Abstract
Purpose
Extracranial ICA imaging has largely focused on the degree of luminal stenosis, but recent advances suggest specific plaque features are crucial in stroke risk assessment. We evaluated the current state of reporting carotid plaque features on neck CTAs at an academic institution.
Methods
In this retrospective observational study, we included neck CTAs performed on patients over age 50 with any reported carotid plaque. We evaluated reports for mention of the following: degree of luminal stenosis, soft plaque, calcified plaque, plaque thickness, quantification of soft and calcified plaque, plaque ulceration, and increased risk associated with specific features. We used Fisher’s exact test to compare how often each feature was mentioned.
Results
We included a total of 651 reports from unique patients (mean age, 68.1 ± 13.3 years). A total of 639 reports (98.1%) explicitly mentioned degree of stenosis per NASCET criteria. Specific plaque features were less frequently characterized: soft plaque in 116 (17.8%); calcified plaque in 166 (25.5%); quantification of the amount of soft plaque and calcified plaque in 24 (3.7%) and 16 (2.5%) reports, respectively; plaque thickness in 12 (1.8%); plaque ulceration in 476 (73.1%); and increased risk associated with plaque in 2 (0.3%). Degree of stenosis was statistically more likely to be mentioned than any other plaque feature (p < 0.001).
Conclusion
Currently, nearly all reports mention the degree of luminal stenosis on neck CTAs while a significant minority mention specific plaque features. Despite mounting evidence of the importance of carotid plaque features in stroke risk assessment, radiology reports do not routinely report these findings.
Keywords: Carotid stenosis, Atherosclerosis, CT angiography
Introduction
Computed tomography angiography (CTA) of the head and neck is commonly performed to evaluate the cervical carotid arteries, especially in the acute setting. One of the most common indications for this examination is for the workup of stroke-like neurologic symptoms, specifically to evaluate the carotid artery. CTA is commonly used to evaluate carotid artery disease because it is the most accurate cross-sectional imaging technique compared to the reference standard of digital subtraction angiography (DSA) in the evaluation of degree of luminal stenosis [1, 2]. Degree of luminal stenosis is commonly measured by methodology laid out by the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [3]. Luminal stenosis is an important part of assessing the extracranial carotid artery because it has been used to determine eligibility for surgical interventions in the setting of flow-limiting stenosis, in part because it has served as inclusion criteria for many clinical trials for carotid revascularization [3–6].
While assessing the degree of stenosis plays a critical role in interpreting CTA examinations, there are other plaque features that can also be characterized on CTA [7, 8]. There has been a paradigm shift in our understanding of how carotid artery disease leads to cerebrovascular ischemia with mounting interest in specific plaque features [9, 10]. Certain plaque features, such as intraplaque hemorrhage or plaque ulceration, are strongly associated with cerebrovascular ischemia and some of these high-risk features can be assessed on CTA neck examinations [7, 11]. Conversely, other plaque features, such as heavy calcification, are less likely to be associated with cerebrovascular ischemia [7, 8, 12]. In this study, our goal was to quantify the frequency with which specific carotid plaque imaging features were explicitly mentioned in radiology reports of CTA neck examinations at an academic institution.
Materials and methods
In this retrospective observational study, we screened all CTA neck examinations performed at the University of Utah Health over a 1-year period from 1/1/2018 to 12/31/2018. Inclusion criteria were CTA examinations of the neck performed at the University of Utah Health system on patients over the age of 50 at the time of the examination. We excluded any patients whose reports explicitly stated that no plaque at all was present in the carotid bifurcation in order to only include patients who were most likely to have carotid plaque. We excluded reports signed by two attending neuroradiologists who perform dedicated research on carotid plaque imaging so our sample was representative of most academic neuroradiologists. We included only the first report if a patient had multiple reports. The radiology reports were read in their entirety to evaluate for mention or discussion of the following specific carotid imaging features [8]: (1) explicit mention of degree of stenosis per NASCET criteria; (2) mention of non-calcified plaque characteristics including any of the following terms: soft, fibrofatty, non-calcified plaque, or any other wording to suggest non-calcified plaque; (3) any quantification of soft/non-calcified plaque using terms such as large, small, or proportion of plaque which is soft with or without measurement of plaque; (4) mention of plaque thickness; (5) mention of presence of calcified plaque; (6) any quantification of calcified plaque using terms such as large, small, or proportion of plaque which is calcified with or without measurement of plaque; (7) mention of plaque ulceration; and (8) mention of any risk conferred with any of plaque features. Each of these components was assessed in a binary, yes/no fashion blinded to patient demographic information and the imaging study. A subset of 30 radiology reports was re-evaluated by a “Certificate of Added Qualification (CAQ)”–certified neuroradiologist to evaluate the interobserver reliability of the data extraction methods. We compared the frequencies of reporting each plaque characteristic using Fisher’s exact test. The interobserver reliability was calculated with a Cohen’s kappa statistic comparing the interpretations of the two readers. This study was approved by the Institutional Review Board and informed consent was waived.
All CTA examinations were performed for clinical purposes with a 64-section scanner (Definition or Definition AS; Siemens). Images were obtained from the aortic arch to the circle of Willis at a thickness of 0.625 mm. Multiplanar reformats were created. The examinations were interpreted by one of 14 board- and CAQ-certified neuroradiologists within the department at the University of Utah. Radiology reports were created using PowerScribe 360.
Results
There were 2001 CTA neck examinations performed over a 1-year period in patients over the age of 50 at our institution. Out of those 2001 CTA neck examinations, we excluded 1035 whose reports specifically stated there was no atherosclerotic plaque at the carotid bifurcations, 302 reports dictated by two neuroradiologists with specific research interest in carotid plaque imaging, and 13 reports which were repeat examinations. We included a total of 651 reports on unique patients. The average patient age was 68.1 ± 13.3 years and 269 examinations were on female (41.3%) patients. There were 285 (43.8%) neck CTA examinations performed on inpatients, 283 (43.5%) were performed on patients in the Emergency Department, and 83 (12.7%) were performed on outpatients. As far as indications for performing the examination, 386 (59.3%) of the examinations were performed for stroke-like symptoms, 78 (11.7%) were performed for trauma, 57 (8.8%) were performed to follow-up known findings, such as known carotid stenosis, 50 (7.5%) were performed for the evaluation of intracranial hemorrhage, 39 (5.9%) were performed to evaluate for headache, 32 (4.8%) were performed to evaluate for vasculitis, and 9 (1.4%) were performed for other reasons.
Nearly all reports 639 (98.1%) explicitly mentioned degree of stenosis per NASCET criteria (Table 1). There were 116 (17.8%) reports that mentioned soft, fibrofatty, or non-calcified plaque and there were 24 (3.7% of all reports) with any sort of quantification of the amount of soft, fibrofatty, or non-calcified plaque (Fig. 1). Similarly, 12 (1.8%) described or quantified plaque thickness. The presence of calcified plaque was mentioned in 166 (25.5%) reports and 16 (2.4%) reports had any sort of quantification of calcified plaque (Fig. 2). The presence or absence of plaque ulceration was mentioned in 476 (73.1%) reports (Fig. 3). Out of all the reviewed CTA neck examinations, only 2 (0.3%) described any increased risk associated with the plaque features within the findings or impression portions of the radiology report. There were significant differences in the number of reports that mentioned degree of NASCET stenosis compared to other plaque characteristics (p < 0.001 for all comparisons; Table 1). Similarly, there were significant differences in the numbers of reports describing plaque ulceration and calcified plaque compared to other plaque features (p ≤ 0.001; Table 1).
Table 1.
Comparison of reports describing plaque characteristics
| Carotid plaque imaging characteristic | Number of radiology reports describing plaque characteristic (percent) | p value compared to reports describing degree of NASCET stenosis* | p value compared to reports describing plaque ulceration* | p value compared to reports describing calcified plaque* |
|---|---|---|---|---|
| Luminal stenosis per NASCET criteria | 639 (98.1) | NA | <0.001 | <0.001 |
| Soft plaque | 116 (17.8) | <0.001 | <0.001 | 0.001 |
| Quantification of soft plaque | 24 (3.7) | <0.001 | <0.001 | <0.001 |
| Calcified plaque | 166 (25.5) | <0.001 | <0.001 | NA |
| Quantification of calcified plaque | 16 (2.4) | <0.001 | <0.001 | <0.001 |
| Plaque thickness | 12 (1.8) | <0.001 | <0.001 | <0.001 |
| Plaque ulceration | 476 (73.1) | <0.001 | NA | <0.001 |
| Increased risk | 2 (0.3) | <0.001 | <0.001 | <0.001 |
Using Fisher’s exact test. NASCET North American Symptomatic Carotid Endarterectomy Trial
Fig. 1.

Neck CTA in a 72-year-old male shows large, soft/fibrofatty plaque in the proximal left internal carotid artery (ICA) with peripheral calcification (a, white arrow) with less than 50% stenosis by NASCET criteria. In this case, the report mentioned “atherosclerotic plaque at the left ICA with less than 50% stenosis” without describing the presence or thickness of the soft/fibrofatty plaque. Neck CTA in another patient, a 69-year-old male, which demonstrates approximately 50% stenosis in the proximal right ICA secondary to large, soft/fibrofatty plaque (b, black arrow). In both of these cases, the report described the 50% stenosis by NASCET criteria but did not describe the plaque
Fig. 2.
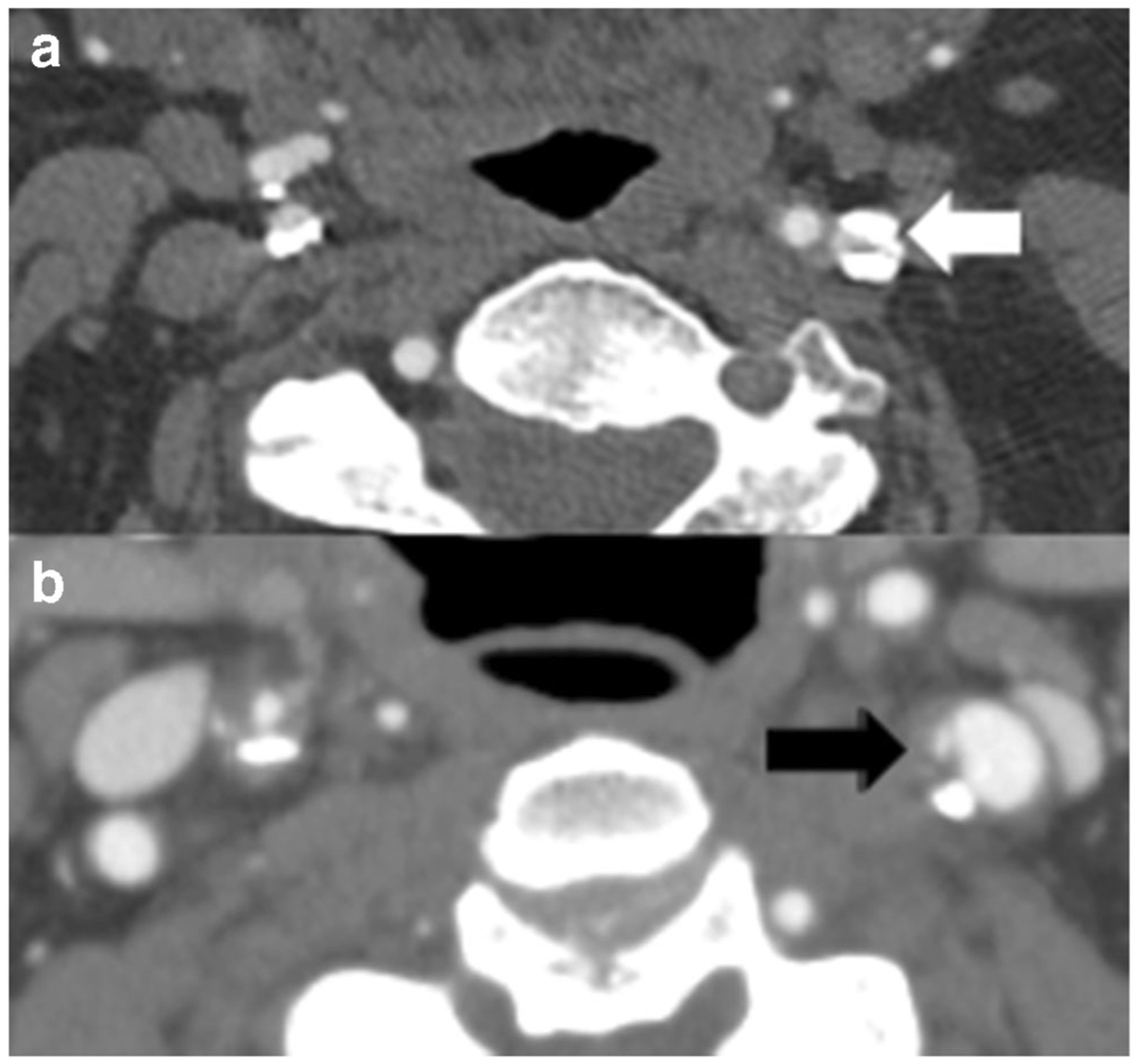
Neck CTA in an 83-year-old male with large heavily calcified plaque (a, white arrow) in the left proximal internal carotid artery (ICA) resulting in 80% stenosis by NASCET criteria. A neck CTA in a different patient with mixed calcified and non-calcified plaque on the left with plaque ulceration medially (b, black arrow). Though there is a greater degree of stenosis in the patient depicted in a, the heavy calcification may confer some stability to the plaque compared to the non-stenosing plaque with plaque ulceration in the patient in b. Without reporting of the carotid plaque characteristics, this information may not be accurately communicated to referring clinicians
Fig. 3.
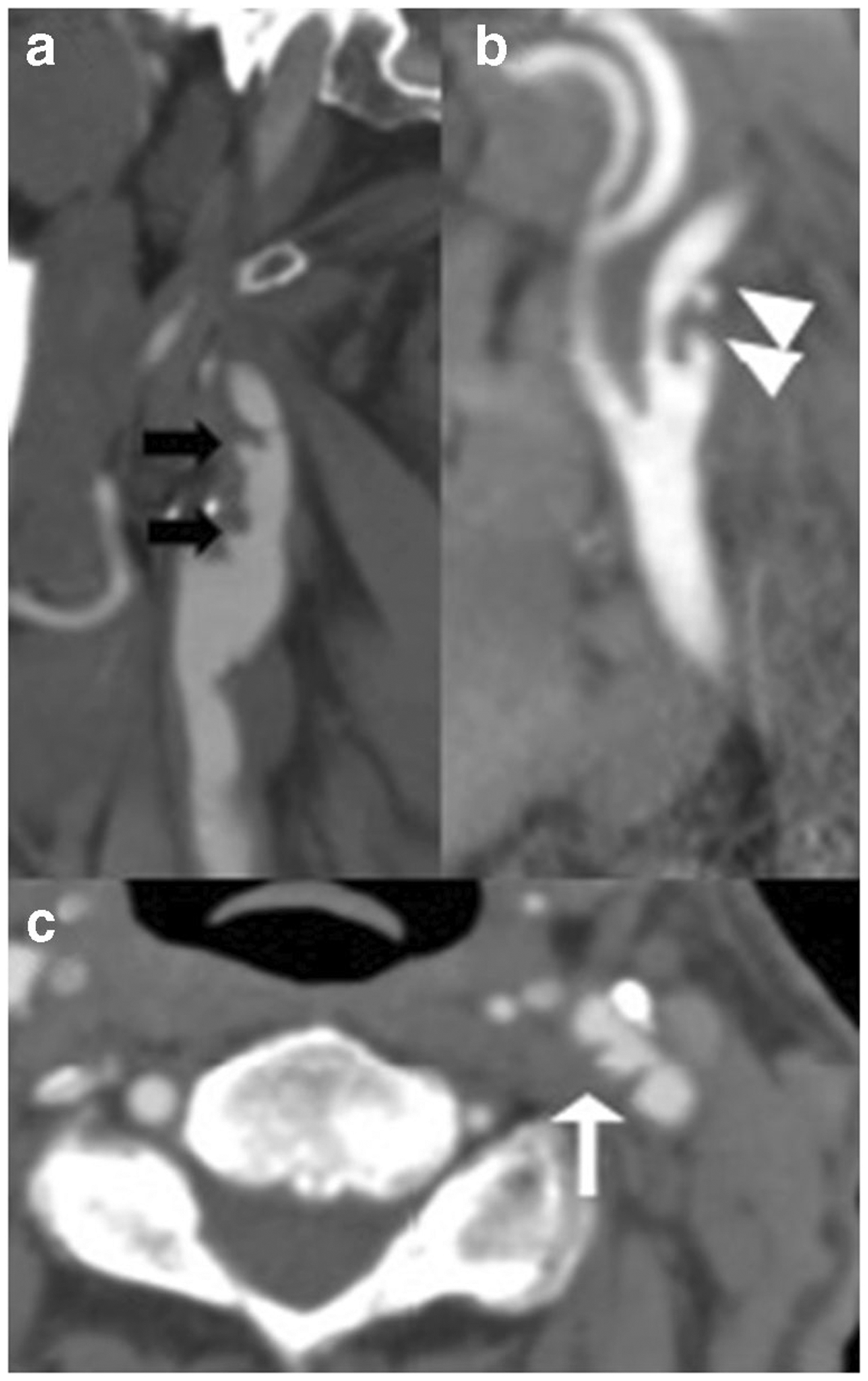
a Sagittal CTA reformation in a 62-year-old man demonstrating multiple ulcerations (black arrows) in a long, predominantly fibrofatty plaque throughout the proximal right internal carotid artery. b Sagittal CTA reformation in a 72-year-old man demonstrating two focal vascular outpouchings (white arrowheads) in the proximal left internal carotid artery, consistent with plaque ulcerations. c Axial CTA in a 68-year-old woman demonstrating focal plaque ulceration (white arrow) in the left carotid bifurcation
The agreement between the two readers on a subset of 30 radiology reports was almost perfect with kappa statistics > 0.81 for all classifications.
Discussion
In a cross-sectional assessment of neck CTA radiology reports at an academic institution, we showed that nearly all reports described the degree of luminal stenosis, but a statistically significant minority of reports described specific plaque features or provided any sort of carotid plaque quantification. Very few reports described any sort of risk associated with any of the plaques. The findings from our study are not surprising given the focus on degree of luminal stenosis in the risk assessment of those with carotid artery disease. More than half of the examinations in our study were performed for concern for cerebrovascular ischemia and most were performed in a high acuity setting, such as the emergency department or inpatient setting. These are both expected findings, given one of the primary reasons to obtain a neck CTA is to evaluate the cervical carotid arteries as a potential etiology in the setting of ischemic stroke.
There was little mention of specific plaque components on CTA examinations, with statistically significant fewer reports describing specific carotid plaque features than degree of luminal stenosis. There were very few reports which mentioned soft or fibrofatty plaque and even fewer reports describing the plaque size even qualitatively, both significantly less than reporting degree of luminal stenosis. Unsurprisingly, reports were statistically more likely to report plaque calcification compared to many other plaque characteristics, including soft or fibrofatty plaque. This finding highlights the high accuracy of CT in the evaluation of calcification, which is an area that CT can outperform US and MR imaging techniques for plaque evaluation [13, 14]. Despite being described more frequently than other plaque features, calcified plaques were still described in a relatively small proportion of reports and significantly less commonly than the degree of luminal stenosis. These findings are not surprising given the relatively recent interest in plaque components as a contributor to cerebrovascular ischemia. Soft or fibrofatty plaque on CTA has recently been associated with intraplaque hemorrhage, an marker of increased plaque vulnerability strongly associated with stroke that is more directly visualized on MR [8, 15–17]. While soft and fibrofatty plaque is not totally specific for intraplaque hemorrhage, it is also independently associated with increased risk of cerebrovascular ischemia [8]. That many of these features were not regularly described on CTA is also not surprising given that most of the plaque components which we assess on imaging are more commonly studied on other imaging techniques, including MR and US, with less attention to CTA [10, 11, 18–20]. Both MR and US have more prospective data correlating certain plaque characteristics, such as intraplaque hemorrhage and echolucent plaque, with future and recurrent stroke [11, 18, 21].
Our findings also highlight the success of certain initiatives in radiology reporting. Nearly every report explicitly described the measurement of NASCET stenosis. Since the presence of a statement regarding methods for measuring carotid stenosis in a radiology report is directly tied to billing and reimbursement in the USA, the near universality of mentioning NASCET stenosis is anticipated [22]. In addition to its inclusion because of billing requirements, degree of luminal stenosis based on NASCET criteria has become an important treatment stratifier, likely in part due to it serving as inclusion criteria for multiple trials [4–6]. Locally, there are no standardized reporting templates across the institution, but many of the templates used by resident and fellow trainees have a statement within the template stating “there is no plaque ulceration,” which likely accounts for the significantly higher number of reports describing the presence of plaque ulceration compared to other plaque features. Plaque ulceration is an important indicator of plaque instability and vulnerability and is associated with cerebrovascular ischemia [8] so its inclusion in a template is appropriate. This also emphasizes the ability of a template to influence reported findings, as others have demonstrated [23, 24]. With increased attention on standardized reporting within the radiology literature, especially with many referring providers preferring standardized reporting, more standardization may be seen within our future [25–28]. Structured reporting may have a role in standardizing carotid plaque reporting and future studies evaluating this possibility would be useful.
Given the lack of consensus on management of patients with high-risk carotid plaque features, the role of reporting specific features is unclear. Currently, there are no official guidelines recommending imaging or reporting of carotid plaque features. As of now, it is unclear exactly which carotid plaque characteristics to report and if radiologists should comment on the degree of increased stroke risk these plaque characteristics confer. Although there are no randomized controlled studies using carotid plaque features as inclusion criteria for surgical revascularization, there are multiple studies demonstrating increased risk of cerebrovascular ischemia associated with high-risk carotid plaque features [8, 11, 18, 29]. It is standard of care at many centers for radiologists to routinely report on findings whose significance has not been proven through randomized controlled trial evidence [30]. Despite the absence of such randomized trial data, the body of existing high-quality observational data may justify incorporating carotid plaque characteristics (beyond luminal stenosis alone) into routine radiologic reporting, though future studies may be necessary to confirm the utility of reporting plaque characteristics [9, 10].
Limitations of our study include that it is a cross-sectional sample in a single academic institution with dedicated board- and CAQ-certified neuroradiologists. Reports from radiologists without neuroradiology fellowship training were not assessed, but we would expect similar or less frequent description of plaque features among such a sample of readers. Similarly, we did not assess reporting at other academic institutions regarding plaque features so can only comment on our own institutional findings. There may also be geographic differences in reporting NASCET criteria, given that billing and reimbursement differ internationally, but this was not assessed in our study. Another limitation is that we did not evaluate the presence of carotid plaque in the imaging examinations to see how many of those with carotid plaque had the features mentioned. We believe that this limitation does not significantly undermine the main aim of our study which is focused on describing and analyzing radiologist reporting practices. We agree that, as the definitions of carotid plaque features on CTA are further standardized, studies evaluating diagnostic accuracy of plaque characterization compared to an accepted reference standard will be increasingly important [8].
Conclusion
In summary, our findings suggest that current reporting practices on CTA neck examinations largely focus on degree of luminal stenosis, with more limited mention of other plaque features. Though traditionally the degree of luminal stenosis has been considered the most critical feature in the evaluation of cervical carotid arteries, carotid plaque imaging has received more attention in its contribution to cerebrovascular ischemia in recent years. At our institution currently, other plaque features are not as commonly reported as degree of luminal stenosis. Our findings suggest there is room for more detailed assessment of carotid plaque characteristics in radiology reports for neck CTA examinations, especially in those patients being assessed for stroke-like symptoms.
Funding
This work was supported by the Association of University Radiologists - GE Radiology Research Academic Fellowship, NIH R01HL144541 and R21HL145427, and American Heart Association (grant number 17SDG33460420).
Footnotes
Conflict of interest The authors declare no relevant conflicts of interest.
Ethics approval All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was waived for this retrospective study.
References
- 1.Bash S, Villablanca JP, Jahan R, Duckwiler G, Tillis M, Kidwell C, Saver J, Sayre J (2005) Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. Am J Neuroradiol 26(5):1012–1021 [PMC free article] [PubMed] [Google Scholar]
- 2.Josephson SA, Bryant SO, Mak HK, Johnston SC, Dillon WP, Smith WS (2004) Evaluation of carotid stenosis using CT angiography in the initial evaluation of stroke and TIA. Neurology 63(3): 457–460. 10.1212/01.wnl.0000135154.53953.2c [DOI] [PubMed] [Google Scholar]
- 3.Collaborators* NASCET (1991) Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 325(7):445–453 [DOI] [PubMed] [Google Scholar]
- 4.Rothwell P, Eliasziw M, Gutnikov S, Fox A, Taylor D, Mayberg M, Warlow C, Barnett H, Collaboration CET (2003) Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 361(9352):107–116 [DOI] [PubMed] [Google Scholar]
- 5.Group ECSTC (1998) Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 351(9113):1379–1387 [PubMed] [Google Scholar]
- 6.Walker MD, Marler JR, Goldstein M, Grady PA, Toole JF, Baker WH, Castaldo JE, Chambless LE, Moore WS, Robertson JT (1995) Endarterectomy for asymptomatic carotid artery stenosis. Jama 273(18):1421–14287723155 [Google Scholar]
- 7.Baradaran H, Gupta A (2020) Carotid vessel wall imaging on CTA. Am J Neuroradiol 41(3):380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baradaran H, Al-Dasuqi K, Knight-Greenfield A, Giambrone A, Delgado D, Ebani E, Kamel H, Gupta A (2017) Association between carotid plaque features on CTA and cerebrovascular ischemia: a systematic review and meta-analysis. Am J Neuroradiol 38: 2321–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saba L, Yuan C, Hatsukami T, Balu N, Qiao Y, DeMarco J, Saam T, Moody A, Li D, Matouk C (2018) Carotid artery wall imaging: perspective and guidelines from the ASNR Vessel Wall Imaging Study Group and expert consensus recommendations of the American Society of Neuroradiology. Am J Neuroradiol 39(2): E9–E31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinjikji W, Huston J, Rabinstein AA, Kim G-M, Lerman A, Lanzino G (2016) Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. 124(1):27. 10.3171/2015.1.jns142452 [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI, Sanelli PC (2013) Carotid plaque MRI and stroke risk. Stroke 44(11):3071–3077 [DOI] [PubMed] [Google Scholar]
- 12.Kwee RM (2010) Systematic review on the association between calcification in carotid plaques and clinical ischemic symptoms. J Vasc Surg 51(4):1015–1025 [DOI] [PubMed] [Google Scholar]
- 13.Trelles M, Eberhardt K, Buchholz M, Schindler A, Bayer-Karpinska A, Dichgans M, Reiser M, Nikolaou K, Saam T (2013) CTA for screening of complicated atherosclerotic carotid plaque—American Heart Association type VI lesions as defined by MRI. Am J Neuroradiol 34(12):2331–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Weert TT, Ouhlous M, Meijering E, Zondervan PE, Hendriks JM, van Sambeek MR, Dippel DW, van der Lugt A (2006) In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol 26(10): 2366–2372 [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Mtui E, Baradaran H, Salama G, Pandya A, Kamel H, Giambrone A, Sanelli P (2015) CT angiographic features of symptom-producing plaque in moderate-grade carotid artery stenosis. Am J Neuroradiol 36(2):349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saba L, Francone M, Bassareo P, Lai L, Sanfilippo R, Montisci R, Suri J, De Cecco C, Faa G (2018) CT attenuation analysis of carotid intraplaque hemorrhage. Am J Neuroradiol 39(1):131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenmenger LB, Aldred BW, Kim S-E, Stoddard GJ, de Havenon A, Treiman GS, Parker DL, McNally JS (2016) Prediction of carotid intraplaque hemorrhage using adventitial calcification and plaque thickness on CTA. Am J Neuroradiol 37(8):1496–1503. 10.3174/ajnr.A4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A, Kesavabhotla K, Baradaran H, Kamel H, Pandya A, Giambrone AE, Wright D, Pain KJ, Mtui EE, Suri JS (2015) Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke 46(1):91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott McNally J, Yoon HC, Kim SE, Narra KK, McLaughlin MS, Parker DL, Treiman GS (2015) Carotid MRI detection of intraplaque hemorrhage at 3T and 1.5 T. J Neuroimaging 25(3): 390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, Small R, Davies JW, Kerwin WS, Hatsukami TS (2001) In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 104(17):2051–2056 [DOI] [PubMed] [Google Scholar]
- 21.Hosseini AA, Kandiyil N, MacSweeney ST, Altaf N, Auer DP (2013) Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Ann Neurol 73(6): 774–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Services CfMaM (2020) Radiology: stenosis measurement in carotid imaging reports - National Quality Strategy Domain: Effective Clinical Care. https://qpp.cms.gov/docs/QPP_quality_measure_specifications/CQM-Measures/2019_Measure_195_MIPSCQM.pdf. Accessed 30 Aug 2020
- 23.Schwartz LH, Panicek DM, Berk AR, Li Y, Hricak H (2011) Improving communication of diagnostic radiology findings through structured reporting. Radiology 260(1):174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson DB, Towbin AJ, Pryor RM, Donnelly LF (2013) Improving consistency in radiology reporting through the use of department-wide standardized structured reporting. Radiology 267(1):240–250 [DOI] [PubMed] [Google Scholar]
- 25.Sistrom CL, Honeyman-Buck J (2005) Free text versus structured format: information transfer efficiency of radiology reports. Am J Roentgenol 185(3):804–812 [DOI] [PubMed] [Google Scholar]
- 26.Naik SS, Hanbidge A, Wilson SR (2001) Radiology reports: examining radiologist and clinician preferences regarding style and content. Am J Roentgenol 176(3):591–598 [DOI] [PubMed] [Google Scholar]
- 27.Brook OR, Brook A, Vollmer CM, Kent TS, Sanchez N, Pedrosa I (2015) Structured reporting of multiphasic CT for pancreatic cancer: potential effect on staging and surgical planning. Radiology 274(2):464–472 [DOI] [PubMed] [Google Scholar]
- 28.Nörenberg D, Sommer WH, Thasler W, D’Haese J, Rentsch M, Kolben T, Schreyer A, Rist C, Reiser M, Armbruster M (2017) Structured reporting of rectal magnetic resonance imaging in suspected primary rectal cancer: potential benefits for surgical planning and interdisciplinary communication. Investig Radiol 52(4): 232–239 [DOI] [PubMed] [Google Scholar]
- 29.Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, Koeppel T, Hoffmann U, Reiser MF, Bamberg F (2013) Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol 62(12):1081–1091 [DOI] [PubMed] [Google Scholar]
- 30.Staunton M (2007) Evidence-based radiology: steps 1 and 2—asking answerable questions and searching for evidence. Radiology 242(1):23–31 [DOI] [PubMed] [Google Scholar]


