neutralization assays with live Alpha, Beta, and Delta variants and Omicron variant pseudovirus.
Methods
Study Design and Participants
This study was an extension to the phase 1 main study, which was a prospective, open-label, dose-escalation study to evaluate the safety and immunogenicity of a SARS-CoV-2 vaccine in adults aged 20–49 years [7]. The extension study was conducted at the National Taiwan University Hospital from September 2020 and included 45 healthy adults from 20 to 49 years of age. Details of the participants’ inclusion and exclusion criteria can be found in the original phase 1 main study [7]. Randomization and blinding were not performed as this was an open-labeled study. The trial protocol and informed consent form were approved by the Taiwan Food and Drug Administration and the ethics committee of the National Taiwan University Hospital. The trial was conducted in compliance with the principles of the Declaration of Helsinki and Good Clinical Practice and safety data was monitored by an independent data and safety monitoring board (DSMB).
Procedure and Outcomes
During the phase 1 main study, three different dose levels were employed: low dose (LD; 5 μg), medium dose (MD; 15 μg), and high dose (HD; 25 μg) [7]. Each dose was adjuvanted with same amount of CpG 1018 (750 μg) and aluminum hydroxide (375 μg) regardless of the amount of S-2P protein. Booster doses were administered in the same manner as the previous two doses (intramuscular injection of 0.5 mL of the vaccine in the deltoid region of non-dominant arm) on day 209 with MD used as the booster dose for the LD and MD group, while HD was used for the HD group (Fig. 1).
Fig. 1.
CONSORT flow diagram for the study
The study timeline is shown in Fig. S1 in the supplementary material. The interim analysis of the main study examined the immunogenicity and safety of MVC-COV1901 for up to 28 days (day 57) after the second dose [7]. To investigate the immune durability of MVC-COV1901 prior to the booster dose, blood samples were drawn on days 119 (90 days after the second dose) and 209 (180 days after the second dose; on the day of booster dose). After the administration of booster dose on day 209, immunogenicity data were collected via three on-site visits for blood samples on days 237, 329, and 389 (28, 120, and 180 days after the booster dose) and safety data were collected via telephone call on day 216 (1 week after the booster dose) and during the on-site visits.
The objectives of this extension study were to evaluate the safety of a booster dose of MVC-COV1901 within 28 days after the booster vaccination, and to evaluate the immunogenicity in terms of neutralizing antibody titers and anti-spike immunoglobulin G (IgG) antibody titers at 28, 90, and 180 days after the booster dose. Safety was assessed by incidences of solicited AEs for up to 7 days after each vaccination and unsolicited AEs for up to 28 days after each vaccination. Other AEs such as serious adverse events (SAEs) and adverse events of special interest (AESI) were recorded within the study period. Immunogenicity and antibody persistence for up to 6 months after the booster dose was assessed by neutralizing assay with the wild-type (Wuhan) SARS-CoV-2 virus and IgG titers in terms of geometric mean titer (GMT), GMT ratio, and seroconversion rate (SCR). Neutralizing assays with the Alpha, Beta, and Delta variant viruses and the Omicron variant pseudovirus were conducted with samples from 4 weeks after the booster dose.
Laboratory Methods
Anti-SARS-CoV-2 spike IgG levels were measured by enzyme-linked immunosorbent assay (ELISA) using customized 96-well plates coated with S-2P antigen and converted to binding antibody unit per milliliter (BAU/mL) [6, 7]. Neutralizing antibody levels against live SARS-CoV-2 virus were measured as previously performed [6] with live SARS-CoV-2 of Wuhan wild-type (hCoV-19/Taiwan/4/2020, GISAID EPI_ISL_411927), Alpha (B.1.1.7, hCoV-19/Taiwan/792, GISAD EPI_ISL_1381386), Beta (B.1.351, hCoV-19/Taiwan/1013), and Delta (B.1.617.2, hCoV-19/Taiwan/1144). Briefly, twofold serial dilutions of serum samples were mixed with equal volumes of 100 TCID50/50 μL of virus and incubated at 37 °C for 1 h. After incubation, the mixtures were added to Vero E6 cells and incubated at 37 °C for 4–5 days. The results were converted to WHO standardized international unit per milliliter (IU/mL) using previously established methods [6]
Pseudovirus with spike proteins of Wuhan wild-type or Omicron variant was constructed and neutralization assays were performed as in the phase 1 clinical study [7]. Twofold serial dilutions of serum samples were mixed with equal volumes of pseudovirus and incubated before adding to the HEK-293-hAce2 cells. Fifty percent inhibition dilution titers (ID50) were calculated with uninfected cells as 100% neutralization and cells transduced with virus as 0% neutralization. The mutations for the Omicron variant (BA.1) used in the spike sequence for pseudovirus construction are A67V, del69-70, T95I, G142D, del143-145, del211, L212I, ins214EPE, G339D, S371L, S373P, S375F, S477N, T478K, E484A. Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, D796Y, N856K, Q954H, N969K, L981F according to the most common combination of mutations listed on Coronavirus3D (https://coronavirus3d.org/).
Statistical Analysis
Small sample size is typical of exploratory stage phase 1 clinical studies of the safety and immunogenicity of vaccines and the sample size of 45 subjects with 15 subjects in each group was arbitrarily determined and not derived from statistical estimation methods. All results are presented using descriptive statistics. Seroconversion rate (SCR) was defined as the percentage of subjects with at least fourfold increases from baseline. GMT ratio was defined as geometric mean of fold increase of post-vaccination titers over the baseline titers (day 1). The 95% confidence interval values were calculated with two-sided unpaired t test for the GMT ratio and binomial distribution estimation for SCR. As a result of the small sample size, Kruskal–Wallis test (two-tailed, alpha = 0.05) with corrected Dunn’s multiple comparisons test were calculated to compare immunogenicity results between different dosing groups and variants. These analysis were performed using Prism 6.01 (GraphPad).
Antibody half-life and 95% confidence intervals were calculated using exponential decay models from day 43 to day 209 after two doses, and from day 237 after the third dose [8]. A mixed linear regression model with random intercept was used for fitting in the following form:
where a and b are the fixed effects, intercept and decay rate, respectively, uj is the random intercept for each participant I, and eij represents random errors for participant i at study day j.
Half-life was derived from the following equation:
where t1/2 is the day when the titer has decayed to half of its starting value and bhat is the estimated model decay rate.
Results
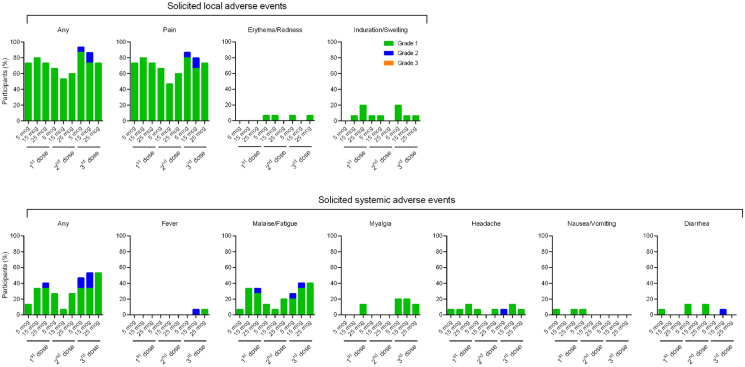
Out of the 45 participants from the phase 1 main study, 41 participants remained in the study through day 389, with 14, 13, and 14 participants in the LD, MD, and HD groups, respectively (Fig. 1; Table 1). Adverse events (AEs) are summarized in Table S1 in the supplementary material. Solicited AEs are shown in Fig. 2 and tabulated in Table S2 in the supplementary material. No SAE (grade 3 AEs or higher) or AESI related to the vaccine have been reported and reactogenicity after the third dose for all participants was comparable to that after the previous two doses. The most common solicited local and systemic effects after any dose were pain and malaise/fatigue, respectively. In terms of solicited local AEs, booster dose resulted in slightly more elevated incidences of pain compared to the first dose and the second dose. Two events of fever, one of grade 2 severity in the MD group and another one of grade 1 severity in the HD group were recorded after the booster dose, whereas no event of fever was reported after their previous first two doses.
Table 1.
Demographic characteristics of the participants
| Demographic characteristics | LD | MD | HD | Total |
|---|---|---|---|---|
| No. of participants (at the start) | 15 | 15 | 15 | 45 |
| Age | ||||
| Mean (SD), years | 36.7 (8.97) | 33.3 (8.03) | 31.5 (5.78) | 33.8 (7.84) |
| Min, max | 26.0 49.0 | 20.0, 47.0 | 24.0, 40.0 | 20.0, 49.0 |
| Gender | ||||
| Male, no. (%) | 7 (46.7%) | 9 (60.0%) | 12 (80.0%) | 28 (62.2%) |
| Female, no. (%) | 8 (53.3%) | 6 (40.0%) | 3 (20.0%) | 17 (37.8%) |
| BMI (kg/m2) | ||||
| Mean (SD) | 23.18 (3.394) | 23.30 (3.084) | 23.17 (2.429) | 23.22 (2.928) |
| Min, Max | 17.04, 29.03 | 17.80, 28.20 | 18.84, 27.84 | 17.04, 29.03 |
Fig. 2.
Solicited local and systemic adverse events after first, second, and third dose of MVC-COV1901
The immunogenicity data for the entire study period are presented in Table S3 in the supplementary material and Fig. 3. Significant anti-SARS-CoV-2 IgG levels persisted in all groups until day 209 at less than 10% of peak levels after the second dose. They were boosted above peak post second dose levels by the third dose of MVC-COV1901 and maintained by day 389 at approximately one-third of the peak post booster level for MD and HD groups (Fig. 3a and Table S3). Six months after the second dose and just prior to the booster shot, neutralizing antibodies expressed as neutralizing GMTs, were reduced 3.9, 6.2, and 8.9-fold for LD, MD, and HD groups, respectively (Fig. 3b and Table S3). Neutralizing antibody GMTs then increased during the 4 weeks after the booster dose until day 237 by 28.9, 10.3, and 11.9-fold for LD, MD and HD groups, respectively, and then waned by 6 months after the booster (day 389) by 10.5, 3.0, and 3.2-fold for LD, MD, and HD groups, respectively. Notably, neutralizing antibodies in the MD group declined by 84% and 67% within 6 months after two doses and 6 months after the booster, respectively (Fig. 3b). Using mixed linear models to estimate the exponential decay, we calculated the half-life of MD as 12 days (95% CI 11–14 days) after the second dose and 44 days (95% CI 31–76 days) after the booster dose. On day 389, even though the neutralizing titers had dropped, they remained at multiple baseline titers with GMT ratios of 4.8, 7.8, and 12.3 for LD, MD, and HD groups, respectively (Table S3). In terms of seroconversion, all dosage groups achieved 100% seroconversion at 4 weeks after the booster dose (day 237) (Table S3). However, at 180 days after the booster dose (day 389), the SCR deduced from neutralizing antibodies dropped to 60%, 73.3%, and 93.3% for LD, MD, and HD groups, respectively; while in terms of IgG titers, SCR remained at higher levels at 93.3%, 86.7%, and 93.3 for LD, MD, and HD groups, respectively (Table S3).
Fig. 3.
Immunogenicity of MVC-COV1901 during the study course. Arrows at the bottom indicate the times of vaccination. a Anti-SARS-CoV-2 IgG antibody titer expressed in binding antibody titers (BAU/mL) and b neutralizing antibody titer against live wild-type SARS-CoV-2 virus expressed in WHO international units (IU/mL) for LD, MD, and HD groups at various time points. Results are expressed as symbols representing GMT and error bars represent 95% confidence interval. The arrows in b point to numerical values for neutralizing titers within the MD group
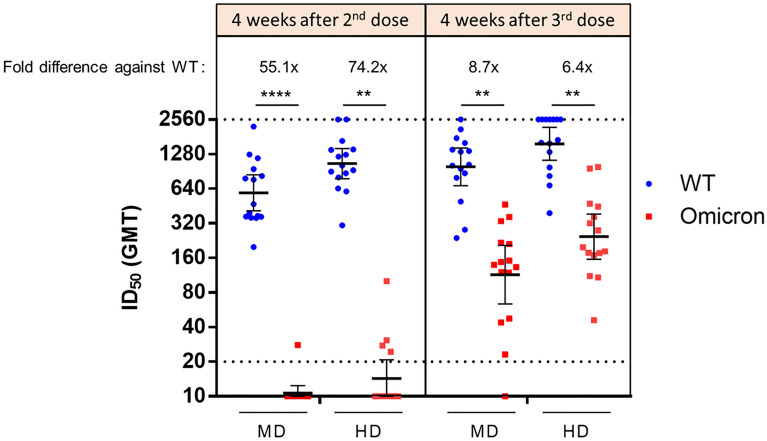
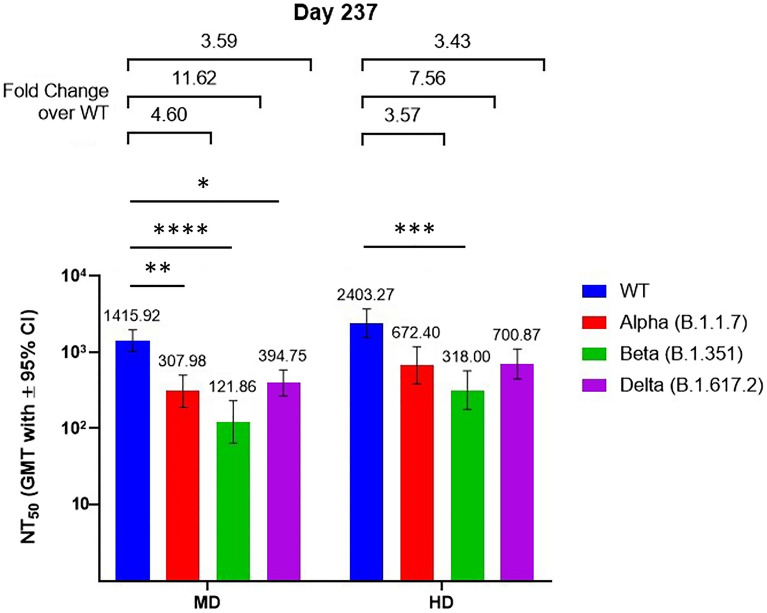
Serums samples from day 57 (4 weeks after second dose) and day 237 (4 weeks after booster dose) of MD and HD groups were subjected to wild-type and Omicron variant pseudovirus neutralization assay. Compared to the wild-type, the Omicron variant exhibited 55.1- and 74.2-fold decreases in neutralizing antibody titer levels for the MD and HD groups, respectively (Fig. 4). Administration of booster dose improved these reductions in neutralizing titers to decreases of only 8.7- and 6.4-fold in the MD and HD groups, respectively. After the second dose, only one individual in the MD group and four individuals in the HD group had detectable neutralizing titer against the Omicron variant pseudovirus. In contrast, in post-booster dose samples, all individuals in the HD group had detectable neutralizing titer while only one individual in the MD group had undetectable neutralizing titer against the Omicron variant (Fig. 4). The samples from day 237 were further assayed for live virus neutralization of the Alpha, Beta, and Delta variants (Fig. 5). Four weeks after the booster dose and regardless of the dosage group, the neutralizing titer levels seen for the Alpha and Delta variants were similar while they were lower for the Beta variant.
Fig. 4.
Pseudovirus neutralization assay of pseudovirus with spike proteins of Wuhan wild-type (WT) or Omicron variant with serum samples from the MD and HD groups taken on days 57 (4 weeks after second dose) and 237 (4 weeks after the third dose). Blue and red symbols show individual titer values. Thick horizontal bars represent GMTs and associated error bars represent 95% confidence intervals. Fold differences between GMTs are shown numerically above the upper dotted line. Dotted lines indicate the starting dilution (20; lower dotted line) and the final dilution (2560; upper dotted line) for the assay, and all values below 20 are tabulated as 10. Statistical significance was calculated with Kruskal–Wallis test with corrected Dunn’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Fig. 5.
Live virus neutralization assay with VoCs using serum samples of MD and HD groups taken on day 237 (4 weeks after the third dose). Results are presented as bars representing GMTs and associated error bars represent 95% confidence intervals. Fold differences between GMTs and associated confidence levels are shown above the data bars. Statistical significance was calculated with Kruskal–Wallis test with corrected Dunn’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Discussion
To our knowledge, this is the first report that addresses the ability of a prefusion stabilized SARS-CoV-2 S-2P protein-based vaccine booster shot to increase immunogenicity and maintain longer-term immunogenicity against wild-type and/or viral variants. Although the correlates of protection (CoP) are yet to be established for COVID-19 vaccines, neutralizing antibody titers appear to highly correlate with vaccine efficacy against symptomatic COVID-19 infection by prototypical strains in the early stage of the pandemic as described by Khoury et al. [9]. According to the above modelling, a level of neutralizing NT50 of 54 IU/mL could render 50% vaccine efficacy against the prototype strain, which is the desired WHO target product profile for COVID-19 vaccine approval [9]. Our study also took into account the waning neutralization over time and impact of variants in their efficacy estimation, and with the current Omicron variant. It was proposed that the tenfold decrease of binding to variant curve could be used to estimate the efficacy against the Omicron variant with efficacy derived from the original CoP curve [8]. In the phase 1 extension study described here, the residual neutralizing titer 6 months after the primary two doses of MVC-COV1901 was 79.4 IU/mL for the MD group, which exceeds the aforementioned 54 IU/mL. However, whether such a level of neutralizing titer could be translated into expected vaccine efficacy and effectiveness remains to be tested in a clinical study or in the post-marketing setting. The dynamics of antibody titer after the second dose are similar to other vaccines [10]. Specifically, titers peak approximately 2 weeks after administration and then decline to a still clearly detectable level by day 209. The GMT of neutralizing antibody titers for the MD group mimicked these dynamics as it showed a 6.2-fold reduction (495.9/79.4) at day 209 from the peak seen 14 days after the second dose. These dynamics are therefore comparable to those of the mRNA vaccines [11, 12]. Four weeks after the booster dose, a fold increase of 1.7 (818.3/495.9) over the previous peak was noted. This result shows that antibody response against SARS-CoV-2 can be boosted by the third dose. The durability of booster dose was clearly greater than that of the second dose. Approximately one-third of IgG level and neutralizing antibody levels in MD and HD groups were retained by day 389 relative to the peak level on day 237, while the comparable residual levels were less than 10% after the second dose (Fig. 3). The longevity of neutralizing antibodies induced by the booster dose was also seen with the half-life of the booster dose for the MD group (44 days versus 12 days for the second dose). However, the sample size of the current study is small and the accuracy of the half-life calculations may be enhanced using larger sample size in future studies. The small sample size also precluded the use of information criteria to evaluate the model selection for half-life estimation.
Notably, the benefit of the booster appears to extend to the Omicron variant as evidenced by impressive improvements by the booster of neutralization titer against Omicron pseudovirus (Fig. 4). However, compared to wild-type pseudovirus, this neutralization level is 8.7-fold lower for the MD group and only 6.4-fold lower for the HD group, suggesting that further boosters or boosters with higher doses may further improve Omicron neutralization titers. This result is in line with recent data that Omicron variant is refractory to the majority of anti-SARS-CoV-2 monoclonal antibody drugs and convalescent sera, and that two doses of currently available vaccines perform poorly against neutralization of the Omicron variant [13, 14]. These results are also seen when second dose and booster neutralization capacity was studied with the recombinant protein subunit vaccine NVX-CoV2373 and Moderna/Pfizer mRNA vaccines [4, 14].
Some limitations apply to this study. First, as a result of difficulty in obtaining live Omicron virus in Taiwan, we only tested Omicron pseudovirus constructs which may not accurately reflect the neutralizing ability against the live Omicron variant. We also did not directly compare the neutralization of the Alpha, Beta, and Delta VoCs after the second dose and third dose, although we have previously reported the neutralizing ability of two doses of MVC-COV1901 against Alpha and Beta variants [15]. Third, we only investigated the effect of booster shots on antibody immunity but not on cellular immunity. Nevertheless, we have shown that two doses of MVC-COV1901 could induce Th1-skewed T cell responses by assaying interferon-gamma and interleukin-4 (IL-4) levels in our phase 1 main study [7]. Finally, the sample size studied (n = 15 for each dosing level) was small because this was an extension to the phase 1 clinical trial. We also acknowledge that this small sample size may not be sufficient to observe rare AEs associated with the booster dose. However, our large-scale phase 2 clinical trial and the ongoing monitoring of post-injection safety by the V-Watch program of Taiwan Centers for Disease Control have robustly demonstrated the tolerability and safety of MVC-COV1901 among the general population, with lower incidence and severity of AEs than that of the mRNA vaccines and AZD1222 [6, 16]. The current findings should be able to usefully guide establishment of vaccination policy for future dosing.
Conclusions
The data presented here support the exploration of MVC-COV1901 vaccine in further clinical development targeting a third dose booster as protection against variants of concern.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study, and the journal’s Rapid Service Fees, were funded by Medigen Vaccine Biologics Corporation and Taiwan Centers for Disease Control, Ministry of Health and Welfare. Medigen Vaccine Biologics (the study sponsor) had a role in study design, data analysis, and data interpretation, but had no role in data collection, or writing of the clinical report. Taiwan CDC of the Ministry of Health and Welfare had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Medical Writing, Editorial, and Other Assistance
The Institute of Biomedical Sciences, Academia Sinica performed the neutralization assay. Members of Medigen Vaccine Biologics Corp. assisted in manuscript editing and revision.
Author Contributions
Concept, design, and leading the clinical trial: Szu-Min Hsieh. Acquisition and interpretation of data: Szu-Min Hsieh and Shan- Chwen Chang. Drafting of the manuscript: Szu-Min Hsieh, Hao-Yuan Cheng, Chia En Lien, and Shan- Chwen Chang. Laboratory assays and data analysis: Shin-Ru Shih.
Disclosures
Hao-Yuan Cheng and Chia En Lien are employees of Medigen Vaccine Biologics Corporation and have received grants from the Taiwan Centers of Disease Control, Ministry of Health and Welfare. Szu-Min Hsieh, Shan- Chwen Chang, and Shin-Ru Shih declared no conflict of interest. All authors have reviewed and approved of the final version of the manuscript.
Compliance with Ethics Guidelines
The trial protocol and informed consent form were approved by the Taiwan Food and Drug Administration and the Research Ethics Committee of National Taiwan University Hospital. The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. An independent data and safety monitoring board (DSMB) was established to monitor safety data and the trial conduct.
Data Availability
All data generated or analyzed during this study are included as supplementary information files.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Szu-Min Hsieh, Email: hsmaids@hotmail.com.
Chia En Lien, Email: allenlien@medigenvac.com.
References
- 1.Dong E, Du H, Gardner L. COVID-19 Dashboard by the Centre for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html. Accessed March 6, 2022.
- 2.UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing: Update on hospitalization and vaccine effectiveness for Omicron VOC-21NOV-01 (B.1.1.529). December 31, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1044481/Technical-Briefing-31-Dec-2021-Omicron_severity_update.pdf. Retrieved January 3, 2022
- 3.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallory R, Formica N, Pfeiffer S, et al. Immunogenicity and safety following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a phase 2 randomized placebo-controlled trial. medRxiv 2021.12.23.21267374. 10.1101/2021.12.23.2126737.
- 5.Munro AP, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh SM, Liu MC, Chen YH, et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide- adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh SM, Liu WD, Huang YS, et al. Safety and immunogenicity of a recombinant stabilized prefusion SARS-CoV-2 spike protein vaccine (MVC-COV1901) adjuvanted with CpG 1018 and aluminum hydroxide in healthy adults: a phase 1, dose-escalationstudy. EClinicalMedicine. 2021 doi: 10.1016/j.eclinm.2021.100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antia A, Ahmed H, Handel A, et al. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 2018;16:e2006601. doi: 10.1371/journal.pbio.2006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;17:1–7. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 10.Cromer D, Juno JA, Khoury D, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21(6):395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doria-Rose N, Suthar MS, Makowski M, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384(23):2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erice A, Varillas-Delgado D, Caballero C. Decline of antibody titers 3 months after two doses of BNT162b2 in non-immunocompromised adults. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 variant Omicron to antibody neutralization. bioRxiv. 2021.12.14.472630. 10.1101/2021.12.14.472630.
- 14.Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. New Engl J Med. 2022;386(5):492–494. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lien CE, Kuo TY, Lin YJ, et al. Evaluating the neutralizing ability of a CpG-adjuvanted S-2P subunit vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern. Clin Infect Dis. 2021;ciab711. 10.1093/cid/ciab711. [DOI] [PMC free article] [PubMed]
- 16.Taiwan Centers for Disease Control. V-Watch Bulletin Report, December 21, 2021. https://www.cdc.gov.tw/File/Get/paX3nGQmQ3qQo-BUQKMShQ. Accessed March 9, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included as supplementary information files.