Abstract
Two routes to the antimalarial drug Pyronaridine are described. The first is a linear sequence that includes a two-step, one-pot transformation in an aqueous surfactant medium, leading to an overall yield of 87%. Alternatively, a convergent route utilizes a telescoped three-step sequence involving an initial neat reaction, followed by two steps performed under aqueous micellar catalysis conditions affording Pyronaridine in 95% overall yield. Comparisons to existing literature performed exclusively in organic solvents reveal a 5-fold decrease in environmental impact as measured by E Factors.
The antimalarial drug Pyronaridine
was developed in the 1970s and 1980s in response to the rise in chloroquine-resistant Plasmodium.1 In addition to effectively
treating malaria, it showed significantly lower toxicity compared
to chloroquine,1b,2 and when administered in combination
with other antimalarials such as sulfadoxine and pyrimethamine little-to-no
resistance to the drug was observed.3 Current
research into repurposing pyronaridine for the treatment of nonmalarial
parasitic diseases,4 cancer,5 and bacterial6 and
viral7 infections has shown promise, including
its potential in the ongoing fight against the SARS-CoV-2 virus responsible
for the COVID-19 pandemic.7b Nonetheless,
its primary purpose remains as a treatment for malaria, especially
in tropical regions of the world. However, from the perspective of
pharmaceutical companies, justifying the sale of drugs to the developing
world can be fiscally challenging;8 hence,
the development of a synthetic route which minimizes cost is imperative
to incentivize the production of Pyronaridine. Moreover, increasing
pressure from governmental restrictions, such as the REACH regulation
in the EU,9 requires chemical manufacturers
to reduce their environmental footprint, providing additional incentive
for the development of a greener route to Pyronaridine.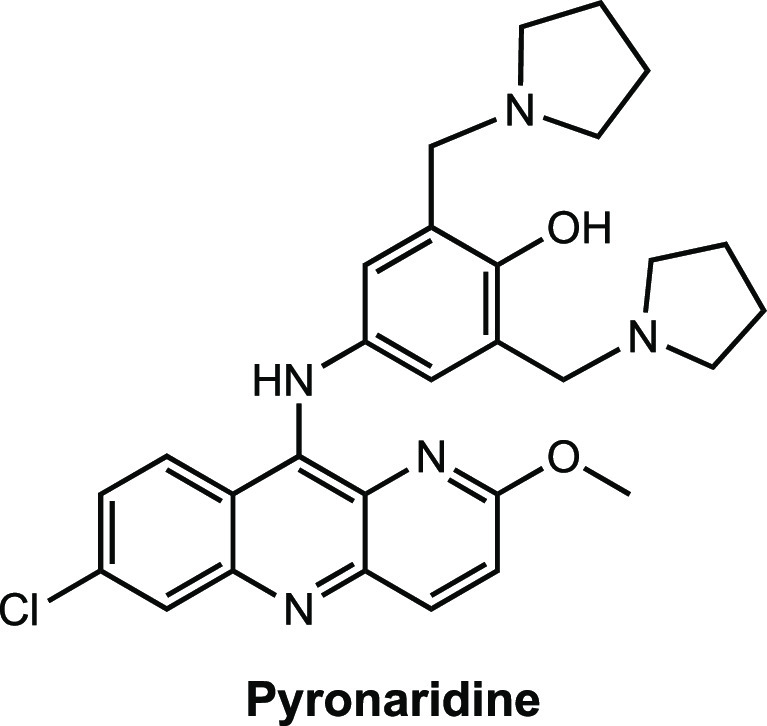
Fortunately, both aims are achievable by utilizing an environmentally responsible approach that relies on reactions run in Nature’s “solvent”: water.10 Such a “switch”11 is enabled using aqueous micellar catalysis, which simply involves the presence of an appropriately engineered and readily available micelle-forming nonionic surfactant that promotes solubilization of typically water-insoluble substrates.12 Furthermore, use of a common aqueous medium enables telescoping of reactions, thereby eliminating wasteful intermediate workups,13 while gaining in both time and pot economies.14 We now describe our recent efforts in collaboration with the Bill and Melinda Gates Foundation that have led to two inexpensive, scalable, and environmentally attractive routes to Pyronaridine.
Overview
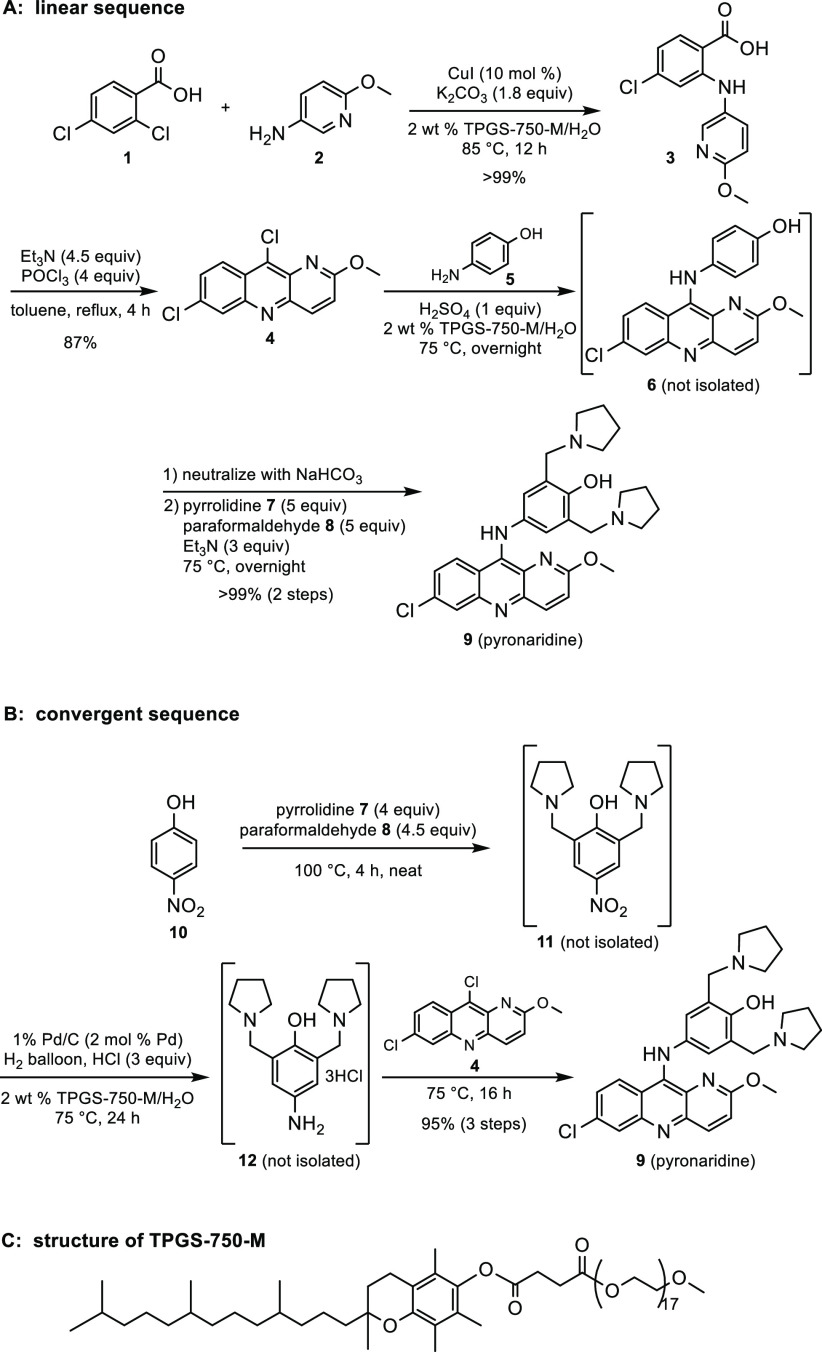
Both linear and convergent routes were developed for consideration toward scaling up a synthesis of Pyronaridine (Scheme 1A and B). Each shares the same initial Cu(I)-catalyzed Ullmann coupling between commercially available 2,4-dichlorobenzoic acid 1 and aminopyridine 2 to arrive at adduct 3, run under aqueous micellar catalysis conditions derived from amphiphile TPGS-750-M15 (shown in Scheme 1C). Subsequent cyclization/deoxychlorination to afford the dichlorotricyclic heteroaromatic 4 required the use of especially water-sensitive POCl3 and was, therefore, carried out in recyclable toluene. The linear route continues directly from here via a two-step, one-pot acid-induced SNAr involving 4 and p-aminophenol 5 leading to intermediate 6. Without isolation, 6 undergoes a double Mannich-like reaction. Both reactions take place smoothly in aqueous solution to cleanly afford Pyronaridine (9).
Scheme 1. Overview of Linear and Convergent Routes to Pyronaridine.
Alternatively, the convergent route employs a three-step tandem sequence involving an initial double Mannich-like reaction using nitrophenol 10 as educt to install the two required methylpyrrolidine residues to arrive at 11. In this case, the reaction could be performed in the complete absence of any reaction medium (i.e., under neat conditions). Nitro group reduction of 11 to 12 is then readily effected in aqueous surfactant solution. An acid-induced SNAr reaction between 4 and 12 merges both components to afford Pyronaridine, 9.
Ullmann Coupling to Acid 3
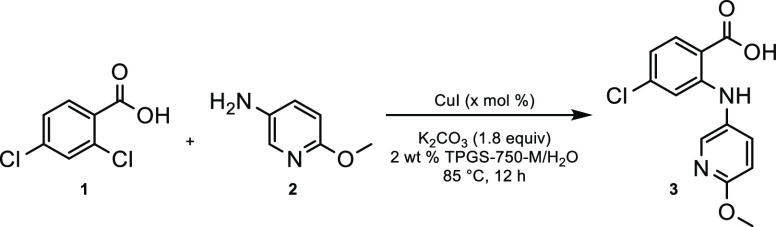
The Ullmann reaction between acid 1 and aminopyridine 2 to form 3 was performed in an aqueous solution of 2 wt % TPGS-750-M15 at a global concentration of 1 M. Purification involved acidification of the aqueous medium to pH 4.0, followed by collection of the precipitated product 3 via filtration. Copper salts were most efficiently removed by centrifugation rather than by suction filtration as the latter approach created a large amount of foam. Unreacted aryl acid 1 could be isolated by further acidification of the filtrate to pH 1, collection by filtration, and recrystallization from EtOH and water. Optimization studies involving the amount of catalytic copper and the role of the surfactant are shown in Table 1. Increasing the loading of CuI from 5 to 10 mol % led to a moderate increase in yield (entry 2). The importance of the surfactant was also apparent, as in its absence the yield was reduced accordingly (entries 3 and 4); thus, the conditions in entry 2 were selected. Some surfactant remained in the precipitated product, although the yields in entries 1 and 2 do not reflect this. Importantly, the product could be used without further purification and the residual surfactant did not impact the yield or purity of the subsequent cyclization/deoxychlorination step (87% overall yield for both steps, vide infra). It is noteworthy that product 3 was obtained with very high regioselectivity (i.e., no substitution at the para-position was observed by HPLC).
Table 1. Optimization Studies of Ullmann Coupling.
| entry | CuI (mol %) | surfactant | isolated yield (%) |
|---|---|---|---|
| 1 | 5 | TPGS-750-M | 91 |
| 2 | 10 | TPGS-750-M | >99 |
| 3 | 5 | none; on water | 73 |
| 4 | 10 | none; on water | 82 |
Cyclization/Deoxychlorination to Aryl Chloride 4
Cyclization and concomitant deoxychlorination of compound 3 to form heteroaromatic 4 was achieved using POCl3. Et3N was included to prevent evolved HCl from demethylating the methoxy group and leading to impurity I (Figure 1) following subsequent deoxychlorination.16 Although water could not be used in this step due to the water-sensitive nature of POCl3, toluene served nicely and could be recovered following isolation of product 4, thereby limiting organic waste generation. The product was obtained in 87% yield.
Figure 1.
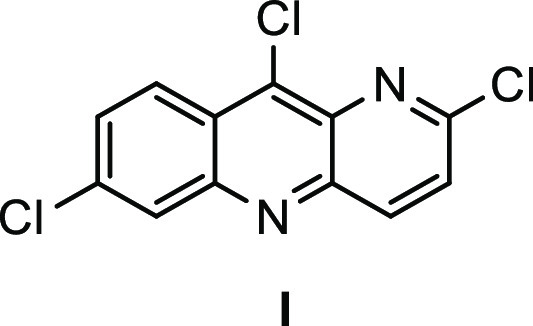
Potential impurity I resulting from demethylation.
Linear Sequence
SNAr Reaction to Phenol 6
The SNAr reaction between 4 and aminophenol 5 was performed under acidic conditions (pH = 1) and proceeded smoothly using the ideal equimolar quantities of each coupling partner. Product 6 precipitated from the reaction mixture and was obtained in quantitative yield. Following washing with water, the product was obtained in high purity.
Mannich-like Reaction to Form Pyronaridine
Initial screening of the double Mannich-like reaction between 6 and excess (10 equiv) pyrrolidine 7 and paraformaldehyde 8 under micellar catalysis conditions provided Pyronaridine, 9, in quantitative yield. Reducing the loading of pyrrolidine 7 and paraformaldehyde 8 from 10 to 4 equiv led to a precipitous drop in conversion, from 100% to 33%, due to poor solubility of educt 6. That is, the reaction containing 10 equiv pyrrolidine begins as a homogeneous emulsion, and then soon thereafter, Pyronaridine slowly precipitated to form a free-flowing suspension over the course of the reaction. The reaction containing 4 equiv, however, did not afford an initial homogeneous mixture, and instead, amine 6 formed a clump, thereby limiting the extent of conversion. This suggested that the excess pyrrolidine was acting either as a cosolvent, a base, or a combination thereof. The addition of 10 v/v % 2-MeTHF as cosolvent containing 4 equiv of 7 and 8 did not improve the overall conversion. However, use of five equivalents each of 7 and 8 in combination with three equivalents of Et3N showed the same initial solubilization and eventual precipitation of 9. In this manner, Pyronaridine could be isolated in quantitative yield.
Two-Step, One-Pot Conversion of 4 to Pyronaridine
The SNAr followed by the Mannich-like reactions could be performed in water in a one-pot fashion. An initial SNAr reaction between 4 and 5 was performed to afford adduct 6. The reaction mixture was then neutralized with NaHCO3, after which was added 7, 8, and Et3N. Pyronaridine 9 was thereby obtained in quantitative yield over both steps. The overall linear sequence, therefore, is performed in four individual steps in three pots. The overall efficiency has been increased from 43%16 to 87% using this process.
Convergent Sequence
Mannich-like Reaction to Form Nitroarene 11
The Mannich-like reaction between p-nitrophenol 10, pyrrolidine 7, and paraformaldehyde 8 was performed under neat conditions. Following addition of 10 and 7 to the pot, the exotherm was effectively mitigated by slow portion-wise addition of 8 at 5 °C. Once all of the reagents had been added, the mixture was heated to 100 °C for 4 h. The remaining pyrrolidine was removed by codistillation with methanol, whereupon nitro compound 11 was obtained in quantitative yield. No over- or under-substitution was observed.
Reduction of Nitroarene 11 to Aniline 12
The nitro group reduction could be performed in aqueous surfactant solution using Pd/C and atmospheric hydrogen pressure.17 It was necessary to include HCl, as the free-base aniline is highly unstable. Because product 12 is water-soluble, isolation from the aqueous solution is not straightforward. Fortunately, it was found that the subsequent SNAr reaction could be performed in a tandem fashion (see sequence below).
SNAr Reaction with 12 Leading to Pyronaridine
The SNAr reaction was performed in 2 wt % aqueous TPGS-750-M under acidic conditions using a 1:1 ratio of coupling partners 4 and 12. Pyronaridine 9 precipitated from the aqueous reaction mixture, and was isolated via centrifugation followed by washing the solid with water leading to the targeted drug in 78% isolated yield. Interestingly, this yield is lower than would be expected from the 3-step tandem sequence in which Pyronaridine is obtained in 95% yield (vide infra). This suggests that in situ formation of the aniline salt, as in the tandem sequence, is preferrable to direct use of the isolated trihydrochloride salt.
Tandem Three-Step Sequence to Pyronaridine
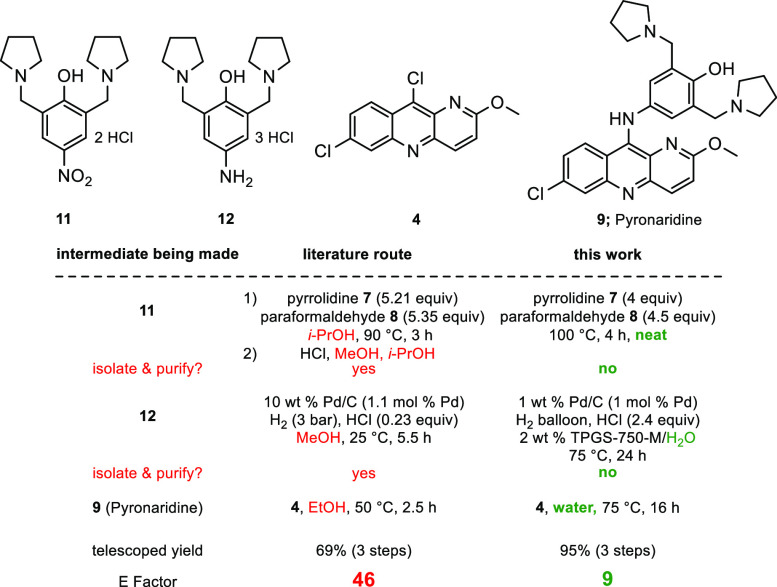
Beginning with nitrophenol 10 and having heteroaromatic 4 in hand (vide supra), both pyrrolomethylene groups were installed to yield intermediate 11. Stripping with methanol removed excess pyrrolidine, to which was then added an aqueous 2 wt % solution of TPGS-750-M. Introduction of HCl and Pd/C under an atmosphere of H2 led to nitro group reduction to give intermediate 12. The Pd/C was then removed via filtration of the reaction mixture through a short plug of Celite. To this filtrate was then added heteroaryl chloride 4, and the mixture was allowed to react at 75 °C for 16 h. Upon completion, the solution was basified to pH 8 with aqueous NH4OH and the precipitated Pyronaridine (9) was collected via filtration, washed with water, and isolated in an overall yield of 95% over three steps. Since Pyronaridine is offered as its tetraphosphate salt, opportunities for further purification exist, although they were not pursued in these studies.
The convergent process involves a three-step sequence from educt 10 that avoids isolation of intermediates and proceeds in an overall yield of 95%. Clearly, this approach has significant advantages that include: (1) avoidance of waste-generating organic solvents; (2) both time14a and pot14b economies, avoiding workup normally associated with each step; (3) the overall efficiency of the process has been increased from the literature value of 69%18 to 95%.
E Factors
To quantify the reduction in environmental impact relative to a literature protocol,18 an Environmental Factor (E Factor)19 was determined, calculated as the ratio of the mass of waste generated to the mass of product. This was evaluated for the three-step tandem sequence leading to Pyronaridine 9, starting with p-nitrophenol 10 (Scheme 1B). This led to a 5-fold decrease in E Factor to a very low value of 9, exemplifying the environmental friendliness of the described sequence. Overall, some of the major comparisons between an existing literature route18 and the current, far greener synthesis are summarized in Scheme 2.
Scheme 2. Comparisons with Existing Route18.
Summary
Two economically attractive and environmentally responsible approaches to the synthesis of the antimalarial drug Pyronaridine have been disclosed which should reduce the cost barrier to its production for eventual distribution in various regions in the world. The convergent route appears to be the most effective in terms of both “greenness” and overall efficiency, but only by virtue of its scale up, currently underway, will its preferred status be confirmed.
Acknowledgments
Financial support provided by the Bill & Melinda Gates Foundation (INV-005858) and National Science Foundation Graduate Research Fellowship (Grant No. 1650114) is warmly acknowledged with thanks. Insight and guidance provided by both BMGF consultants John Dillon and Trevor Laird throughout this project are very much appreciated.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c00944.
Experimental procedures, optimization details, and analytical data (NMR, HPLC, and MS) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Zheng X. Y.; Xiao Y.; Gao F. H.; Chen C. Synthesis of 7351, a New Antimalarial Drug. Yao Xue Xue Bao 1979, 14, 736–737. [PubMed] [Google Scholar]; b Fu S.; Xiao S.-H. Pyronaridine: A New Antimalarial Drug. Parasitology Today 1991, 7, 310–313. 10.1016/0169-4758(91)90267-R. [DOI] [PubMed] [Google Scholar]
- Croft S. L.; Duparc S.; Arbe-Barnes S. J.; Craft J. C.; Shin C.-S.; Fleckenstein L.; Borghini-Fuhrer I.; Rim H.-J. Review of Pyronaridine Anti-Malarial Properties and Product Characteristics. Malar. J. 2012, 11, 270. 10.1186/1475-2875-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lapierre J. Paludisme à Plasmodium falciparum polychimiorésistant traité avec succès par la benzonaphthyridine. La Nouvelle Presse Medicale 1982, 11, 673. [PubMed] [Google Scholar]; b Rottmann M.; Jonat B.; Gumpp C.; Dhingra S. K.; Giddins M. J.; Yin X.; Badolo L.; Greco B.; Fidock D. A.; Oeuvray C.; Spangenberg T. Preclinical Antimalarial Combination Study of M5717, a Plasmodium falciparum Elongation Factor 2 Inhibitor, and Pyronaridine, a Hemozoin Formation Inhibitor. Antimicrob. Agents Chemother. 2020, 64, e02181-19. 10.1128/AAC.02181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Pradines B.; Briolant S.; Henry M.; Oeuvray C.; Baret E.; Amalvict R.; Didillon E.; Rogier C. Absence of Association BetweenPpyronaridine In Vitro Responses and Polymorphisms in Genes Involved in Quinoline Resistance in Plasmodium falciparum. Malar. J. 2010, 9, 339. 10.1186/1475-2875-9-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Wang W.; Yao J.; Wang T.; Li S.; Qi W.; Han S.; Ren Y.; Dang Z.; Han X.; Guo G.; Guo B.; Wang L.; Duan L.; Zhang W. Old Drug Repurposing for Neglected Disease: Pyronaridine as a Promising Candidate for the Treatment of Echinococcus granulosusIinfections. EBioMedicine 2020, 54, 102711. 10.1016/j.ebiom.2020.102711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly B. Pyronaridine: An Update of its Pharmacological Activities and Mechanisms of Action. Biopolymers 2021, 112, e23398 10.1002/bip.23398. [DOI] [PubMed] [Google Scholar]
- Mori G.; Orena B. S.; Franch C.; Mitchenall L. A.; Godbole A. A.; Rodrigues L.; Aguilar-Pérez C.; Zemanová J.; Huszár S.; Forbak M.; Lane T. R.; Sabbah M.; Deboosere N.; Frita R.; Vandeputte A.; Hoffmann E.; Russo R.; Connell N.; Veilleux C.; Jha R. K.; Kumar P.; Freundlich J. S.; Brodin P.; Aínsa J. A.; Nagaraja V.; Maxwell A.; Mikušová K.; Pasca M. R.; Ekins S. The EU Approved Antimalarial Pyronaridine Shows Antitubercular Activity and Synergy with Rifampicin, Targeting RNA Polymerase. Tuberculosis 2018, 112, 98–109. 10.1016/j.tube.2018.08.004. [DOI] [PubMed] [Google Scholar]
- a Lane T. R.; Massey C.; Comer J. E.; Anantpadma M.; Freundlich J. S.; Davey R. A.; Madrid P. B.; Ekins S. Repurposing the Antimalarial Pyronaridine Tetraphosphate to Protect Against Ebola Virus Infection. PLoS Negl Trop Dis 2019, 13, e0007890 10.1371/journal.pntd.0007890. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Krishna S.; Augustin Y.; Wang J.; Xu C.; Staines H. M.; Platteeuw H.; Kamarulzaman A.; Sall A.; Kremsner P. Repurposing Antimalarials to Tackle the COVID-19 Pandemic. Trends in Parasitology 2021, 37, 8–11. 10.1016/j.pt.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer M. Pharmaceuticals and the Developing World. J. Eco. Perspect. 2002, 16, 67–90. 10.1257/089533002320950984. [DOI] [PubMed] [Google Scholar]
- Understanding REACH . ECHA.https://echa.europa.eu/regulations/reach/understanding-reach (accessed on 9/10/2021).
- For recent representative examples featuring chemistry in water, see; a Ansari T. N.; Sharma S.; Hazra S.; Jasinski J. B.; Wilson A. J.; Hicks F.; Leahy D. K.; Handa S. Shielding Effect of Nanomicelles: Stable and Catalytically Active Oxidizable Pd(0) Nanoparticle Catalyst Compatible for Cross-Couplings of Water-Sensitive Acid Chlorides in Water. J. Am. Chem. Soc. Au 2021, 1, 1506–1513. 10.1021/jacsau.1c00236. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sharma S.; Ansari T. N.; Handa S. HPMC: A Biomass-Based Semisynthetic Sustainable Additive Enabling Clean and Fast Chemistry in Water. ACS Sustain. Chem. Eng. 2021, 9, 12719. 10.1021/acssuschemeng.1c04607. [DOI] [Google Scholar]; c Sak H.; Mawick M.; Krause N. Sustainable Gold Catalysis in Water Using Cyclodextrin-tagged NHC-Gold Complexes. ChemCatChem. 2019, 11, 5821–5829. 10.1002/cctc.201901658. [DOI] [Google Scholar]; d Kawase M.; Matsuoka K.; Shinagawa T.; Hamasaka G.; Uozumi Y.; Shimomura O.; Ohtaka A. Suzuki-Miyaura Cross-Coupling Reaction with Potassium Aryltrifluoroborate in Pure Water Using Recyclable Nanoparticle Catalyst. Synlett 2022, 33, 57–61. 10.1055/a-1661-3152. [DOI] [Google Scholar]
- Lipshutz B. H. When Does Organic Chemistry Follow Nature’s Lead and Make the Switch. J. Org. Chem. 2017, 82, 2806–2816. 10.1021/acs.joc.7b00010. [DOI] [PubMed] [Google Scholar]
- a Cortes-Clerget M.; Yu J.; Kincaid J. R. A.; Walde P.; Gallou F.; Lipshutz B. H. Water as the Reaction Medium in Organic Chemistry: From Our Worst Enemy to Our Best Friend. Chem. Sci. 2021, 12, 4237–4266. 10.1039/D0SC06000C. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lipshutz B. H.; Ghorai S.; Cortes-Clerget M. The Hydrophobic Effect Applied to Organic Synthesis: Recent Synthetic Chemistry “in Water. Chem.—Eur. J. 2018, 24, 6672–6695. 10.1002/chem.201705499. [DOI] [PubMed] [Google Scholar]; c Lipshutz B. H. Catalyst: Imagine Doing Chemistry at No Cost··· to the Environment!. Chem. 2018, 4, 2004–2007. 10.1016/j.chempr.2018.08.031. [DOI] [Google Scholar]
- a Cortes-Clerget M.; Kincaid J. R. A.; Akporji N.; Lipshutz B. H.. Surfactant Assemblies as Nanoreactors for Organic Transformations. In Supramolecular Catalysis: New Directions and Developments; van Leeuwen P. W. M. N.; Raynal M.; Wiley-VCH GmbH: Weinheim, Germany, 2022; pp 467–487. [Google Scholar]; b Akporji N.; Singhania V.; Dussart-Gautheret J.; Gallou F.; Lipshutz B. Nanomicelle-Enhanced, Asymmetric ERED-Catalyzed Reductions of Activated Olefins. Applications to 1-Pot Chemo- and Bio-Catalysis Sequences in Water. Chem. Commun. 2021, 57, 11847–11850. 10.1039/D1CC04774D. [DOI] [PubMed] [Google Scholar]
- a Hayashi Y. Time Economy in Total Synthesis. J. Org. Chem. 2021, 86, 1–23. 10.1021/acs.joc.0c01581. [DOI] [PubMed] [Google Scholar]; b Hayashi Y. Pot Economy and One-Pot Synthesis. Chem. Sci. 2016, 7, 866–880. 10.1039/C5SC02913A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshutz B. H.; Ghorai S.; Abela A. R.; Moser R.; Nishikata T.; Duplais C.; Krasovskiy A.; Gaston R. D.; Gadwood R. C. TPGS-750-M: A Second-Generation Amphiphile for Metal-Catalyzed Cross-Couplings in Water at Room Temperature. J. Org. Chem. 2011, 76, 4379–4391. 10.1021/jo101974u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. W.; Lee S. K.; Cho J. H.; Yoon S. S. Improved Manufacturing Process for Pyronaridine Tetraphosphate. Bull. Korean Chem. Soc. 2014, 35, 521–524. 10.5012/bkcs.2014.35.2.521. [DOI] [Google Scholar]
- Li X.; Thakore R. R.; Takale B. S.; Gallou F.; Lipshutz B. H. High Turnover Pd/C Catalyst for Nitro Group Reductions in Water. One-Pot Sequences and Syntheses of Pharmaceutical Intermediates. Org. Lett. 2021, 23, 8114–8118. 10.1021/acs.orglett.1c03258. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhang Z.; Wu A.; Yang X.; Zhu Y.; Zhao N. A Novel Process for Antimalarial Drug Pyronaridine Tetraphosphate. Org. Process Res. Dev. 2014, 18, 349–353. 10.1021/op400357f. [DOI] [Google Scholar]
- a Sheldon R. A. The E Factor: Fifteen Years On. Green Chem. 2007, 9, 1273–1283. 10.1039/b713736m. [DOI] [Google Scholar]; b Sheldon R. A. The E Factor 25 years on: the Rise of Green Chemistry and Sustainability. Green Chem. 2017, 19, 18–43. 10.1039/C6GC02157C. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.