Introduction
Stroke is a major cause of morbidity and mortality worldwide, with carotid atherosclerosis contributing to a significant proportion of ischemic strokes.1,2 Detailed imaging assessment of extracranial carotid artery disease is critical for the appropriate risk stratification and management of those presenting with cerebrovascular ischemia. When imaging carotid atherosclerosis, the two important factors are the degree of luminal stenosis and the assessment of plaque components. Luminal stenosis has been the basis for patient inclusion in multiple randomized stroke prevention trials and the results of these studies established stenosis severity as the primary imaging-based method for carotid disease risk stratification.3–6 However, recent mounting evidence of the contribution of individual plaque components to vulnerable carotid plaque has challenged the primacy of luminal stenosis measures alone as the imaging biomarker of choice for stroke risk assessment. Imaging of luminal stenosis and carotid plaque can be performed with a number of techniques including ultrasound, digital subtraction angiography, CT angiography, and high-resolution MR. Accurate characterization of both luminal stenosis and plaque characteristics is the primary objective of imaging the extracranial carotid artery and will be the focus of this review.
Normal anatomy and Imaging Technique
Most carotid atherosclerotic plaque develops near the bifurcation and proximal internal carotid artery (ICA). Hemodynamic forces and stress plays a critical role in the development of atherosclerosis at the carotid bifurcation, in addition to other local and systemic inflammatory and thrombotic processes.7 The carotid bifurcation is usually between the C3–C5 level but can vary slightly in position and may have differing angles among individuals and even among sides in an individual patient.8
Carotid Ultrasound
B-mode ultrasonography, a relatively inexpensive examination without ionizing radiation, is often the first-line examination to evaluate for carotid stenosis.9 It is a commonly used screening exam which can indirectly assess the degree of carotid stenosis and evaluate specific carotid plaque features. Limitations of carotid ultrasound include its dependence on the sonographer or technologist performing the exam, inherent limitations secondary to patient habitus and anatomy, its flow dependence which can limit evaluation in the setting of systemic hemodynamic alterations, and inability to discern low levels of stenosis.9–13
In addition to B-mode sonographic techniques, contrast-enhanced ultrasonography (CEUS) can also be used in the assessment of carotid plaque. In CEUS, sonography is performed after the intravenous injection of microbubble contrast agent which remains intravascular for the duration of the exam and allows for the evaluation of the carotid lumen and plaque neovascularity.14–16
Computed Tomography Angiography
Computed tomography angiography (CTA) of the neck is commonly performed in the acute stroke setting and is an accurate method to evaluate carotid stenosis. CTA is a widely available imaging technique that can be quickly performed without specialized equipment or post-processing software yet still has the ability to directly visualize luminal stenosis and many plaque features. Major considerations with CTA examinations are ionizing radiation and the need to inject intravenous contrast, limiting its use in patients with renal insufficiency. In addition to standard multidetector CTA, dual-energy/dual source CT can also be performed to evaluate carotid plaque. Dual-energy CT allows for improved delineation between calcification associated with a carotid plaque and the increased density from luminal contrast.17
Magnetic resonance imaging
MR-based imaging is well-established for the evaluation of carotid artery plaque.18,19 MR angiography (MRA) can be performed to evaluate the degree of luminal stenosis and to evaluate for additional plaque features which are known to be high-risk. MR-based imaging evaluation does not involve ionizing radiation and many sequences can be performed without the administration of intravenous contrast. MR evaluation of carotid plaque is limited due to its often-lengthy imaging acquisition time, relatively higher costs, and individual patient contraindications to MR imaging, such as certain types of cardiac devices.
Protocols
Ultrasound
When evaluating carotid atherosclerotic disease using B-mode ultrasound, a 4–7MHz linear array transducer is used to evaluate the distal CCAs, the carotid bifurcations, and the cervical ICAs. A complete examination of the carotid arteries includes assessment with grayscale imaging, color Doppler imaging, and spectral Doppler velocity evaluation.9,13 Peak systolic velocity (PSV) in the ICA along with the presence of plaque are the most important factors in assessing carotid stenosis.13 Additional Doppler parameters, such as ICA/CCA ratios, can also be used to confirm and/or assist in the diagnosis of carotid stenosis. Power Doppler may be used when there is critical stenosis or near-occlusion to evaluate for the presence of any flow.9
CTA
Multi-detector CTA is the most commonly performed type of CTA examination when evaluating cervical vasculature. After the administration of nonionic iodinated contrast using a power injector (4mL/s flow rate), helical mode CT scanning with a multidetector scanner is performed. Imaging is performed from the aortic arch to the C1 ring for a neck CTA examination with sub-mm, typically 0.625mm, resolution.20,21 Sagittal, coronal, and sometimes oblique reconstructions are performed for complete evaluation of the carotid bifurcations. Dual energy CT examinations can also be performed and have been shown to be useful for differentiating calcified plaque from the luminal contrast.17 Currently, dual energy CT is not frequently used in the clinical setting.
MRA/MRI
There is more variability in protocols when imaging the cervical vasculature using MR-based techniques.20 Protocols range from simple, relatively quickly performed non-contrast MRA of the neck to more time-intensive examinations using multiple sequences and dedicated carotid neck coils. All protocols should have at least 1 sequence centered on the carotid bifurcation and extending 3–4cm longitudinally. Most protocols include a time-of-flight sequence to assess for flow directionality. Contrast-enhanced sequences are also commonly performed in order to more fully assess for stenosis at the origins of the great vessels and vertebral arteries and for more accurate evaluation of luminal stenosis. Many institutions include some kind of sequence with blood suppression to evaluate for plaque burden. Often a 3D T1-weighted sequence, such as MPRAGE, is performed to evaluate for intraplaque high-intensity signal, a marker for intraplaque hemorrhage. Either 1.5T or 3T scanners can be used, but 3T scanners have improved SNR.20
Imaging Findings/Pathology
There are two main findings being imaged with extracranial vascular disease in the carotid artery: Luminal stenosis and plaque features. While luminal stenosis has historically been the focus of imaging, plaque features are becoming increasingly important in the management of patients with carotid artery disease.
Luminal Stenosis
One of the most important findings to report on extracranial vessel imaging is the degree of luminal stenosis. On MRA and CTA, stenosis can be defined by a number of different measurement techniques, but most commonly by criteria set by the North American Symptomatic Carotid endarterectomy trial (NASCET).6,22 This method requires measurement of the area of the greatest degree of luminal narrowing and then measurement of a non-stenotic region in the normal mid to distal ICA, not in an area where there is post-stenotic dilatation (Figure 1).22 While these measurements were initially validated using digital subtraction angiography (DSA), there is good correlation with CTA and contrast-enhanced MRA images so measurement is made in a similar fashion.22,23 Other schemes for measuring luminal stenosis are not as commonly used in the United States but include the European Carotid Surgery Trial (ESCT) and common carotid (CC) methods of measurement.24 Luminal stenosis can also be estimated using a technique suggested by Bartlett et al25 on CTA, in which the narrowest measurement in mm is associated with degree of stenosis by NASCET criteria. In this method, 2.2 mm corresponds to a stenosis of approximately 50% and 1.3 mm to a stenosis of 70%.25
Figure 1.
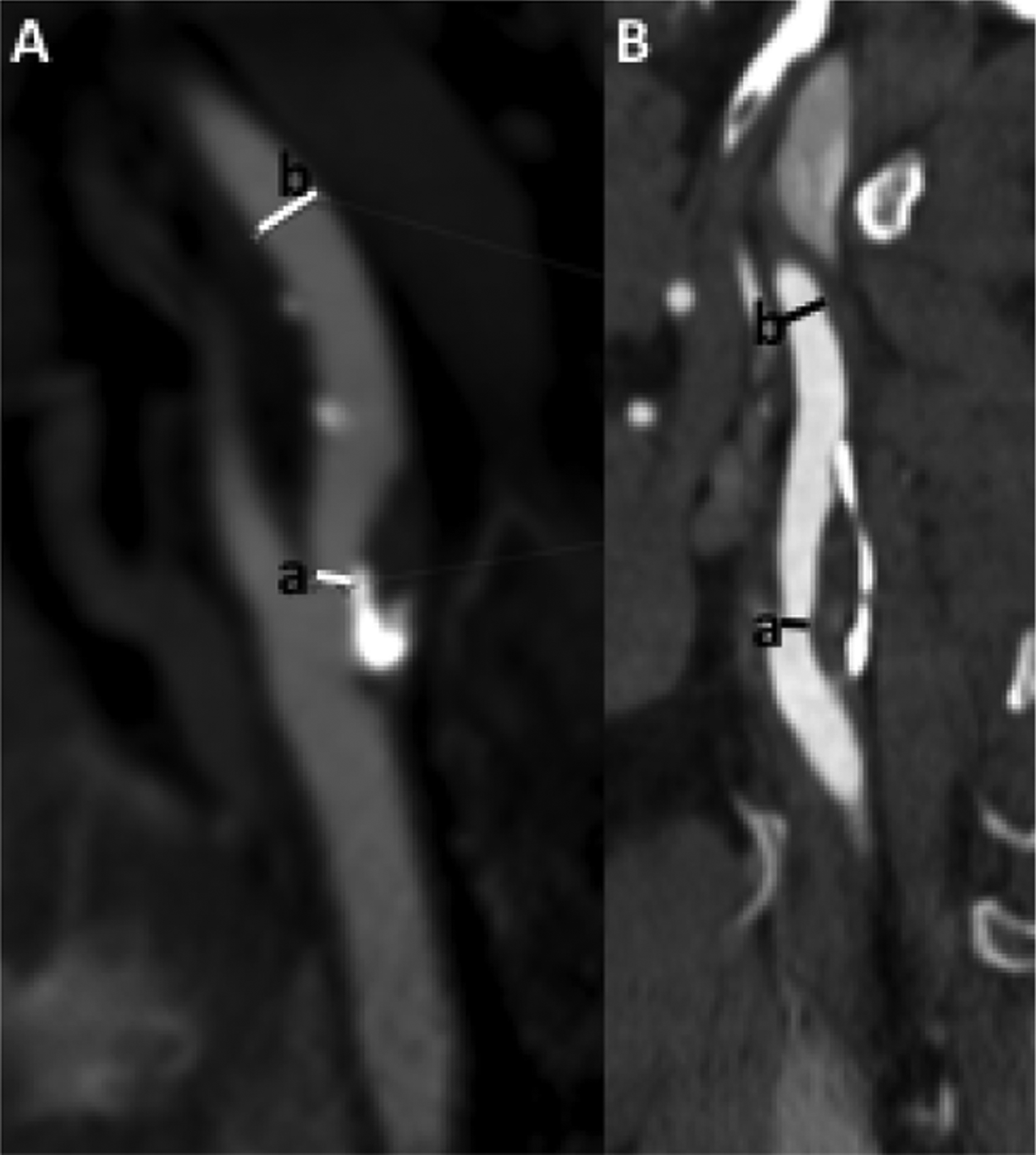
Measuring stenosis by NASCET criteria involves first identifying and measuring the area of narrowest luminal diameter (a) and then measuring the luminal diameter in the normal more distal cervical ICA (b). These measurements should be made in plane with the vessel, accounting for vessel tortuosity. NASCET stenosis percentage equals (b-a)/b.
For US, on the other hand, luminal measurements of the area of greatest narrowing cannot be directly made. Instead, the degree of stenosis is inferred based on blood velocity in the ICA and the ratio of PSVs in the CCA and ICA and the plaque burden.13 US allows for a “real-time,” flow-dependent assessment of stenosis. Issues include any states that can alter the flow, such as aortic stenosis or regurgitation.
Plaque Features
In addition to the degree of luminal stenosis, the plaque composition is also essential to evaluate and discuss. The histopathologic findings in carotid plaque evolution and development has been extensively studied. Atherosclerotic plaque develops along a spectrum from mild fatty deposition in asymptomatic young arteries to complex, irregular plaques prone to releasing thromboemboli.26 The features identifiable on plaque vessel wall imaging are correlates to histopathologic features.27–29 Many plaque characteristics have a typical appearance on each of the most frequently performed imaging modalities (Table 1). We will review some of the most commonly encountered plaque features on routine imaging.
Table 1.
Description of the most commonly encountered plaque features and their typical imaging findings.
| Plaque Feature | Definition/histopathologic correlate | US finding | CTA finding | MR finding |
|---|---|---|---|---|
| Calcification | Plaque calcification | Echogenic plaque with posterior acoustic shadowing | Plaque with density of at least over 130 HU | Profound hypointensity on all sequences |
| Intraplaque hemorrhage | Hemorrhagic plaque components | Echolucent plaque | Plaque with low attenuation in the 40–50 HU range | Hyperintense on all T1 and TOF sequences |
| Lipid-rich necrotic core | Necrotic core within lipid-rich plaque which contains macrophages and inflammatory cells | Echolucent plaque | Plaque with low attenuation in the 40–50 HU range | Hyperintense on T1 weighted sequences; Isointense on TOF |
| Plaque ulceration | Ulceration of the surface of the plaque leading to exposure of plaque materials to the lumen | Concavity along the surface of the plaque greater than 2 × 2 mm with Color Doppler signal within the concavity | Extension of hyperdense contrast beyond the vessel wall into the plaque by at least 1 mm | Surface irregularity on most MR sequences with extension of contrast beyond the lumen wall on MRA |
HU=Hounsfield units; TOF=time-of-flight
Calcified plaque
Calcification is one of the most commonly encountered plaque components (Figure 2). The presence of calcified plaque has a lower association with cerebrovascular ischemia30–32 leading some to believe that calcified plaque is a “lower-risk” plaque feature and may offer some more stability to the plaque surface with decreased likelihood of generating thromboemboli compared to plaques which lack dense calcification.33 It is most readily visualized on CTA by an area of increased HU in the region of the carotid bifurcation. On MR, plaque calcification can be visualized with high sensitivity and specificity as an area of hypointensity on all pulse sequences.18,34 On US, calcified plaque is correlated with increased echogenicity and posterior acoustic shadowing. At times, the posterior acoustic shadowing from a large echogenic, calcified plaque may obscure evaluation of the entire plaque, limiting evaluation (Figure 2).
Figure 2.
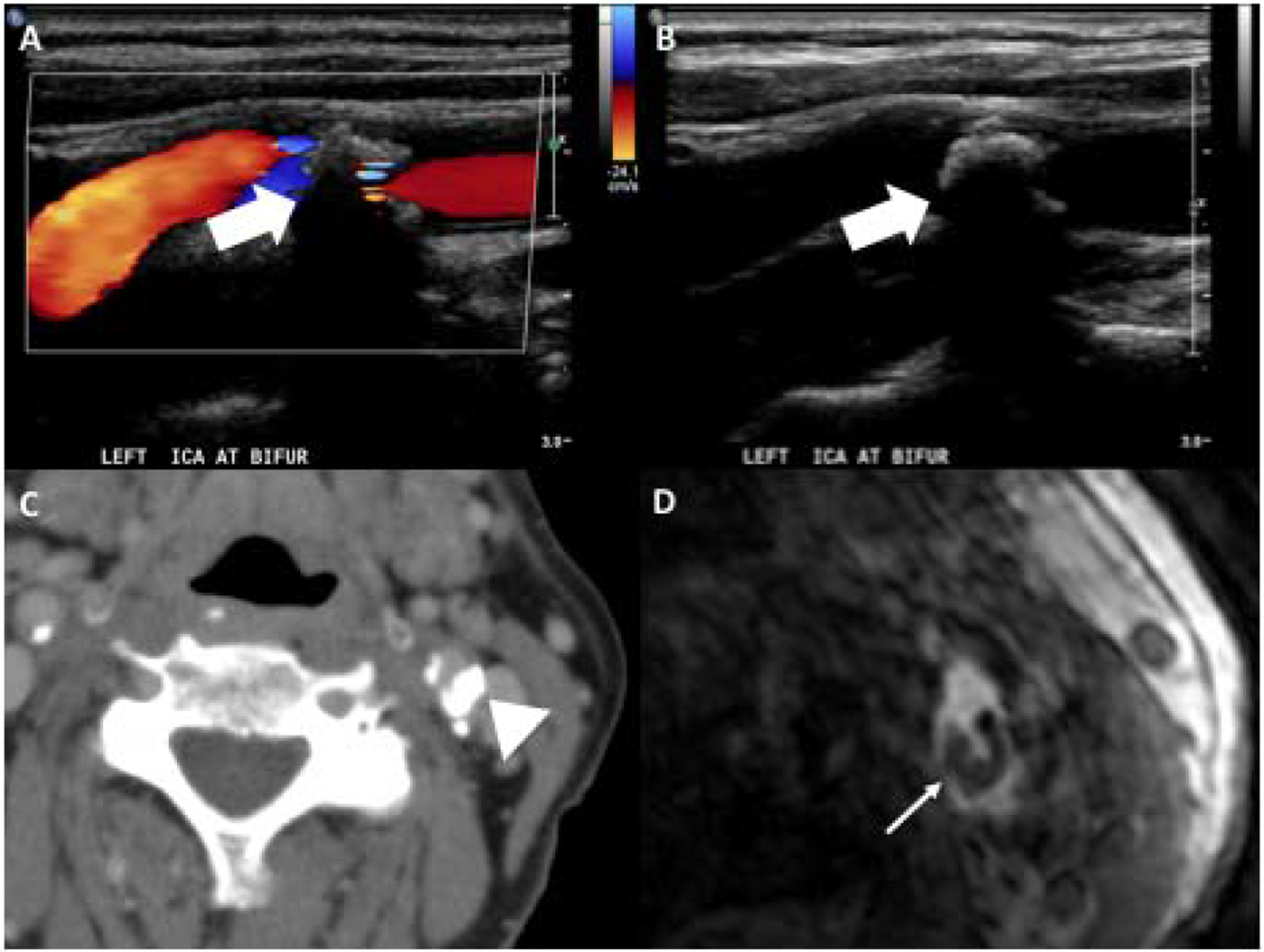
85-year-old female with large calcified plaque at the left carotid bifurcation as seen on US, CT, and MR. Doppler (A) and gray scale (B) US images demonstrate large echogenic plaque with posterior acoustic shadowing (white large arrows). The posterior acoustic shadowing limits appreciation of the size of the plaque. CT on the same patient (C) shows large calcified plaque in the posterior aspect of the left carotid bifurcation (arrowhead). Axial slice of 3D MR TOF (D) shows area of hypointensity in the posterior aspect of the left carotid bifurcation corresponding to the plaque calcification (narrow white arrow).
Intraplaque hemorrhage
Intraplaque hemorrhage (IPH) is one of the most well-known “high-risk” plaque features on imaging (Figure 3). IPH has been studied extensively and is known to confer increased risk of future and recurrent stroke.19,35 There is robust evidence that IPH can be accurately evaluated on MR imaging.34,36 IPH has distinctive imaging features on MR, namely intrinsic T1 hyperintensity, often seen on MPRAGE or other sequences. This high-intensity signal can also be appreciated on TOF sequences.37 Recognizing IPH on CT and US can be more difficult. There is evidence that “soft” plaque seen on CTA and echolucent plaque seen on US, likely has some component of IPH, as both of these plaque features confer increased risk.30,31,38–40
Figure 3.
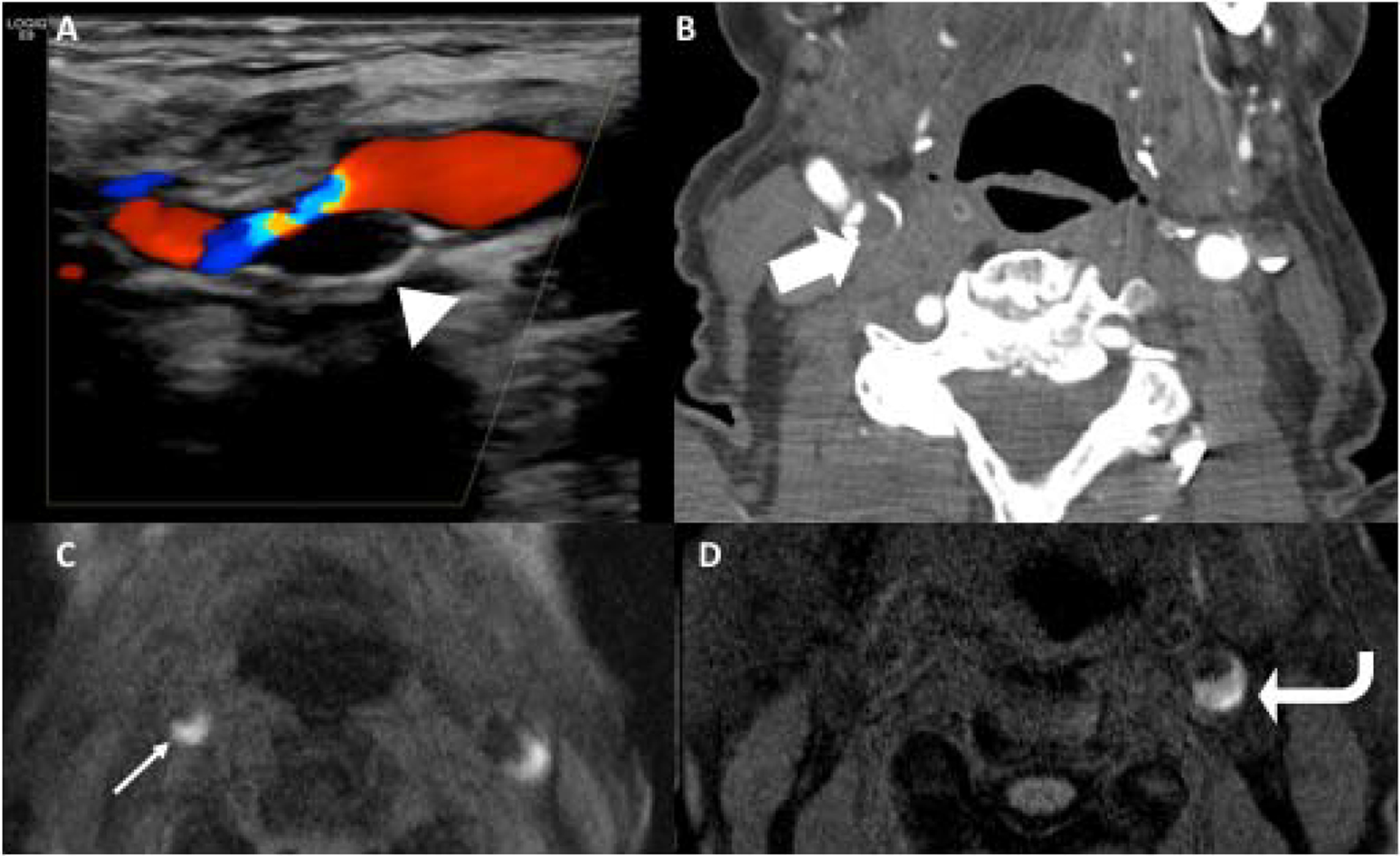
A-C show US, CTA, and axial 3D MPRAGE MR on the same patient. This 87-year-old female with acute right sided stroke (not pictured) had large echolucent plaque in the right carotid bifurcation on US (arrowhead), large soft/fibrofatty plaque on CTA (large block arrow), and crescentic T1 hyperintense plaque on 3D MPRAGE in the right (narrow white arrow) and left proximal internal carotid arteries (ICAs), consistent with intraplaque hemorrhage. D is another patient who presented with an acute left sided infarction with large crescentic T1 hyperintense plaque in the proximal left ICA (curved arrow), consistent with intraplaque hemorrhage. The signal is greater than 2x the intensity of adjacent sternocleidomastoid muscle.
Lipid-rich necrotic core
Lipid-rich necrotic core (LRNC) is another “high-risk” plaque feature than can be appreciated on imaging. LRNC is also highly associated with future and recurrent stroke.19 Histopathologically, LRNC is indicative of complex plaque with a necrotic lipid-rich core. LRNC is most readily identifiable on MR imaging by its TI hyperintense appearance and isointensity on TOF sequences. It has less characteristic findings on CTA and US with its imaging appearance overlapping with IPH. Although it is more challenging to differentiate LRNC and IPH on CTA or US, precise distinction may not be critical from a clinical perspective as LRNC and IPH both confer increased risk of future and recurrent stroke.19,21
Plaque ulceration
Plaque ulceration is a high-risk marker because it indicates instability of the plaque surface and exposes the unstable plaque to flowing luminal blood. Ulceration indicates plaque surface irregularity which increases the likelihood of thrombus formation. Plaque ulceration is defined as an extension of luminal contrast into a plaque, measuring at least 1–2mm.21 Plaque ulceration is well visualized on CTA (Figure 4) and can have varying degrees of severity ranging from focal areas of contrast extension to large cavitating ulcers. Plaque ulceration can also be identified on contrast-enhanced MRA with high specificity and sensitivity.41 US can also depict plaque ulceration though with less sensitivity than the other cross-sectional techniques.
Figure 4.
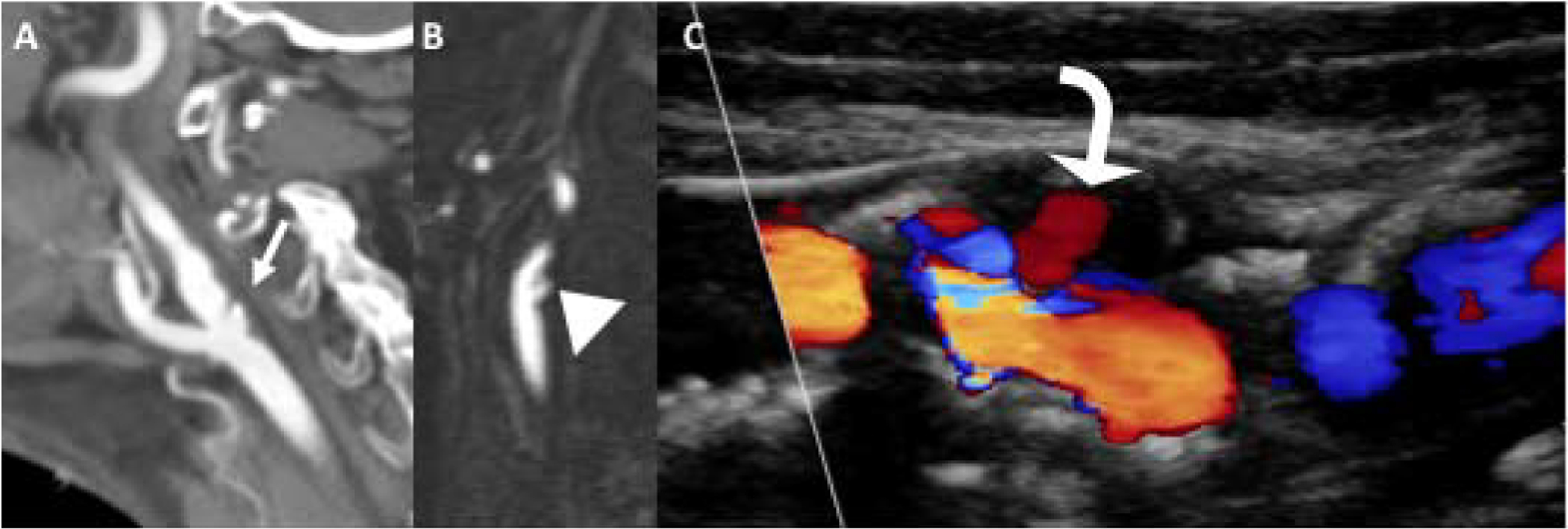
Plaque ulceration. Sagittal reformations of CTA (A) and contrast-enhanced MRA (B) demonstrate large plaque ulcerations (white arrow and arrowhead) in the proximal ICAs in two different patients. Carotid US (C) demonstrating Doppler flow (curved arrow) within a large echolucent plaque in the proximal ICA, compatible with large plaque ulceration.
Clinical Applications
Atherosclerosis of the extracranial carotid arteries is a substantial contributor to ischemic stroke, leading to about 15–20% of ischemic strokes.1,2 Risk stratification is critical in identifying those who are at highest risk of stroke. Imaging-based risk assessment has evolved over recent years with increased attention to carotid plaque features, rather than strictly focusing on degree of stenosis.20,42 Carotid atherosclerosis is thought to lead to ischemic strokes by two main methods: 1) flow reduction in the setting of a stenotic plaque and 2) artery-to-artery thromboembolism from plaque surface irregularities.43 Stenosing plaque is a large contributor to ischemic strokes and has received the most attention traditionally, but small thromboemboli from unstable or irregular plaque surface is emerging as an important cause of ischemic stroke. While both stenosis and plaque can result in ischemic events, there is no direct correlation of plaque size/volume and degree of stenosis.44–46 There is mounting evidence that many high-risk plaque features identified via imaging directly contribute to cerebrovascular ischemia and that these features can contribute to ischemic strokes/TIAs without associated stenosis.47–49 In a recent meta-analysis of 64 studies enrolling 20,751 patients, the pooled prevalence of high-risk plaques was 26.5% (95% CI, 22.9%−30.3%), and the incidence of ipsilateral ischemic cerebrovascular events was higher in patients with high-risk plaques (4.3 events per 100 person-years; 95% CI, 2.5–6.5 events per 100 person-years) than in those without high-risk plaques (1.2 events per 100 person-years; 95% CI, 0.6–1.8 events per 100 person-years), with an odds ratio of 3.0 (95% CI, 2.1–4.3).50
Differential Diagnosis
Atherosclerotic plaque is the most common pathology visualized in the region of the carotid bifurcation and is generally readily identifiable. There are a few other processes with imaging findings similar to atherosclerotic plaque which may be considered.
Dissection
ICA dissections can have overlapping findings with carotid artery atherosclerotic plaque, especially if the dissection does not present with the pathognomonic findings of intimal flap and double lumen. Dissections usually occur more distal to the carotid bulb than plaque, usually at least 2–3cm distal from the bifurcation and are commonly seen in the distal cervical ICA, just proximal to the skull base. Also, dissections are typically not associated with calcifications which are commonly seen in the setting of plaque.
Fibromuscular dysplasia
Fibromuscular dysplasia (FMD) can cause arterial stenosis in the ICAs, and may be confused with narrowing secondary to atherosclerotic plaque. FMD causes vessel irregularity and beading that usually spares the carotid bifurcations and is usually seen in the distal cervical ICAs. It is caused by overgrowth of smooth muscle and fibrous tissue. Unlike atherosclerotic plaque, no mural calcifications are seen.
Carotid Web
Carotid webs are linear filling defects in the posterior aspect of the proximal internal carotid artery. They are thought to be a variant of FMD with focal intimal hyperplasia.51 Though they are not as common as atherosclerotic plaque, they are an important cause of ischemic stroke, particularly in younger, female patients.51,52
TIPIC
Transient perivascular inflammation of the carotid artery (TIPIC), previously known as carotidynia, is a distinct clinical entity in which there is wall thickening and inflammatory changes surrounding the carotid bifurcation and proximal ICA.53 TIPIC is a self-limited process in which patients present with neck pain and tenderness in the region of the bifurcation and may have elevated serum inflammatory markers. Imaging findings include thickening and enhancement of the carotid wall along with surrounding fat stranding. The inflammatory changes will resolve on follow-up studies, as opposed to atherosclerotic changes which persist.
Case Study Presentation
We present a case of 71-year-old female who presented to the Emergency Department with acute right sided weakness and was found to have acute infarctions in the left frontal and parietal lobes (Figure 5). She then underwent a CTA head/neck which demonstrated a large predominantly soft/fibrofatty plaque with ulceration. Despite the large size of the soft plaque, there is less than 50% stenosis by NASCET criteria. This patient went on to undergo MRA of the cervical vasculature which revealed high-intensity signal associated with the left-sided carotid plaque, consistent with intraplaque hemorrhage. Although this patient had no significant stenosis by NASCET criteria, her irregular, ulcerated plaque with intraplaque hemorrhage likely contributed to her ischemic stroke. This case highlights the importance of looking beyond the lumen to evaluate the associated vessel wall and plaque characteristics.
Figure 5.
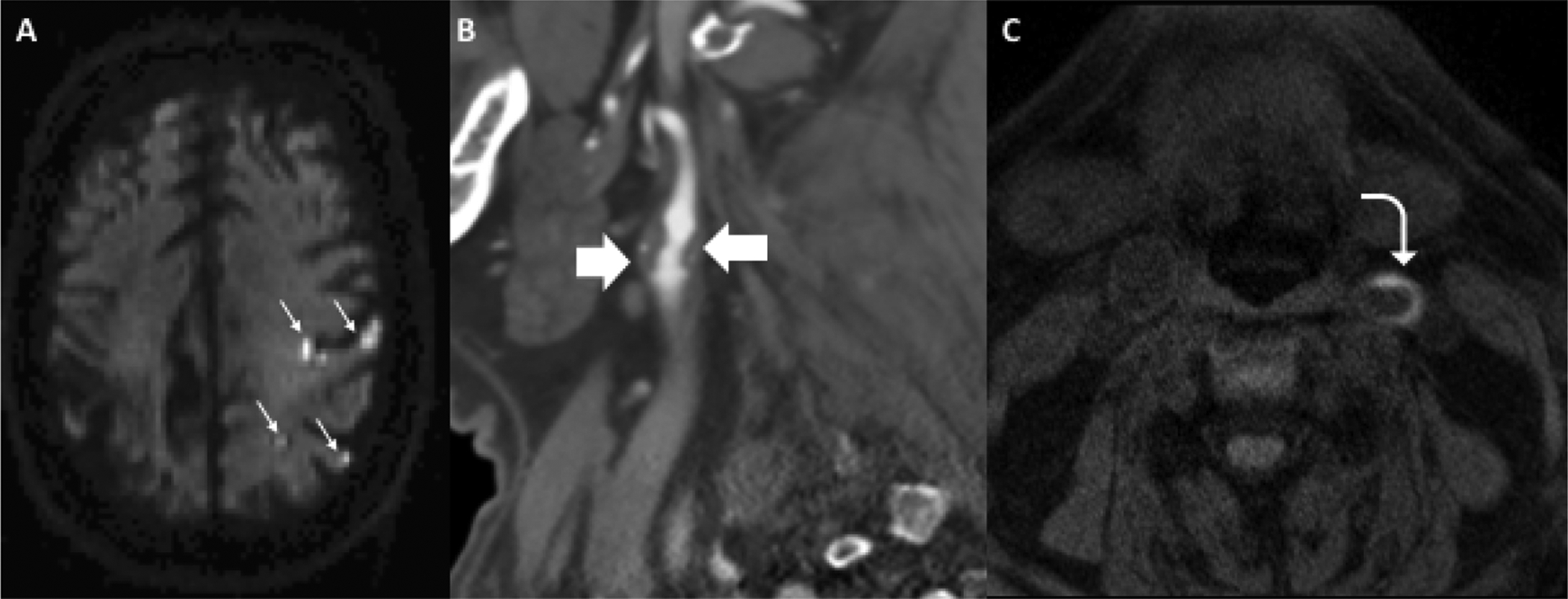
Case study of 71-year-old female presenting with acute right-sided weakness found to have multiple acute infarctions throughout the left cerebral hemisphere on axial DWI (A, small white arrows). Immediately after rapid brain MRI, she had a CTA head and neck which showed a large soft/fibrofatty plaque in the proximal left ICA (B, block arrows) with areas of ulceration resulting in less than 50% stenosis by NASCET criteria. On axial MPRAGE, she had areas of crescentic T1 hyperintensity (C, curved arrow) consistent with intraplaque hemorrhage.
Conclusion/Summary
Extracranial carotid artery imaging is an important part of the workup for cerebrovascular ischemia. The two most important imaging features of carotid atherosclerosis are the degree of luminal stenosis and specific plaque features. Many plaque features can be identified on routine imaging and can be helpful in the risk stratification of those with carotid atherosclerosis.3
Key points:
When imaging carotid atherosclerosis, degree of stenosis and carotid plaque features are the most important findings
Many “high-risk” carotid plaque features are detectable on carotid US, CTA, and MRA
Some plaque features are more closely associated with cerebrovascular ischemia than degree of stenosis
Synopsis:
Carotid atherosclerosis is an important contributor to ischemic stroke. When imaging carotid atherosclerosis, it is essential to describe both the degree of luminal stenosis and specific plaque characteristics as both are risk factors for cerebrovascular ischemia. Carotid atherosclerosis can be accurately assessed using multiple imaging techniques, including ultrasound, CT angiography, and MR angiography. By understanding the underlying histopathology, we can appreciate the specific plaque characteristics on each of these imaging modalities. In this review article, we briefly describe some of the most commonly encountered plaque features, including plaque calcification, intraplaque hemorrhage, lipid-rich necrotic core, and plaque ulceration.
Clinics Care Points –
Bulleted list of evidence-based pearls and pitfalls relevant to the point of care
Carotid plaque characteristics can be assessed on US, CTA, and MRA
Certain plaque features, including intraplaque hemorrhage and plaque ulceration are “high-risk” and are independently associated with stroke, regardless of degree of stenosis
Intraplaque hemorrhage, one of the most high-risk plaque features, is T1 and TOF hyperintense on MR, low attenuation on CTA, and echolucent on US
Both plaque characteristics and luminal stenosis should be described when reporting on carotid atherosclerosis
Disclosures:
The authors have nothing to disclose relevant to the submitted work. Dr. Gupta reports non-financial support from GE Healthcare and non-financial support from Siemens Medical Solutions USA, Inc., outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Sacco RL, Kasner SE, Broderick JP, et al. An Updated Definition of Stroke for the 21st Century. Stroke. 2013;44(7):2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wityk R, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27(11):1974–1980. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell P, Eliasziw M, Gutnikov S, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. The Lancet. 2003;361(9352):107–116. [DOI] [PubMed] [Google Scholar]
- 4.Group ECSTC. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). The Lancet. 1998;351(9113):1379–1387. [PubMed] [Google Scholar]
- 5.Walker MD, Marler JR, Goldstein M, et al. Endarterectomy for asymptomatic carotid artery stenosis. Jama. 1995;273(18):1421–1428. [PubMed] [Google Scholar]
- 6.Collaborators* NASCET. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. New England Journal of Medicine. 1991;325(7):445–453. [DOI] [PubMed] [Google Scholar]
- 7.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Jama. 1999;282(21):2035–2042. [DOI] [PubMed] [Google Scholar]
- 8.Thomas JB, Antiga L, Che SL, et al. Variation in the Carotid Bifurcation Geometry of Young Versus Older Adults. Stroke. 2005;36(11):2450–2456. [DOI] [PubMed] [Google Scholar]
- 9.Tahmasebpour HR, Buckley AR, Cooperberg PL, Fix CH. Sonographic examination of the carotid arteries. Radiographics. 2005;25(6):1561–1575. [DOI] [PubMed] [Google Scholar]
- 10.Glor FP, Ariff B, Hughes AD, et al. Operator dependence of 3-D ultrasound-based computational fluid dynamics for the carotid bifurcation. IEEE Transactions on Medical Imaging. 2005;24(4):451–456. [DOI] [PubMed] [Google Scholar]
- 11.Mead GE, Lewis SC, Wardlaw JM. Variability in Doppler ultrasound influences referral of patients for carotid surgery. European Journal of Ultrasound. 2000;12(2):137–143. [DOI] [PubMed] [Google Scholar]
- 12.Gaitini D, Soudack M. Diagnosing carotid stenosis by Doppler sonography: state of the art. Journal of ultrasound in medicine. 2005;24(8):1127–1136. [DOI] [PubMed] [Google Scholar]
- 13.Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: grayscale and Doppler ultrasound diagnosis—Society of Radiologists in Ultrasound consensus conference. Ultrasound quarterly. 2003;19(4):190–198. [DOI] [PubMed] [Google Scholar]
- 14.Piscaglia F, Nolsøe C, Dietrich Ca, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall in der Medizin-European Journal of Ultrasound. 2012;33(01):33–59. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer JE, Samsó JJ, Serrando JR, Valenzuela VF, Montoya SB, Docampo MM. Use of ultrasound contrast in the diagnosis of carotid artery occlusion. Journal of vascular surgery. 2000;31(4):736–741. [DOI] [PubMed] [Google Scholar]
- 16.Akkus Z, Hoogi A, Renaud G, et al. New quantification methods for carotid intra-plaque neovascularization using contrast-enhanced ultrasound. Ultrasound in medicine & biology. 2014;40(1):25–36. [DOI] [PubMed] [Google Scholar]
- 17.Das M, Braunschweig T, Mühlenbruch G, et al. Carotid plaque analysis: comparison of dual-source computed tomography (CT) findings and histopathological correlation. European Journal of Vascular and Endovascular Surgery. 2009;38(1):14–19. [DOI] [PubMed] [Google Scholar]
- 18.Saam T, Ferguson M, Yarnykh V, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(1):234–239. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Baradaran H, Schweitzer AD, et al. Carotid Plaque MRI and Stroke Risk. Stroke. 2013;44(11):3071–3077. [DOI] [PubMed] [Google Scholar]
- 20.Saba L, Yuan C, Hatsukami T, et al. Carotid artery wall imaging: perspective and guidelines from the ASNR Vessel Wall Imaging Study Group and expert consensus recommendations of the American Society of Neuroradiology. American Journal of Neuroradiology. 2018;39(2):E9–E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baradaran H, Gupta A. Carotid Vessel Wall Imaging on CTA. American Journal of Neuroradiology. 2020;41(3):380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox AJ. How to measure carotid stenosis. Radiology. 1993;186(2):316–318. [DOI] [PubMed] [Google Scholar]
- 23.Anzidei M, Napoli A, Zaccagna F, et al. Diagnostic accuracy of colour Doppler ultrasonography, CT angiography and blood-pool-enhanced MR angiography in assessing carotid stenosis: a comparative study with DSA in 170 patients. La radiologia medica. 2012;117(1):54–71. [DOI] [PubMed] [Google Scholar]
- 24.Staikov IN, Arnold M, Mattle H, et al. Comparison of the ECST, CC, and NASCET grading methods and ultrasound for assessing carotid stenosis. Journal of neurology. 2000;247(9):681–686. [DOI] [PubMed] [Google Scholar]
- 25.Bartlett E, Walters T, Symons S, Fox A. Quantification of carotid stenosis on CT angiography. American Journal of Neuroradiology. 2006;27(1):13–19. [PMC free article] [PubMed] [Google Scholar]
- 26.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–1374. [DOI] [PubMed] [Google Scholar]
- 27.de Weert TT, Ouhlous M, Meijering E, et al. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(10):2366–2372. [DOI] [PubMed] [Google Scholar]
- 28.Trelles M, Eberhardt K, Buchholz M, et al. CTA for screening of complicated atherosclerotic carotid plaque—American Heart Association type VI lesions as defined by MRI. American Journal of Neuroradiology. 2013;34(12):2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke SE, Hammond RR, Mitchell JR, Rutt BK. Quantitative assessment of carotid plaque composition using multicontrast MRI and registered histology. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2003;50(6):1199–1208. [DOI] [PubMed] [Google Scholar]
- 30.Gupta A, Kesavabhotla K, Baradaran H, et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke. 2015;46(1):91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baradaran H, Al-Dasuqi K, Knight-Greenfield A, et al. Association between Carotid Plaque Features on CTA and Cerebrovascular Ischemia: A Systematic Review and Meta-Analysis. American Journal of Neuroradiology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwee RM. Systematic review on the association between calcification in carotid plaques and clinical ischemic symptoms. Journal of vascular surgery. 2010;51(4):1015–1025. [DOI] [PubMed] [Google Scholar]
- 33.Shaalan WE, Cheng H, Gewertz B, et al. Degree of carotid plaque calcification in relation to symptomatic outcome and plaque inflammation. Journal of vascular surgery. 2004;40(2):262–269. [DOI] [PubMed] [Google Scholar]
- 34.Den Hartog A, Bovens S, Koning W, et al. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. European Journal of Vascular and Endovascular Surgery. 2013;45(1):7–21. [DOI] [PubMed] [Google Scholar]
- 35.Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. Journal of the American College of Cardiology. 2013;62(12):1081–1091. [DOI] [PubMed] [Google Scholar]
- 36.Ota H, Yarnykh VL, Ferguson MS, et al. Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging: comparison of the diagnostic performance of three T1-weighted sequences. Radiology. 2010;254(2):551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta A, Baradaran H, Kamel H, et al. Intraplaque high-intensity signal on 3D time-of-flight MR angiography is strongly associated with symptomatic carotid artery stenosis. American Journal of Neuroradiology. 2014;35(3):557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajduk M, Pavić L, Bulimbašić S, et al. Multidetector-row computed tomography in evaluation of atherosclerotic carotid plaques complicated with intraplaque hemorrhage. Annals of vascular surgery. 2009;23(2):186–193. [DOI] [PubMed] [Google Scholar]
- 39.Ajduk M, Bulimbasic S, Pavic L, et al. Comparison of multidetector-row computed tomography and duplex Doppler ultrasonography in detecting atherosclerotic carotid plaques complicated with intraplaque hemorrhage. Collegium antropologicum. 2013;37(1):213–219. [PubMed] [Google Scholar]
- 40.Saba L, Francone M, Bassareo P, et al. CT attenuation analysis of carotid intraplaque hemorrhage. American Journal of Neuroradiology. 2018;39(1):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etesami M, Hoi Y, Steinman D, et al. Comparison of carotid plaque ulcer detection using contrast-enhanced and time-of-flight MRA techniques. American Journal of Neuroradiology. 2013;34(1):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brinjikji W, Huston J, Rabinstein AA, Kim G-M, Lerman A, Lanzino G. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. 2016;124(1):27. [DOI] [PubMed] [Google Scholar]
- 43.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Archives of neurology. 1998;55(11):1475–1482. [DOI] [PubMed] [Google Scholar]
- 44.Saam T, Yuan C, Chu B, et al. Predictors of carotid atherosclerotic plaque progression as measured by noninvasive magnetic resonance imaging. Atherosclerosis. 2007;194(2):e34–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rozie S, De Weert T, De Monyé C, et al. Atherosclerotic plaque volume and composition in symptomatic carotid arteries assessed with multidetector CT angiography; relationship with severity of stenosis and cardiovascular risk factors. European radiology. 2009;19(9):2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mono M-L, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovascular diseases. 2012;34(5–6):343–350. [DOI] [PubMed] [Google Scholar]
- 47.Saba L, Montisci R, Sanfilippo R, Mallarini G. Multidetector row CT of the brain and carotid artery: a correlative analysis. Clinical radiology. 2009;64(8):767–778. [DOI] [PubMed] [Google Scholar]
- 48.Gupta A, Mtui E, Baradaran H, et al. CT angiographic features of symptom-producing plaque in moderate-grade carotid artery stenosis. American Journal of Neuroradiology. 2015;36(2):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta A, Baradaran H, Kamel H, et al. Evaluation of computed tomography angiography plaque thickness measurements in high-grade carotid artery stenosis. Stroke. 2014;45(3):740–745. [DOI] [PubMed] [Google Scholar]
- 50.Kamtchum-Tatuene J, Noubiap JJ, Wilman AH, Saqqur M, Shuaib A, Jickling GC. Prevalence of High-Risk Plaques and Risk of Stroke in Patients With Asymptomatic Carotid Stenosis: A Meta-analysis. JAMA Neurol. 2020;77(12):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haussen DC, Grossberg JA, Bouslama M, et al. Carotid Web (Intimal Fibromuscular Dysplasia) Has High Stroke Recurrence Risk and Is Amenable to Stenting. Stroke. 2017;48(11):3134–3137. [DOI] [PubMed] [Google Scholar]
- 52.Compagne KC, van Es AC, Berkhemer OA, et al. Prevalence of carotid web in patients with acute intracranial stroke due to intracranial large vessel occlusion. Radiology. 2018;286(3):1000–1007. [DOI] [PubMed] [Google Scholar]
- 53.Lecler A, Obadia M, Savatovsky J, et al. TIPIC syndrome: beyond the myth of carotidynia, a new distinct unclassified entity. American Journal of Neuroradiology. 2017;38(7):1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]


