Abstract
Direct-acting antivirals (DAAs) achieve high hepatitis C virus (HCV) cure rates and are forgiving to missed doses, but adherence–efficacy relationships have not been well defined. Traditional adherence measures (e.g. pill counts, self-report and pharmacy refills) over-estimate medication adherence. Newer technology-based tools have been used to provide more objective adherence data. Herein, electronic medication diaries (e-diaries), medication events monitoring system (MEMS®) caps, electronic blister packs, electronic pill boxes, video-based directly observed therapy (vDOT), artificial intelligence platforms (AIPs), and ingestible sensor systems are described, and compared based on existing studies using DAA. Percent adherence, predictors of adherence, and HCV cure rates utilizing these technologies are included. DAA adherence with e-diaries was 95–96%, MEMS® caps and ingestible biosensors were between 95% and 97%, blister pack weekly dosing ranged 73–98%, and daily dosing 73–94%, whereas electronic pill boxes ranged between 39% and 89%, vDOT was 98% and AIP 91–96%. Despite a wide range of adherence, high sustained virologic response (SVR) rates (86–100%) were observed across all studies utilizing these different technology-based tools. Current data support the forgiveness of DAA therapies to missed doses using tools that provide more quantitative adherence measures compared with self-report and provide insight on adherence–efficacy relationships for contemporary DAA.
Keywords: adherence, DAA, hepatitis C, SVR, technology-based tools
Introduction
Hepatitis C virus (HCV) infection is caused by a blood-borne flavivirus, an enveloped positive single-sense-strand RNA virus. 1 HCV establishes chronic infection in the majority of those infected (~70%), 2 which may lead to cirrhosis, liver cancer, and/or death if not treated. An estimated 58 million people have chronic HCV infection worldwide, with 1.5 million new cases annually. 2 There is currently no vaccine to prevent HCV infection, but chronic HCV is curable with a variety of effective direct-acting antiviral (DAA) therapies. Sustained virologic response (SVR) is synonymous with cure and is defined as having an undetectable HCV-RNA in peripheral blood (determined with the most sensitive polymerase chain reaction (PCR) technique) 12 weeks after completion of treatment ( i.e. SVR12).3,4 Current DAA therapies appear forgiving to missed doses, based on high SVR rates in clinical trials and real-world settings.5,6 Achieving SVR12 is associated with a number of benefits, including reductions in transmission, liver-related manifestations and all-cause mortality, and improved quality of life. 7
Available DAA agents (Table 1) act on three targets in the HCV lifecycle. NS3/4A protease inhibitors prevent cleavage of enzymes that are essential for HCV replication, whereas NS5A inhibitors block the protein responsible for proper assembly and function of the replication complex, therefore, inhibiting both viral replication and assembly/egress of viral particles. 8 NS5B nucleotide inhibitors suppress viral replication by binding to the active site of the polymerase enzyme and terminating the cycle chain. The non-nucleoside inhibitors (less preferred due to their low potency) bind to an allosteric site thereby preventing polymerase conformational changes.9,10
Table 1.
Summary of HCV DAA treatment regimens by class.
| DAA treatment regimen | NS3/4A protease inhibitor | NS5B nucleotide polymerase inhibitor | NS5A inhibitor |
|---|---|---|---|
| Elbasvir/grazoprevir (EBR/GZR) | x | x | |
| Glecaprevir/pibrentasvir (GLE/PIB) | x | x | |
| Ledipasvir/sofosbuvir (LDV/SOF) | x | x | |
| Sofosbuvir/simeprevir (SOF/SIM) | x | x | |
| Sofosbuvir/velpatasvir (SOF/VEL) | x | x | |
| Sofosbuvir/velpatasvir/voxilaprevir (SOF/VEL/VOX) | x | x | x |
DAA, direct-acting antivirals; HCV, hepatitis C virus.
Combinations of DAAs are used to achieve high SVR rates. 11 DAA treatment is generally 8 or 12 weeks in duration with one to three tablets taken once daily. DAAs are well tolerated. More common side effects (occurring in less than 20% of patients) include fatigue, gastrointestinal symptoms, and headache.12–14
Despite high efficacy rates and good tolerability, some patient populations are less likely to be treated with DAAs due to adherence concerns, such as persons who use drugs (PWUD) or those with mental health comorbidities.15–18 DAAs are clearly forgiving to missed doses based on high rates of SVR12 achieved in clinical trials and in practice, but the precise level of ‘forgiveness’ of DAA therapies to missed doses is unclear. Traditional adherence measures like pill counts, self-report, and pharmacy refills have been used to capture DAA adherence, however, these measures have significant shortcomings and tend to over-estimate adherence for a variety of disease states. 19 Newer technology-based measures for adherence assessments could help to better understand adherence–efficacy relationships with DAAs in addition to offering more detail about dose timing and adherence patterns in persons with HCV.
The purpose of this review is to describe and compare technology-based adherence monitoring approaches used in persons with HCV and DAA adherence as measured by these new approaches. Characteristics of study participants (including drug and alcohol use) are described to evaluate usability and generalizability.
Methodology
Abstracts, posters, presentations, and manuscripts relevant for this review were identified through searches of Google Scholar and PubMed between 2015 and 2021. The following search terms were used: ‘hepatitis C’, ‘HCV’, ‘direct acting antiviral’, ‘DAA’, ‘electronic’, ‘adherence’, ‘technology’. Additional studies were identified by following citations of identified manuscripts, presentations, and posters. Technologies reviewed included electronic medication diaries, medication events monitoring system (MEMS®) caps, electronic blister packs, electronic pill boxes, video-based directly observed therapy (vDOT), artificial intelligence platforms (AIPs), and ingestible biosensor systems.
Electronic medication diary
Electronic diaries20,21 (e-diaries) function like an electronic dose log, allowing participants to manually record drug dosing (and symptoms if desired) using handheld devices, such as phones, tablets, and computers that are wirelessly integrated in a website or an application (Figure 1).20,21 E-diaries also feature alarms to remind participants to voluntarily answer pre-defined study questions, such as adverse events (AEs) and date/time entry of medication dosing. With this technology, participants complete questionnaires electronically and data are sent wirelessly. Once data are entered and accuracy confirmed by participants in the e-diaries, it cannot be changed. 22 Investigators can then access the stored data in designated secure cloud.
Figure 1.
Schematic of electronic diary showing patient access, log drug dosing, and symptoms are logged manually (left), versus trial manager access (right), monitor, and visualize patient data. E-diary measures medication adherence, adverse effects, and provides schedule reminders.
E-diaries were used to monitor adherence to elbasvir–grazoprevir (EBR/GZR) in the phase 3 randomized, double-blind, multicenter, placebo-controlled C-EDGE CO-STAR trial. 23 HCV mono-infected (93%) and HCV/HIV co-infected individuals (7%) were on opioid agonist therapy (OAT). In this study, 301 participants (Table 2) with HCV genotypes 1, 4, and 6 were randomly assigned in a 2:1 ratio to immediate versus deferred EBR/GZR (50 mg/100 mg daily). In total, 201 participants were assigned to the immediate-treatment group (ITG) and received EBR/GZR for 12 weeks, and 100 were assigned to the deferred-treatment group (DTG). The latter received placebo for the first 12 weeks followed by 4 weeks of follow-up prior to the 12-week open-label treatment phase with EBR/GZR. All 100 in the DTG had detectable HCV RNA at week 16, of which 95 started the open-label study medication for active treatment. Substance use was assessed at baseline, day 7, every 2 weeks during treatment and at SVR 4, 8, 12, and 24 via urine drug screening (UDS).
Table 2.
Demographic characteristics of participants.
| Technologies/studies | Participants (n) | Mean/median age (years) | Male sex (%) | Majority of race (%) | Risks factors at baseline |
|---|---|---|---|---|---|
| Electronic medication diary | |||||
| Dore et al. 23 | 301 | 18 or older | 76.4 | White (80.1) | Positive urine drug screening (97.6%), cirrhotic (20.6%), amphetamines, barbiturates, benzodiazepines, buprenorphine, cannabinoids, cocaine, methadone, other opioids, phencyclidine, and propoxyphene |
| Medication events monitoring system | |||||
| Petersen et al. 25 | 60 | 72 | Black (88) | High-school degree or less (63%), psychiatric comorbidity (57%), intravenous drug use 6 months prior (52%), alcohol (10%), marijuana (17%), cocaine (8%), and/or heroin (5%) | |
| Electronic blister packs | |||||
| Litwin et al. 31 | 61 | 53 | 62 | Latino (66) | Medicaid insured (93%), HIV-negative (85%), cirrhotic (1/3), psychiatric comorbidities (74%), medical comorbidities (85%), injection drug use/ methadone (95%), tobacco use (77%), alcohol use (15%), any drug use (58.6%), opiates/prescription drugs (41%), benzodiazepines (34%), and cocaine (31%) |
| Cunningham et al. 5 , Grebely et al. 32 | 103 | 48 | 72 | Receiving opioid agonist therapy (59%), injection drug use (74%), heroin (55%), amphetamines (30%), and other opioids (21%) | |
| Akiyama et al. 33 | 150 | 51 | 65 | Non-Caucasian (92) | Treatment-naïve (89%), drug use (65%), opioids (47%), cocaine (47%), drug injection (75%) |
| Electronic pill boxes | |||||
| Coffin et al. 39 | 31 | 42 | 80.7 | White (74.2) | Injection drug use (45.2%) Heroin (77.4%), prescription opioids (29%), cocaine/crack (22.6%), methamphetamine injection (67.7%), mean injection partners 6.2, syringes sharing 45.2%, ‘cooker’ sharing 35.5% |
| Electronic pill boxes or video-based directly observed therapy | |||||
| Brooks et al. 42 | 60 | 51 | 78 | White (72) | Drug and alcohol use, HIV co-infection (78%), substance use: marijuana (60%), methamphetamine (37%), opioids (22%), cocaine (17%), alcohol use (56% person visits) and 19% were heavily using alcohol ranging from 0 to 17 drinks daily per self-report |
| Artificial intelligence platforms | |||||
| Litwin et al. 45 | 17 | 51 | 70.6 | Latino (76.5) | Polysubstance use (70.6%) (heroin, cocaine, crack cocaine, benzodiazepines and/or prescription opioids) |
| Leo et al. 46 | 35 | 53 | 63 | N/A | N/A |
| Ingestible biosensor systems | |||||
| Sulkowski et al. 49 | 288 | 53 | 67.4 | African-American (42) Caucasian (39) |
Medicaid insurance (64.9%) Psychiatric disorders (61.1%), cirrhosis (10.8%), HIV co-infection (18.8%), drug/substance-use disorder (29.2%), alcohol use disorders (3.7%) |
| Bonacini et al. 55 | 28 | 59 | 61 | Caucasian (39) African-American (36) |
Treatment-naïve (93%), psychiatric comorbidities (46%), history of drug abuse (32%) |
N/A, not available.
By intention to treat, SVR12 was 91.5% (184/201; (95% CI: 86.8%, 95%)) in the ITG group versus 89.5% (85/95; (95% CI: 81.5%, 94.8%)) in the active phase of the DTG. 23 Adherence by e-diary in the initial 12-week treatment period was >95% in 192/199 (96.5%) of ITG participants and 97/97 (100%) of the placebo-phase DTG. Adherence was 91/95 (95.8%) during the active-phase DTG. Positive UDS results were found in >50% of participants for at least one recreational drug, but there was no difference in SVR rates between those who had positive versus negative UDS.
Medication events monitoring system (electronic MEMS® caps)
Medication event monitoring system 24 (Electronic MEMS Caps (MWV Healthcare, Switzerland Ltd, Sion, Switzerland)) is a conventional medication bottle equipped with an electronic chip cap that records the date and time of each bottle opening/closing (Figure 2) 24 Dosing data are recorded in real-time but must be transferred from the cap and stored on a secure and centralized server to be accessed. Encrypted data can be exported from the manufacturer’s server for visualization and analysis at the clinical site. Compatible smartphones or tablets can also be provided to patients to download the stored data from the caps to the pre-installed software. 24
Figure 2.
Schematic of the Medication Event Monitoring System (Electronic MEMS Caps) consisting of medication bottle equipped with an electronic chip cap (left), and the reader (center) for patients to download stored data that are exported to the software for visualization and analysis of adherence patterns by the provider (right).
MEMS Caps were used to monitor DAA adherence in the NIH SYNERGY trial: a three-arm phase 2a clinical trial that investigated whether a third potent DAA added to ledipasvir/sofosbuvir (LDV/SOF) could reduce treatment duration from 12 to 6 weeks. 25 The impact of participants’ adherence to HCV treatment outcomes and risk factors associated with suboptimal adherence were also investigated. Sixty HCV mono-infected treatment-naïve, genotype 1 participants (Table 2) were enrolled into one of three arms (n = 20 participants per arm): (1) LDV/SOF (90 mg/400 mg) once daily for 12 weeks, (2) LDV/SOF plus an investigational protease inhibitor (GS-9451) 80 mg once daily for 6 weeks, and (3) LDV/SOF once daily plus an investigational non-nucleoside reverse transcriptase inhibitor (GS-9669) 500 mg twice daily for 6 weeks.
Adherence was measured using MEMS caps, pill counts, and patient self-report. In participants treated with LDV/SOF alone, adherence by MEMS, pill count, and self-report were 96.7%, 98.2%, and 99.3%, respectively. 25 LDV/SOF + GS-9451 adherence was 97.3%, 98.2%, and 99.3% by MEMS, pill count, and self-report, respectively. LDV/SOF + GS-9669 adherence was 95.0%, 98.9%, and 99.5% by MEMS, pill count, and self-report, respectively. 25 MEMS adherence in all arms was lower than those of self-report. Adherence by MEMS was similar to pill count for the one and two pill(s) per day treatment arms, but significantly lower for three pills per day. Overall, (58/60) 96.6% of the patients in the study achieved SVR. Of those who did not achieve SVR, one was incarcerated and the other missed one dose according to MEMS and no doses by self-report.
The most common self-reported reasons for missing doses included ‘feeling as if the treatment was working’ (38%), ‘forgetting’ (35%), and ‘being away from home’ (32%). 25 MEMS adherence decreased over time from 98.1% (weeks 0–4) to 95% (weeks 8–12) in the 12-week treatment arm. Use of marijuana, cocaine, or heroin in the 6 months before therapy or alcohol use (three drinks daily or five drinks within 2–4 h at any time) within the prior 30 days was associated with lower adherence in the 12-week, but not in the 6-week treatment arms. Self-report tended to overestimate adherence by ~2–4% compared with MEMS caps (and pill counts for the three pills per day regimen), but overall adherence to HCV treatment was high in this study, showing that even individuals with perceived risk factors for non-adherence have very high adherence to HCV treatment.
Electronic blister packs
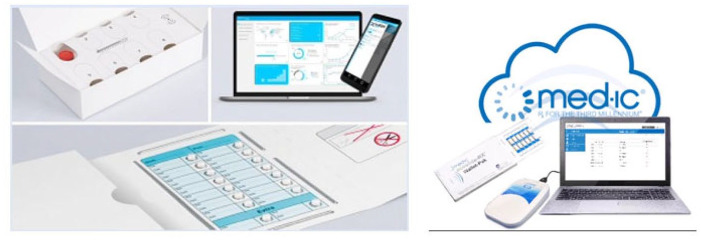
Electronic blister packs 26 are single unit-of-use packaging systems equipped with radio frequency identification (RFID) sensor tag grids that record the date and time of capsule or tablet pill dosing (Figure 3).26–28 Standard blister packs are made of two essential components: (1) the cavity containing the pill and (2) the backing that helps to seal the pill in the package. 29 Each pill may be pushed out by pressing into the back of the cardboard. Smart blister packs developed by Med-ic ECM® (Ottawa, Ontario, Canada) or Schreiner MediPharm (Germany) in partnership with AARDEX (Belgium, Switzerland, and the United States) incorporated sensors into standard blister packs to help trace, record, and improve adherence capturing. Dosing information is automatically stored in the medication package and transmitted to secure cloud when packaging is either scanned to a smartphone compatible app by participants, or to a reader by study personnel when blister packs are returned at follow-up visits.28,30
Figure 3.
Schreiner MediPharm (left) versus Med-ic ECM (right). Schematic of the electronic blister pack system showing tablets pushed out from blister pack (equipped with radio frequency identification tag that records medication type, extraction time, specific cavity) and exportation of data via smartphone/reader for provider adherence analysis and follow-up.
The RISE-II study was a single-arm prospective study that evaluated real-world adherence and SVR rates to LDV/SOF or sofosbuvir/simeprevir (SOF/SIM) in PWID receiving OAT for 8–24 weeks across three study sites. 31 DAA adherence was calculated multiple ways: daily dosing within 6 and 24 h of the assigned dosing time and weekly dosing (number of doses taken/1-week period) with electronic blister packs. In addition, monthly self-report with a visual analog scale (VAS) ranging from 0 to 100% was provided and urine toxicology was obtained via chart review.
This study enrolled 61 participants (Table 2) who were mostly HCV genotype 1 (97%). 25% (N = 15) of participants were treated with SIM/SOF and 75% (N = 46) received LDV/SOF. 31 DAA treatment durations were 8 (13%, N = 5), 12 (82%, N = 50) and 24 (10%, N = 6) weeks. Compensation of US$10 was provided weekly. Mean adherence was 63.4%, 73.4%, 90.2%, and 98.4% for daily dosing within 6 and 24 h of scheduled time by blister pack, weekly by blister pack, and monthly by VAS, respectively. 31 A decrease in doses taken within 24 h was observed as weeks of treatment increased. Daily time-frame adherence by blister pack for weeks 1–4 was 74.8%, versus 74.1% for weeks 5–8 and 71.3% for weeks 9–12. SVR12 was achieved in 98.4% (60/61) of participants. The one participant that did not achieve SVR12 was LTFU after achieving SVR4.
Adherence to fixed dose of sofosbuvir/velpatasvir (SOF 400 mg/VEL 100 mg) and risks factors for imperfect adherence in PWID were investigated in the SIMPLIFY study in Australia.5,32 One hundred and three PWID with recent drug use (within 6 months) and chronic HCV infection took 12 weeks of SOF/VEL in a weekly blister pack. Self-reported adherence (by questionnaire) and pills remaining in the blister pack were also assessed every 4 weeks. Compensation of $30 Australian was given to each participant for returning the blister packs to download adherence data and for treatment follow-up.
Overall median adherence was 94% and 98% daily and weekly, respectively, compared with self-reported adherence which was 99%. Ninety-seven percent (n = 100) completed treatment at 12 weeks with SVR of 94% (97 of 103 (95% CI: 88–98%)).5,32 Among 81 reports of non-adherence during therapy, reasons for non-adherence were: ‘Forgot’ (67%), inaccessibility at dose time (17%), and lost pills (9%). 5 Overall, 34% (n = 35) of participants were <90% adherent to SOF/VEL.5,32 The odds of non-adherence were ~two- to three-fold higher among those injecting stimulants (cocaine and/or amphetamines) during treatment. 5 SVR was the same among those with ⩾90% adherence (94%, 66/70) versus those with less than 90% (94%, 31/33). Ninety-seven of the 100 participants who completed treatment achieved SVR, and 82% (9/11) of the highly non-adherent participants completed treatment and achieved SVR. 32
A separate study, PREVAIL, examined adherence, treatment completion, and SVR12 using either Med-ic blister packs or directly observed therapy (DOT) in a three-arm randomized controlled trial. 33 This included two intervention groups (DOT and group treatment (GT)) and one control group (self-administered individual treatment (SIT)). DOT was performed during methadone visits in opioid treatment programs (OTP), whereas GT group participated in weekly HCV examination and educational sessions where some received peginterferon injections and weekly blister pack medications. All SIT participants received weekly blister pack medications and self-administered them.
A total of 158 participants were included in an adherence sub-study in which 53, 52, and 53 participants were randomly allocated to DOT, GT, and SIT, respectively. Fifty-one, 48, and 51 participants initiated and completed treatment in DOT, GT, and SIT groups, respectively. In total, 98% (147/150) of all participants received methadone DOT four to six times weekly. 33 Most participants (77% (115/150) received DAA treatments, including LDV/SOF (n = 104, 69%), SIM/SOF (n = 11, 7%), and the others received SOF/RBV (n = 17, 11%), telaprevir (TVR)/pegylated interferon/ribavirin (n = 15, 10%), and SOF/pegylated interferon/ribavirin (n = 3, 2%). 33 Overall treatment completion was 97% (95% CI: 92–99%; with no difference between groups: p = 0.53). 33 Overall adherence was 78% (95% CI: 75–81%) across all groups. Adherence was higher in the DOT group (86%) than in the SIT group (75%, p = 0.001). The GT arm was 80% adherent and did not significantly differ from the SIT group. Overall SVR was 94% (95% CI: 89–97%). No significant difference (p = 0.152) was found between group SVR rates: 98% (95% CI: 90–100%) for DOT; 94% (95% CI: 83–99%) for GT, and 90% (95% CI: 79–97%) for SIT. SVR in SIT was numerically, but not statistically, lower than DOT and GT. 33 Limiting data only to DAA treatments, overall SVR was 95% (95% CI: 89–98%; p = 0.056). Group SVR for DAAs alone were 100% (95% CI: 90–100%) for DOT, 95% (95% CI: 83–99%) for GT and 90% (95% CI: 76–97%) for SIT. 33 Among those taking only DAAs, overall adherence, consecutive days of doses taken or missed, and adherence in the first 4 and 8 weeks of treatment (but not weeks 8–12) were associated with SVR. 34
Electronic pill boxes
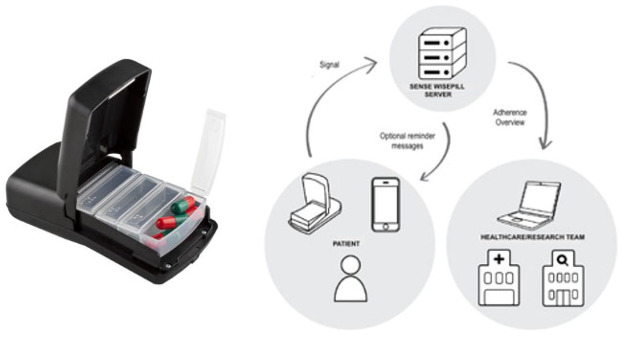
Electronic pill boxes, such as the Wisepill® RT2000 (Cape Town, South Africa), 35 are reusable compartmented pill boxes that wirelessly monitor opening of the outer pill box portion (Figure 4). 35 These are equipped with a SIM card connected to a cellular network and are battery powered. 36 As users open the box to take medication, a cellular signal is sent to the web-based server, and the appropriate healthcare staff can access and transfer the recorded data via a centralized server in real-time. These devices have been used in multiple clinical studies across different therapeutic areas, including HCV.37–39
Figure 4.
Schematic of a pill box – Wisepill RT2000 for medication adherence monitoring. As patients open the box, a cellular signal is sent to the web-based server, and research staff access and transfer the recorded data via a centralized server.
Coffin et al. conducted a randomized (2:1) trial of modified DOT (mDOT) versus Wisepill only based LDV/SOF (90 mg/400 mg) monitoring in persons with HCV and injection drug use. 39 mDOT participants received in-person doses at the clinic on weekdays and weekend doses in a Wisepill® dispenser. Participants randomized to the Wisepill-only arm had seven tablets loaded in their Wisepill device at weekly study visits for self-administration. Thirty-one participants were randomized to either the mDOT intervention (n = 20) or unobserved arm (n = 11). Participants in both arms attended weekly visits for reviews of medication adherence by pill count, concomitant medications, and AE review, and UDS. Compensation of up to US$340 was provided.
Retention rates were 96.6% and 96.3% in the Wisepill-only and mDOT arms for weekly visits, respectively (p = 0.89). 39 mDOT dosing participants attended 89.4% of daily dosing visits. In this study, 91.6% of daily visits were completed and all remaining participants completed treatment (96.8%). Mean adherence was 39.2% in the Wisepill-only arm versus 49.9% for weekend doses in the mDOT arm (p = 0.66). Only 36.4% and 60% of participants in the Wisepill-only and the mDOT arms, respectively, demonstrated >50% adherence with Wisepill (p = 0.27). 39 However, many participants never opened their Wisepill dispensers and in 53% of visits, the dispensers were not returned (p = 0.72). Therefore, replacement Wisepill dispensers were provided. Adherence by pill count was 99.5% in visits involving return of Wisepill dispensers or alternative pill containers. SVR12 was reached for 28/31 (90.3%) participants. 39 Among those who failed to achieve SVR, 1 terminated early, another relapsed, and the other was reinfected. Given the high rate of SVR, it is likely that participants were still taking HCV therapy, but not consistently engaging in use of the Wisepill container in this study.
Video-based platforms
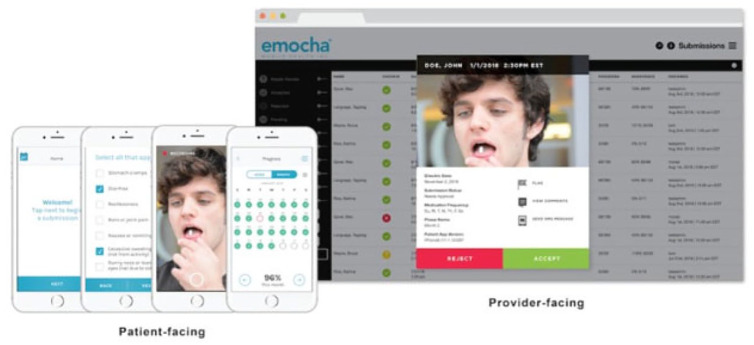
vDOT platforms like emocha® Mobile Health (Baltimore, MD), 40 allow providers to asynchronously and remotely visualize and monitor medication adherence (Figure 5). 40 Patients video-record their medication ingestion using the Emocha application on a smartphone. Other information, such as AEs, can also be entered. Once transmitted into the designated secure cloud, the authorized staff receive the encrypted video and data.40,41
Figure 5.
Emocha—schematic of video directly observed (vDOT) technology illustrating patient-facing side allowing to record, review, and send medication ingestion, side effects, therapy progress, and adherence (left). The provider-facing side is accessible by research personnel for adherence review, analysis and follow-up from electronic devices (right).
The INtensive monitoring of hCv antiviraL adherence in persons Using Drugs (INCLUD) study investigated adherence to LDV/SOF, SVR, and risk factors associated with low adherence in PWUD and alcohol. 42 Sixty participants were randomized to take LDV/SOF by either WOT, Wisepill® or vDOT with a smartphone app (miDOT using emocha® Mobile Health, Baltimore, MD). Participants were stratified by injection drug use (IDU) and cirrhosis status. Self-reported drug use and urine toxicology screen were performed every 2 weeks during the 12-week LDV/SOF treatment period. Compensation of US$20 per visit with an additional US$5/video for adherence monitoring engagement and US$5/week for pill box exchange were given to vDOT and WOT participants, respectively.
Overall and between-visit adherence were calculated by WOT and vDOT. Total median (range) adherence was 96% (1–101%) for the overall population, 89% (49–100%) for WOT, and 98% (30–101%) for vDOT. Median (range) between-visit adherence was 93% (7–100%) with WOT, and 100% (0–107%) with vDOT. 42 During the treatment period, drug use occurred at 94% of the 343 person-visits based on self-report or UDS. SVR rates were 86.7% (52/60) and 94.5% (52/55) by the intent-to-treat (ITT) and as-treated populations, respectively. 42 Of the 31 participants in the WOT study arm, 26 achieved SVR. Out of 29 participants in vDOT, 26 achieved SVR. Overall, high adherence and cure rates were achieved with LDV/SOF using both technologies. Findings from this study indicate LDV/SOF is forgiving since cure was achieved even with imperfect adherence ranging as low as 30%.
Artificial intelligence platforms
AIPs, such as AiCure®, are novel technology-based adherence monitoring approaches that use facial recognition systems to monitor dosing in real-time (Figure 6). 43 AIP is designed for synchronous audio-visual confirmation of medication ingestion by tracing patients’ identity, type of medication, and ingestion behaviors from a smartphone or camera tablet. 37 AIP can also be used to remind patients when and how to take medications by sending timely notifications to patients for improper medication administration, late or missed doses. Encrypted dosing data are stored on participants’ tablets/smartphones and sent wirelessly into web-based dashboards. 44
Figure 6.
Schematic of the artificial intelligence platform (AIP) that uses audio-visual recognition systems to monitor dosing compliance (left) and store encrypted data to the web-based dashboards for provider review in real-time (right).
AIP has been explored as a means of monitoring adherence to HCV therapy in two small pilot studies that both utilized the AiCure platform (New York, NY). The first was an open-label, non-randomized study of 17 PWID during self-administration of LDV/SOF for 8 (n = 2) or 12 weeks (n = 15). 45 All participants completed treatment and mean cumulative adherence by visual AIP was 91.3%. Fifteen of 17 participants (88.2%) achieved SVR12. 45 The two participants who failed to achieve cure despite high adherence rates of 98% and 100%, had stage 3 fibrosis and cirrhosis, respectively. The former received only 8 weeks of LDV/SOF. Study compensation was not described.
In a separate study, 124 members of a health plan initiating HCV treatment were asked to utilize smartphone-based AIP for DAA dosing. 46 Forty participants (32%) responded and agreed to use AIP and were given US$5 per daily DAA dose and US$60 monthly bonuses for adherence >85%. Individuals were treated with one of three DAA regimens: LDV/SOF (n = 26), SOF/VEL (n = 18), or GLE/PIB (n = 49). 46 AIP participants had adherence >95% and 98% of DAA doses were taken within 5 h of the AIP reminder time. To compare DAA adherence with versus without AIP, the proportion of days covered (PDC) based on commercial pharmacy claims was compared among those who used AIP versus a control group of non-AIP participants. PDC data were available for 56 non-participants and 35 of the 40 AIP participants. PDC among participants using AIP was significantly higher (96.2%) than non-participants (87.6%, p = 0.02). 47 There was no difference in PDC between regimens. SVR results were not reported in this study.
Ingestible biosensor systems
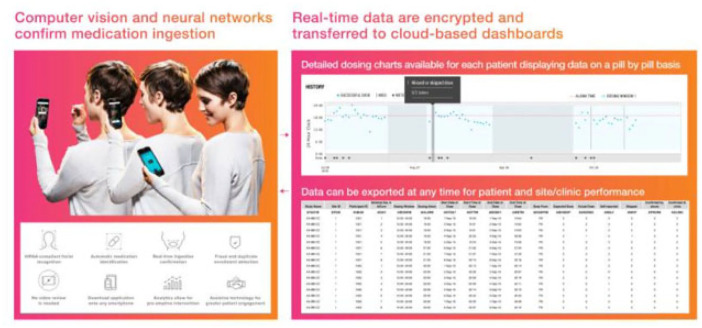
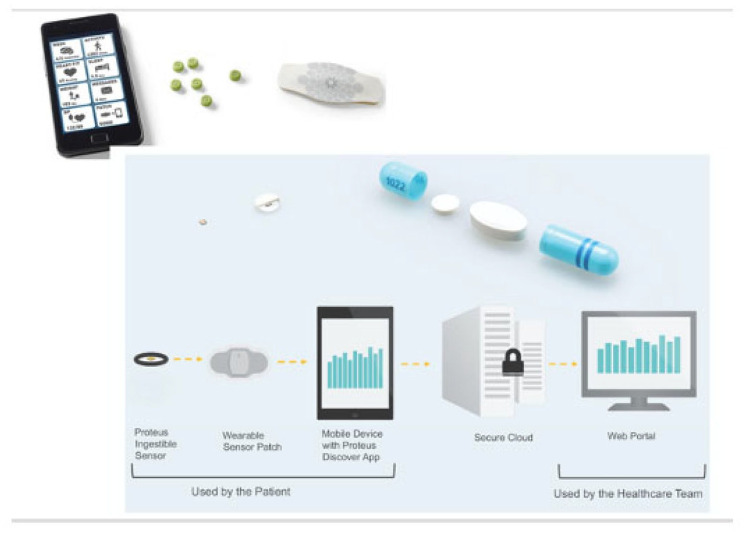
Ingestible biosensor systems are a unique way to measure adherence as they measure actual drug ingestion. Proteus Discover™ (Proteus Digital Health, Inc.; Redwood City, CA)48–50 is an example of an ingestible biosensor system in which medications are co-encapsulated with micro digital ingestible sensors made of active layers, integrated circuit, and insulating skirt disk (Figure 7).48–50 Once ingested, gastric fluid powers a battery followed by the activation of the sensor. 51 The signal and all related activities are detected by the adhesive wearable sensor patch. EtectRx™ (Gainesville, FL) uses a different ingestible sensor system, the ID-Cap™ system. The differences here are (1) the ID-Tag™ is made of magnesium/silver chloride pairs and is embedded in a gelatin capsule 52 and (2) a wearable neck reader reads the radio frequency signal instead of a patch. 53 Both Proteus and EtectRx™ technologies are Food and Drug Administration (FDA)-approved. Adherence data are recorded, stored, and displayed on designated mobile application or a web portal. 54 Patients can manually enter dosing if ingestion is not captured and can also enter the reason for a manual ingestion.
Figure 7.
Proteus Discover—overview of ingestible sensor system (top) depicting sensor-co-encapsulated pill, a wearable patch, and mobile device app from which stored medication adherence, physical, physiological/behavioral data are sent to provider web portal from the secure server once pill is ingested (bottom).
Proteus Discover™ was used to determine DAA efficacy and adherence in persons with HCV and at risk for non-adherence. This prospective, single-arm, open-label, multicenter study was conducted across 18 clinics in the United States among adults initiating oral DAA for chronic HCV treatment. 49 Patients were treated and monitored wirelessly with the sensor co-encapsulated fixed dose of each regimen. The main efficacy outcomes evaluated were SVR12 and medication adherence. A total of 288 participants were enrolled and received DAA treatment for 8 or 12 weeks. Participants received either SOF/VEL (19%, n = 56), LDV/SOF (26%, n = 74), or GLE/PIB (55%, n = 158). Overall, 81.9% (236/288) of the enrolled participants completed the treatment phase. The remainder (n = 52) were LTFU (n = 35) or withdrew from the study (eight participants for ingestible sensor system-related reasons, five for AEs, three for non-AEs, and one ineligible after enrollment).
In all, 218 out of the 236 participants (92.4%) who completed treatment had adherence and SVR data at least 10 weeks after treatment completion available. Of the remaining 18 who did not complete post-treatment follow-up, one did not have HCV RNA measured but the other 17 participants had HCV RNA measures at a median of 5.6 weeks post completion of DAA treatment. Adherence rates in the latter were 88.7% and 94.4% in 16 and 1 participants, respectively. Overall mean adherence was 95% (90.5%, 97.6%). Median (interquartile range (IQR)) adherence per DAA regimen were 96.1% for LDV/SOF, 95.2% for VEL/SOF, and 94.6% for GLE/PIB. 49 Overall, 99.1% achieved SVR, recognizing that this value is based on those with complete data and does not count non-completers as failures.
This study included a survey of participants’ and providers’ satisfaction with the technology, more than 80% of the 230 participants who completed the survey responded with ‘strongly agree’ or ‘somewhat agree’ to perceived usefulness and ‘ease of use’ of the system. Also, 66.7% (12/18) of providers returned their four-question survey on the usefulness of the ingestible sensor system in their treatment decision. Seven percent (20/288) of the enrolled participants experienced nonserious skin AEs (rash, dermatitis, and erythema) related to Proteus patch. Nonserious AEs (diarrhea, nausea, anxiety, and fatigue) deemed possibly related to the ingestible sensor were reported in 1% (3/288) of enrolled participants.
A separate study evaluated adherence to LDV/SOF treatment among patients with HCV infection also using the Proteus platform. 55 In this prospective, observational, open-label, 24-week single-arm pilot study conducted at two study sites, participants who were able to use a smartphone/tablet and had adequate data connectivity at home were eligible to participate. A total of 31 patients were screened, of which 28 were enrolled (Table 2). Eighty-nine percent of participants achieved adherence of least 95%, and the overall mean adherence was 97%. Risk factors associated with lower mean adherence included education level (91% for high school vs 96% for less than high school), psychiatric comorbidity (90% for psychiatric comorbidity vs 96% for those without a psychiatric comorbidity), and a combination of race, level of education, and psychiatric comorbidity (88% for African Americans with high school education or less, and psychiatric comorbidity vs 96% for others). 55
The ingestible sensor system allowed for same-day intervention from providers in 39% of participants. Ninety-three percent of participants achieved SVR12. The other two participants relapsed (one with 90% adherence and the other with 95% adherence suggesting viral resistance). Twenty-six participants responded to the patient satisfaction survey and 21–25 were mostly satisfied with the use of the system device. Ninety-two percent completely or somewhat agreed that system was easy to use; 96% had no issues wearing the patch and sharing data with study personnel, and 85% understood the importance of taking medications as scheduled. Four participants reported AEs (rashes and itching) related to patch usage, ranging from mild to severe, but they all resolved.
Discussion
Several adherence monitoring tools have been studied in persons with HCV, many of which are novel, technology-based approaches to monitor and improve medication adherence, better understand adherence patterns, and allow for real-time interventions if needed. Many of these tools were also evaluated in patient populations historically considered to have adherence challenges. As expected, DAA adherence assessed using pill counts and self-report (>98% and >99%, respectively) was higher in these studies than that observed using technology-based monitoring tools. Adherence ranges among studies with technology-based tools (Tables 3 and 4) were as follows: vDOT (98%), MEMS® caps and ingestible sensors (95–97%), e-diaries (95–96.5%), AIP (91–96%), blister pack weekly adherence (73–98%), blister pack daily adherence (73–94%), and electronic pill boxes (39–89%). Daily adherence measured by blister packs in RISE-II and SIMPLIFY were lower compared with weekly adherence (doses taken more than 24 h apart), suggesting that participants may take all the prescribed doses in a week, but may take more than one pill in a single 24-h period. Use of drugs was associated with lower overall adherence across several studies, but adherence was still high. 33 Also in INCLUD, between-visit dosing was lower with WOT compared with vDOT. 42 These patterns of DAA adherence suggest that patients may take doses but perhaps not as scheduled. Considering homelessness, substance use, and other risk factors, it is possible that pocket dosing could explain the discrepancies, especially if participants found dispensers too bulky to carry when moving between places or planning to be outside of the home at the next scheduled dosing. These electronic technologies were developed to improve upon some issues observed with the standard monitoring methods, such as pill counts, pharmacy refills, and self-report, all of which tend to overestimate adherence and cannot measure medication ingestion. 56 While the technologies may provide a more accurate estimate of HCV medication adherence and even improve adherence, they do have some limitations, reviewed below, and outlined in Table 5, they require participant engagement with the technology, and increased costs. Costs of the technology may vary and are dependent on factors, such as study sample size/number of patients, monitoring duration, whether technology is needed for participants to upload data directly versus only research or clinic personnel, and number of study personnel or clinical providers who require access to the data.
Table 3.
Summary of study characteristics.
| First-author reference | Study type | Sample size (n) | Substance/alcohol use (Y/N) | HCV genotypes, 1–6 (%) | DAA regimens (%) | Number of tablets—dosing frequency/day | Treatment duration in weeks (%) | Adherence monitoring tools | Mean cumulative DAA adherence (%) | SVR12 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dore et al. 23 | Randomized (immediate deferred) HCV treatment |
201 100 |
Y | 1a (75.7) 1b (15) 4 (6) 6 (3) Mixed (0.3) |
EBR/GZR | 1 QD | 12 | Electronic medication diary | 96.5 95.8 |
91.5 89.5 |
| Petersen et al. 25 | Three-arm phase-2a clinical trial | 60 (20/arm) | Y | 1 | LDV/SOF LDV/SOF/GS-9451 LDV/SOF/GS-9669 |
1 QD 2 QD 3 BID |
12 6 6 |
Medication events monitoring system (Electronic MEMS Caps) | 97.6 97.3 95 |
96.6 |
| Litwin et al. 31 | Single-arm prospective trial | 61 | Y | 1 (97.7) 4 (3.3) |
LDV/SOF (75) SIM/SOF (25) |
1 QD 1QD |
8 (8) 12 (82) 24 (10) |
Electronic blister packs (Med-ic ECM®) |
73.4 (daily) 90.2 (weekly) |
98.4 94 |
| Cunningham et al. 5 /Grebely et al. 13 | Single-arm open-label | 103 | Y | 1 (35) 2 (5) 3 (58) 4 (2) |
SOF/VEL | 1 QD | 12 (100) | Electronic blister packs (Med-ic ECM®) |
94 (daily) 98 (weekly) |
94 |
| Akiyama et al. 33 (Prevail study) | Randomized to DOT GT SIT (control) |
150 51 (DOT) 48 (GT) 51 (SIT) |
Y | 1a (85) 1b (15) |
LDV/SOF (69) SIM/SOF (7) SOF/RBV(11) SOF/IFN/RBV (10) TVR/IFN/RBV (2) |
1 QD 1 QD 1 QD 1 QD 1 QD |
8–12 | Electronic blister packs (Med-ic ECM®) | 78 (overall) 86 (DOT) 80 (GT) 75 (SIT) |
95 (overall) 100 95 90 |
| Coffin et al. 39 | Randomized to mDOT or Wisepill |
31 | Y | 1 (100) | LDV/SOF | 1 QD | 8 (100) | Electronic pill boxes (Wisepill®) | 39.2 (weekly dosing), 49.9 (36.6) (weekend dosing) | 89.7 (as treated), 90.3 (ITT) |
| Brooks et al. 42 | Randomized to Wisepill (WOT) or vDOT | 31 29 |
Y | 1a (65) 1b (22) 1 (8) 4/4a (5) |
LDV/SOF (100) | 1 QD | 12 | Electronic pill boxes WOT: Wisepill, or video-based directly observed therapy (vDOT—emocha®) |
89 (WOT)98 (vDOT) |
96 (overall), 83.9 (pill box), 89.7 (vDOT) |
| Litwin et al. 45 | Prospective single-arm, non-randomized | 17 | Y | 1 (100) | LDV/SOF (100) | 1 QD | 8–12 | Artificial intelligence platforms (AIP, AiCure) | 91.3 | 88.2 |
| Leo et al.46 | Invited to use AIP versus usual care | 35 (AIP) 58 (usual care) |
N/A | LDV/SOF SOF/VEL GLE/PIB |
1 QD 1 QD 3 QD |
Artificial intelligence platforms (AIP, AiCure) | 96.2 | N/A | ||
| Sulkowski et al. 49 | Prospective, single-arm, open-label | 288 | Y | 1a (65) 1b (13) 2 (6) 3 (12) 4 (1) |
VEL/SOF (19) LDV/SOF (26) GLE/PIB (55) |
1 QD 1 QD 3 QD |
Ingestible sensors systems (Proteus Digital Health) | 95 | 99.1 | |
| Bonacini et al. 55 | Prospective, observational, open-label, single-arm | 28 | Y | 1a(100) | LDV/SOF (100) | 1 QD | Ingestible sensors systems (Proteus Digital Health) | 97 | 92.9 |
AIP: artificial intelligence platform; BID: twice daily; DOT, directly observed therapy; EBR/GZR: elbasvir/grazoprevir; GLE/PIB: glecaprevir/pibrentasvir; GT: group treatment; HCV: hepatic C virus; IFN: interferon; ITT: intention-to-treat; LDV/SOF: ledipasvir/sofosbuvir; mDOT: modified directly observed therapy; N/A: not available; RBV: ribavarin; SIM/SOF: simeprevir/sofosbuvir; SOF/VEL: sofosbuvir/velpatasvir; SIT: self-administered individual treatment; TVR: telaprevir; vDOT: video directly observed therapy; wDOT: wireless direct observed therapy; QD: once daily.
Table 4.
Adherence and SVR comparison by study and technology tool.
| First author reference | Dore et al. 23 | Petersen et al. 25 | Litwin et al. 31 | Cunningham et al.
5
Grebely et al. 32 |
Akiyama et al.
33
(Prevail study) |
Coffin et al. 39 | Brooks et al. 42 | Litwin et al. 45 | Leo et al. 46 | Sulkowski et al. 49 | Bonacini et al. 55 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adherence monitoring tools | Electronic medication diary | Medication events monitoring system (Electronic MEMS Caps) | Electronic blister packs (Med-ic ECM) | Electronic blister packs (Med-ic ECM) | Electronic blister packs (Med-ic ECM) | Electronic pill boxes (Wisepill) | Electronic pill boxes, WOT: Wisepill or video-based directly observed therapy (vDOT—emocha) | Artificial intelligence platform (AIP, AiCure) | Artificial intelligence platform (AIP, AiCure) | Ingestible sensors systems (Proteus Digital Health) | Ingestible sensors systems (Proteus Digital Health) |
| Mean cumulative DAA adherence (%) | 95.8–96.5 | 95–97.6 | 73.4–90.2 | 94–98 | 75–86 | 39.2–49.9 | 89 (WOT), 98 (vDOT) | 91.3 | 96.2 | 95 | 97 |
| SVR12 (%) | 89.5–91.5 | 96.6 | 94–98.4 | 94 | 90–100 | 89.7–90.3 | 83.9 (WOT), 89.7 (vDOT) | 88.2 | N/A | 99.1 | 92.9 |
DAA, direct-acting antivirals; SVR, sustained virologic response; vDOT, video-based directly observed therapy (emocha); WOT, electronic pill boxes (Wisepill); N/A, not available.
Table 5.
Key points and limitations of HCV technology-based tools.
| E-diary | Electronic MEMS Caps | Electronic blister packs (Med-ic ECM)) | Electronic pill boxes (Wisepill) | Video-based directly observed therapy (vDOT—emocha) | Artificial intelligence platforms0 (AIPs, AiCure) | Ingestible sensors systems (Proteus Digital Health) | |
|---|---|---|---|---|---|---|---|
| Key points | Limit recall bias, incomplete entries and loss of data over
paper diaries Resolve handwriting issues/ data manipulation |
Adapted to non-technologically savvy Automatically record dosing as caps are opened |
Adapted to non-technologically savvy Automatically record dosing as blister is popped |
Adapted to non-technologically savvy Automatically record dosing as boxes are opened |
Confirm medication ingestion More convenient over DOT HIPAA compliant Stores-dosing data |
Confirm medication ingestion Real-time recording, storage, and access of dosing data Improve provider/patient relationship |
Time and cost-effective compared with DOT Confirm medication ingestion Real-time recording and storage of dosing data |
| Limitations | Cannot confirm medication ingestion Limited to those who are comfortable with technology Require power and internet |
Cannot confirm medication ingestion or Remind of previous/future dosing event |
Cannot confirm medication ingestion or Remind of previous/future dosing event |
Cannot confirm medication ingestion orRemind of previous/future dosing event |
Operational or technical challenges (Recording while ingesting Missed recordings due to uncharged devices, low network range, or internet) Privacy and data breach concerns |
Feasibility and technological challenges Frustrations with showing faces, pills in hand, then on tongue, and the empty bottom of the tongue |
Desirability limitations (Crushing pills encapsulated with sensor is not possible ,Patches can come off after showering/perspiration, Rashes and itching AEs related to patch wearing) Logistics of coordinating over-encapsulation of tablets across multiple sites may also be challenging for large-scale uptake |
AEs, adverse events; DOT, directly observed therapy; HCV, hepatitis C virus; HIPAA: Health Insurance Portability and Accountability Act; MEMS, medication events monitoring system.
E-diaries present the advantage of limiting recall bias, incomplete entries, and loss of data compared with paper diaries. 57 These also help resolve handwriting issues and manipulation of data since date and time data entered by users are stamped electronically. Both adherence and SVR measured by CRF health in this review paper were higher than 90%. However, medication ingestion is not verifiable by the provider. The devices could also be limited to those who are comfortable with use of technology, have an electronic device and power to charge the device daily in addition to having access to reliable Internet for the reported data to be viewable by the provider.
MEMS, blister packs and pill box technologies seem more adapted and convenient to users even for those who are not technologically savvy, as data are recorded at each opening event of the cap, box, or pop of the blister. However, like e-diaries, these three technologies do not confirm medication ingestion. In addition, they cannot remind users of the time the medication was previously taken, 26 nor can they provide reminders to take the next dose. Adherence and SVR measured by MEMS were each higher than 95%. Of these three technologies, the lowest SVR was recorded with Wisepill (39–89%). Although adherence measured by Wisepill consistently appeared lower compared with those measured by all other technologies, high SVR was still achieved across all adherence assessments with DAA regimens including among PWID.
vDOT resulted in the highest adherence (98%) of all other monitoring tools in this review and was associated with lower odds of missing doses between visits. It might be more adapted and accurate compared with some tools in that drug ingestions are video-recorded and uploaded encrypted to secure server for visualization by study personnel. Participants may also feel more comfortable video-recording in settings of their convenience versus direct observation by the personnel at a clinic. However, limitations of this tool can include operational or technical challenges such as difficulty recording while ingesting medications, missed recordings due to uncharged electronic devices, low network range or Internet when in remote areas therefore preventing uploading of the recordings. Also, the use of this technology might be limited in developing countries due to low or expensive technological infrastructures. Privacy and data breach concerns could limit participants willingness of using vDOT app. Cost of the technology could also be a limitation. As alternatives, live video DOT, such as TimeStamp, FaceTime, Skype, or Tango could be used, but an advantage of emocha is that it is Health Insurance Portability and Accountability Act (HIPAA)-compliant and stores the dosing data in a centralized database, allowing staff to view at their convenience.
Mean cumulative adherence in the AIP studies were 91.3% 45 and 96.2%, 47 respectively. These are relatively higher than the adherence measured by blister packs in the 51 participants in the DOT arm of the PREVAIL study (86%) 33 and that of the SIT arm (75%). 33 Small sample size related to AIP, lack of randomization, and monetary incentives may have impacted results and limited generalizability of study findings. There may also be feasibility and technological challenges with AIP. Some patients may not engage with AIP due to frustrations with the requirements to show their faces, pills in hand, then on tongue, and the empty bottom of the tongue. In the reviews of AiCure Google Play Mobile application software, 58 users complained about the app crashing, resulting in delayed or missed reporting of dosing events due to rebooting the phone. Others disliked the alarm that cannot be silenced when medications cannot be taken. Despite these critiques, in a study of ischemic stroke patients, 83% of those randomized to use AIP to monitor anti-coagulant dosing rated AIP as ‘extremely good’ as a medication management tool and means to improve provider/patient relationship. 44 Uptake of AIP-based technology to monitor DAA adherence will likely depend on its ease of use and reliable access to technology.
The two studies using ingestible biosensor systems suggested that this platform may be used to support adherence and optimize cure rates in difficult to treat HCV populations and those with pre-existing conditions. A study suggests ingestible sensor systems might be cost-effective in comparison with in-person DOT approaches. Proteus Digital Health was found to be time and cost-effective compared with in-person 7- or 3-day DOT among persons receiving tuberculosis treatment as the cost of Proteus was 1/3 of the 7-day DOT, and approximately 2/3 the cost of 3-day DOT considering the public health facility’s cost-to-treat. 59 This technology might present some desirability limitations that could come with swallowing a sensor along with the medication. For example, crushing pills encapsulated with sensor is not possible, and the size of some medication tablets may prohibit the ability to use this technology. In addition, the potential challenges arise for having to wear a patch often, which can easily come off after showering or due to perspiration, without replacement, leading to loss of data available for therapy management unless the participant has the option to manually enter the doses. Rashes and itching AEs related to patch wearing could also limit the choice of this technology over others. The logistics of coordinating over-encapsulation of tablets across multiple sites may also be challenging for large-scale uptake.
The evaluation of the technologies used to monitor HCV adherence in this article focused on providing general descriptions of each technology along with key findings from HCV treatment studies that reported adherence measures alongside SVR results. It would be helpful to have participants’ feedback on the technologies to compare advantages and limitations for real-world applications. Future studies should compare technologies side-by-side based on cost (application costs and provider/researcher costs), functionality, usability, quality, satisfaction, and AEs according to users’ feedback, as well as adherence and SVR for a more comprehensive comparison. Such data were not readily available or reported, limiting this review. Overall, some adherence monitoring technologies provide more objective evidence of medication ingestion (such as AIP, vDOT, ingestible sensors) in comparison with other approaches. Though adherence results were variable across studies, high SVR rates were observed across many of these studies (Table 3), supporting the forgiveness of DAA therapies to missed doses. However, it is important to highlight that forgiveness rates might not be the same across all DAA regimens due to differences in pharmacokinetic properties of the drugs (e.g. longer or shorter half-lives). The choice of a monitoring tool should consider multiple factors. While high SVR rates do not indicate a need for widespread implementation of technology-based tools to monitor DAA adherence in routine clinical practice, these tools may prove very useful in a variety of research and clinical care scenarios.
Footnotes
Ethics approval and consent to participate: Our review did not require ethics board review and approval.
Author contribution(s): Yeba H. Adje: Writing – original draft.
Kristina M. Brooks: Resources; Writing – review & editing.
Jose R. Castillo-Mancilla: Writing – review & editing.
Peter L. Anderson: Writing—review & editing.
David L. Wyles: Writing – review & editing.
Jennifer J. Kiser: Funding acquisition; Supervision; Writing – review & editing.
ORCID iDs: Yeba H. Adje  https://orcid.org/0000-0002-1259-9474
https://orcid.org/0000-0002-1259-9474
Jose R. Castillo-Mancilla  https://orcid.org/0000-0003-1242-1745
https://orcid.org/0000-0003-1242-1745
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (grant no. R01DA040499 to JJK). Contents are the authors’ sole responsibility and do not necessarily represent official views by the NIH.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PLA has received consulting fees from Merck and ViiV. The remaining authors declared no conflicts of interest.
Availability of data and material: This review includes publications available in peer-reviewed journals, presentation at scientific conferences, and websites.
Contributor Information
Yeba H. Adje, Department of Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Kristina M. Brooks, Department of Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Jose R. Castillo-Mancilla, Division of Infectious Diseases, Department of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
David L. Wyles, Division of Infectious Diseases, Department of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA Denver Health and Hospital Authority, Denver, CO, USA.
Peter L. Anderson, Department of Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Jennifer J. Kiser, Department of Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, 12850 E. Montview Blvd., V20-C238, Aurora, CO 80045, USA.
References
- 1. Bartenschlager R, Sparacio S. Hepatitis C virus molecular clones and their replication capacity in vivo and in cell culture. Virus Res 2007; 127: 195–207. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Hepatitis C, https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
- 3. Deutsch M, Hadziyannis SJ. Old and emerging therapies in chronic hepatitis C: an update. J Viral Hepat 2008; 15: 2–11. [DOI] [PubMed] [Google Scholar]
- 4. Langness JA, Nguyen M, Wieland A, et al. Optimizing hepatitis C virus treatment through pharmacist interventions: identification and management of drug-drug interactions. World J Gastroenterol 2017; 23: 1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cunningham EB, Amin J, Feld JJ, et al. Adherence to sofosbuvir and velpatasvir among people with chronic HCV infection and recent injection drug use: the SIMPLIFY study. Int J Drug Policy 2018; 62: 14–23. [DOI] [PubMed] [Google Scholar]
- 6. Read P, Gilliver R, Kearley J, et al. Treatment adherence and support for people who inject drugs taking direct-acting antiviral therapy for hepatitis C infection. J Viral Hepat 2019; 26: 1301–1310. [DOI] [PubMed] [Google Scholar]
- 7. Dolatimehr F, Karimi-Sari H, Rezaee-Zavareh MS, et al. Combination of sofosbuvir, pegylated-interferon and ribavirin for treatment of hepatitis C virus genotype 1 infection: a systematic review and meta-analysis. Daru 2017; 25: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kiser JJ, Burton JR, Jr, Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol 2013; 10: 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han B, Martin R, Xu S, et al. Sofosbuvir susceptibility of genotype 1 to 6 HCV from DAA-naïve subjects. Antiviral Res 2019; 170: 104574. [DOI] [PubMed] [Google Scholar]
- 10. Bartenschlager R, Lohmann V, Penin F. The molecular and structural basis of advanced antiviral therapy for hepatitis C virus infection. Nat Rev Microbiol 2013; 11: 482–496. [DOI] [PubMed] [Google Scholar]
- 11. Elberry MH, Darwish NHE, Mousa SA. Hepatitis C virus management: potential impact of nanotechnology. Virol J 2017; 14: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. González-Grande R, Jiménez-Pérez M, González Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol 2016; 22: 1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and Velpatasvir for HCV genotype 2 and 3 Infection. N Engl J Med 2015; 373: 2608–2617. [DOI] [PubMed] [Google Scholar]
- 14. Heo YA, Deeks ED. Sofosbuvir/velpatasvir/voxilaprevir: a review in chronic hepatitis C. Drugs 2018; 78: 577–587. [DOI] [PubMed] [Google Scholar]
- 15. Ma J, Non L, Amornsawadwattana S, et al. Hepatitis C care cascade in HIV patients at an urban clinic in the early direct-acting antiviral era. Int J STD AIDS 2019; 30: 834–842. [DOI] [PubMed] [Google Scholar]
- 16. Jain MK, Thamer M, Therapondos G, et al. Has access to hepatitis C virus therapy changed for patients with mental health or substance use disorders in the direct-acting-antiviral period? Hepatology 2019; 69: 51–63. [DOI] [PubMed] [Google Scholar]
- 17. Zeremski M, Zibbell JE, Martinez AD, et al. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol 2013; 19: 7846–7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soriano V, Gallego L. Viral hepatitis: treating hepatitis C in injection drug users. Nat Rev Gastroenterol Hepatol 2013; 10: 568–569. [DOI] [PubMed] [Google Scholar]
- 19. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med 2015; 5: 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Electronic diary (E-diary) med-Ic, 2021, https://www.informationmediary.com/nfc-smart-packaging-devices/ediary-nfc-symptom-recorder/
- 21. Electronic diary by signant health CRF, 2021, https://www.crfhealth.com/?q=about-crf-health/news/press-releases&page=9
- 22. Giffin NJ, Lipton RB, Silberstein SD, et al. The migraine postdrome: an electronic diary study. Neurology 2016; 87: 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dore GJ, Altice F, Litwin AH, et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016; 165: 625–634. [DOI] [PubMed] [Google Scholar]
- 24. Electronic MEMS caps (MWV healthcare/AARDEX Group), https://www.aardexgroup.com/solutions/mems-adherence-software/
- 25. Petersen T, Townsend K, Gordon LA, et al. High adherence to all-oral directly acting antiviral HCV therapy among an inner-city patient population in a phase 2a study. Hepatol Int 2016; 10: 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conn VS, Ruppar TM, Chan KC, et al. Packaging interventions to increase medication adherence: systematic review and meta-analysis. Curr Med Res Opin 2015; 31: 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Electronic blister packs Schreiner-MediPharm, 2018, https://www.healthcarepackaging.com/issues/regulatory/press-release/13294889/schreiner-medipharm-smart-blister-pack-for-clinical-trials
- 28. Digital tools (Smart Blister by Schreiner MediPharm) help manage and track process, patient compliance, 2020, https://www.printedelectronicsnow.com/contents/view_breaking-news/2018-06-07/schreiner-medipharm-develops-smart-blister-pack-to-enhance-medication-adherence/
- 29. What are blister packs? 2020, https://drugpackage.com/whats-new/what-are-blister-packs/
- 30. Medication Adherence Experts–Information Mediary Corp. Med-ic® Rx for the third millenium®, 2020, https://www.informationmediary.com/nfc-smart-packaging-devices/med-ic-electronic-medication-blister/
- 31. Litwin AH, Agyemang L, Akiyama M, et al. High rates of sustained virological response in people who inject drugs treated with all-oral direct acting antiviral regimens, 2016, https://na.eventscloud.com/file_uploads/ec626d74ab97207d900ddc93eeb18144_166_AlainLitwin.pdf
- 32. Grebely J, Dalgard O, Conway B, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol 2018; 3: 153–161. [DOI] [PubMed] [Google Scholar]
- 33. Akiyama MJ, Norton BL, Arnsten JH, et al. Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy: a randomized controlled trial. Ann Intern Med 2019; 170: 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heo M, Pericot-Valverde I, Rennert L, et al. Hepatitis C virus direct-acting antiviral treatment adherence patterns and sustained viral response among people who inject drugs treated in opioid agonist therapy programs. Clin Infect Dis 2021; 73: 2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wisepill Technologies. 2021, https://www.wisepill.com
- 36. Haberer JE, Kahane J, Kigozi I, et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav 2010; 14: 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keutzer L, Wicha SG, Simonsson US. Mobile health apps for improvement of tuberculosis treatment: descriptive review. JMIR Mhealth Uhealth 2020; 8: e17246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Craker L, Tarantino N, Whiteley L, et al. Measuring antiretroviral adherence among young people living with HIV: observations from a real-time monitoring device versus self-report. AIDS Behav 2019; 23: 2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coffin PO, Santos GM, Behar E, et al. Randomized feasibility trial of directly observed versus unobserved hepatitis C treatment with ledipasvir-sofosbuvir among people who inject drugs. PLoS ONE 2019; 14: e0217471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holzman SB, Atre S, Sahasrabudhe T, et al. Use of smartphone-based video directly observed therapy (vDOT) in tuberculosis care: single-arm, prospective feasibility study. JMIR Form Res 2019; 3: e13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Emocha Mobile Health – vDOT platform, https://www.biospace.com/article/releases/emocha-expanding-remote-monitoring-service-for-covid-19-exposed-healthcare-professionals/
- 42. Brooks KM, Castillo-Mancilla JR, Morrow M, et al. Adherence to direct-acting antiviral therapy in people actively using drugs and alcohol: the INCLUD study. Open Forum Infect Dis 2021; 8: ofaa564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bain EE, Shafner L, Walling DP, et al. Use of a novel artificial intelligence platform on mobile devices to assess dosing compliance in a phase 2 clinical trial in subjects with schizophrenia. JMIR Mhealth and Uhealth 2017; 5: e7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Labovitz DL, Shafner L, Reyes Gil M, et al. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke 2017; 48: 1416–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Litwin AH, Shafner L, Norton B, et al. Artificial intelligence platform demonstrates high adherence in patients receiving fixed-dose ledipasvir and sofosbuvir: a pilot study. Open Forum Infect Dis 2020; 7: ofaa290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leo S, Gentry-Brown K, Makanji H, et al. Impact of a smartphone-based artificial intelligence platform on hepatitis C adherence in a real-world population. AMCP, 2019, https://www1.magellanrx.com/documents/2019/04/research_impact-of-smartphone-based-artificial-intelligence-platform-on-hepatitis-c-adherence-in-a-real-world-population.pdf/
- 47. Leo S, Brown-Gentry K, Makanji H, et al. Impact of a smartphone-based artificial intelligence platform on hepatitis C adherence in a real-world population (Abstract). AMCP, 2019, https://www.jmcp.org/doi/pdf/10.18553/jmcp.2019.25.3-a.s1
- 48. Browne SH, Behzadi Y, Littlewort G. Let visuals tell the story: medication adherence in patients with type II diabetes captured by a novel ingestion sensor platform. JMIR Mhealth and Uhealth 2015; 3: e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sulkowski M, Luetkemeyer AF, Wyles DL, et al. Impact of a digital medicine programme on hepatitis C treatment adherence and efficacy in adults at high risk for non-adherence. Aliment Pharmacol Ther 2020; 51: 1384–1396. [DOI] [PubMed] [Google Scholar]
- 50. Frias J, Virdi N, Raja P, et al. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open-label, cluster-randomized pilot clinical trial. J Med Internet Res 2017; 19: e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hafezi H, Robertson TL, Moon GD, et al. An ingestible sensor for measuring medication adherence. IEEE Trans Biomed Eng 2015; 62: 99–109. [DOI] [PubMed] [Google Scholar]
- 52. First digital pill approved to worries about biomedical ‘big brother’. The New York Times, 13 November 2020, https://www.nytimes.com/2017/11/13/health/digital-pill-fda.html
- 53. EtectRx™. Breakthrough ID-cap system from etectRx™ selected to measure adherence to HIV treatment in new University of Colorado study, 2020, https://etectrx.com/breakthrough-id-cap-system-from-etectrx-selected-to-measure-adherence-to-hiv-treatment-in-new-university-of-colorado-study/
- 54. Digimeds to optimize adherence in patients with hepatitis c and increased risk for nonadherence, https://ClinicalTrials.gov/show/NCT03164902
- 55. Bonacini M, Kim Y, Pitney C, et al. Wirelessly observed therapy to optimize adherence and target interventions for oral hepatitis C treatment: observational pilot study. J Med Internet Res 2020; 22: e15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spinelli MA, Haberer JE, Chai PR, et al. Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Curr HIV/AIDS Rep 2020; 17: 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kajander K, Lätti M, Hatakka K, et al. An electronic diary versus a paper diary in measuring gastrointestinal symptoms. Dig Liver Dis 2007; 39: 288–289. [DOI] [PubMed] [Google Scholar]
- 58. AiCure®. Google store reviews, 2020, https://play.google.com/store/apps/details?id=com.aicure.aiview.clinical.master.mobile&hl=en_US
- 59. Au-Yeung KY, DiCarlo L. Cost comparison of wirelessly vs. directly observed therapy for adherence confirmation in anti-tuberculosis treatment. Int J Tuberc Lung Dis 2012; 16: 1498–1504. [DOI] [PubMed] [Google Scholar]