Abstract
Psychedelics are a class of drugs that produce unique subjective effects via agonist actions at the 5-hydroxytryptamine 2A receptor (5-HT2A). The 5-HT2A-mediated head twitch response (HTR) in rodents is used as a reliable proxy for psychedelic drug activity in humans, but existing methods for measuring HTRs require surgery or time-consuming visual scoring. In the present work, we validated a simple noninvasive method for quantitating HTRs using computer-based analysis of experimental video recordings. Male C57BL/6J mice received injections of the 5-HT2 receptor agonist (±)2,5-dimethoxy-4-iodoamphetamine (DOI; 0.03–3 mg/kg, s.c.) and were placed into cylindrical arenas. High frame rate videos were recorded via cameras mounted above the arenas. Antagonist experiments, which entailed pretreatment with the 5-HT2A antagonist M100907 (0.01 or 0.1 mg/kg s.c.) prior to DOI (1 mg/kg s.c.), were also recorded. The experimental videos were analyzed for HTRs using a newly developed feature of a commercial software package and compared to visual scoring carried out by trained observers. As expected, DOI produced dose-related increases in HTRs, which were blocked by M100907. Computer scoring was positively correlated with visual scoring, and no statistical difference between the two methods was found. The software captured nearly all visually observed HTRs, false positives induced by other behaviors (e.g., grooming) were rare and easily identified, and results were improved by optimizing lighting conditions. Our findings demonstrate the utility of combining high frame rate video recordings with commercial software analyses to measure HTRs, validating an additional reliable method to study psychedelic-like drug activity in mice.
Keywords: head twitch response, mice, computer software, psychedelics, 5-HT2A
The head twitch response (HTR) is a reliable unconditioned behavioral response displayed in rodents andother mammals, after administration of agonists of the 5-hydroxytryptamine 2A receptor (5-HT2A).1,2 The HTR was first observed in mice given intravenous lysergic acid diethylamide (LSD) and it was noted by the researchers that this behavioral response may be a reliable tool to study the effects of LSD and other known psychedelics such as mescaline and psilocin.3 HTR behavior in mice was later suggested to be a screening tool that could be used to predict psychedelic activity in humans,4 which was recently confirmed in studies showing a strong positive relationship between potency for HTR induced by a wide range of psychedelics in mice and the doses in humans that produce psychedelic subjective drug effects.5 Importantly, HTR in mice can discriminate psychedelic from non-psychedelic 5-HT2A agonists acting on cortical receptor populations.6 Due to these observations, the HTR in mice has been successfully used to assess the potential psychedelic activity of a wide range of compounds, test the efficacy of antipsychotic drugs, and study changes in 5-HT2A function.1,5,7
To facilitate measuring the HTR in various contexts, many research groups have developed methods to quantify this behavior in mice. The simplest and most widely used methods for quantifying HTRs involve either direct visual observation8−12 or a review of video recordings of rodents after administration of 5-HT2A agonists13−17 to tally the number of behavioral responses for each subject (i.e., visual scoring). Although visual scoring of HTRs is a viable strategy, it is time-consuming, suffers from potential observer bias, and requires the training of multiple behavioral raters. Methods employing visual scoring have been improved with the introduction of video-based tracking and magnetometer detection of HTRs, both of which require mice to be anesthetized for surgical placement of an object (plastic piece or magnet) on the dorsal surface of the head to facilitate recording and scoring.18,19 Magnetometer recording methods for detecting HTRs have been further refined to be semiautomated,19 and more recently fully automated, to capture visual counts with high accuracy.20,21 In addition to surgical placement of magnets to measure HTRs in magnetometer detection systems, data also support the use of magnets glued to aluminum ear-tags in these systems.22 While magnetometer detection systems have been well characterized for testing a wide range of psychedelic as well as non-psychedelic drugs, all available modern automated methods require surgical implantation of magnets or at least anesthesia for proper ear-tag placement.19−22
The current study was designed to validate a simple noninvasive method for quantifying HTRs using computer software analysis of video recordings. This novel scoring method takes advantage of a new feature of a commercially available software package (TopScan–Clever Sys Inc., Reston, VA) to allow automated behavioral scoring of mouse HTR events from high frame rate video recordings (Figure 1A–F; Supporting Information Videos 1 and 2). To test the hypothesis that computer software scoring of HTRs can recapitulate visual scoring by trained observers, pharmacological studies using a known 5-HT2 agonist, (±)2,5-dimethoxy-4-iodoamphetamine (DOI), were conducted in mice. The relationship between visual scoring and computer software-based scoring was evaluated to support the validation of a new computer software-based tool to score HTR in mice.
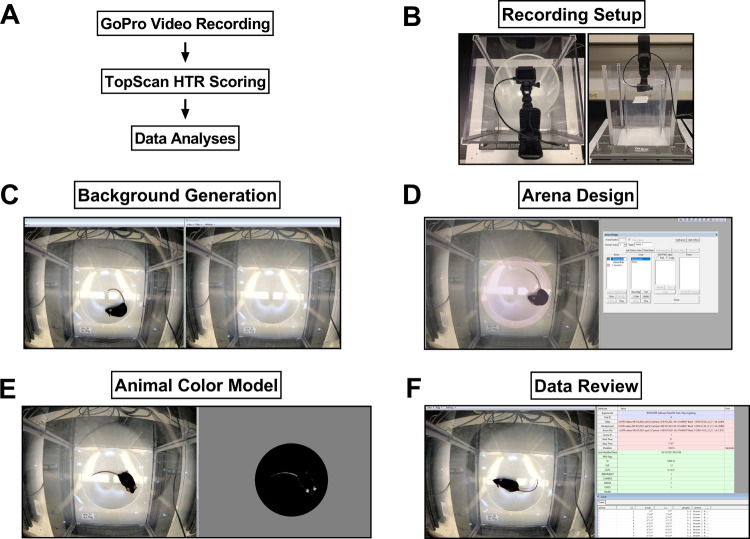
Figure 1.
Computer software-based HTR scoring method for recording and analyzing components. (A) Workflow for recording and analyses with the software-based system. (B) Aerial and side-view of the recording setup for analyzing HTRs in mice. Images of setting up the background (C), arena (D), and animal color model (E) files to run software scoring on recorded videos of mouse HTR experiments. (F) Image depicting the review of a video with experimental details and event details listed.
Results and Discussion
Comparison of Computer Software and Visual Scores in Dose–Response and Antagonist Studies with DOI
The experiments comparing computer scoring of HTRs to visual scoring were based on the well-established ability of DOI to induce HTRs in rodents.1 For dose–response experiments, C57BL/6J mice received s.c. doses of DOI (0.03–3 mg/kg) or their saline vehicle. For antagonist experiments, 1 mg/kg of DOI was administered 30 min after pretreatment with the selective 5-HT2A antagonist M100907 (0.01 or 0.1 mg/kg). HTRs were recorded for 30 min after DOI administration (Figure 1A,B). HTRs were tallied by two trained observers, as well as by a novel computer-based software video analysis method. Visual scores from both trained observers were positively correlated across the dose–response and antagonist pharmacological studies (Figure 2A), thus demonstrating that scoring from visual inspection of experimental videos is consistent across raters.
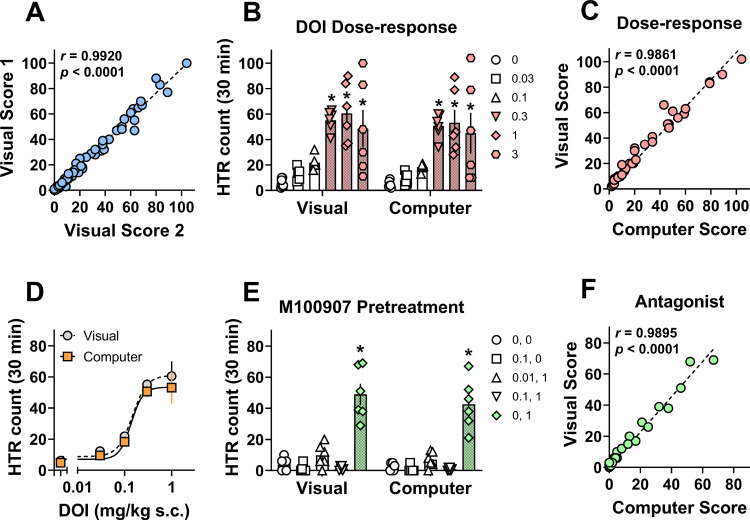
Figure 2.
Comparison of computer software and visual scoring methods to detect pharmacological effects of DOI in mice. (A) Correlation between visual scores tallied by two trained raters for dose–response and antagonist studies. HTRs were recorded for 30 min after DOI administration. (B, E) Two-way ANOVA comparisons of visual vs computer scores with Tukey’s post-test for dose–response and antagonist experiments. Filled values and bars with asterisks represent statistically significant within scoring method treatment differences (p < 0.05) versus respective vehicle controls (0 or 0, 0). (C) Correlation between average visual and computer scores for HTRs produced in dose–response studies with DOI (0.03–3 mg/kg s.c.). (D) Dose–response curves for DOI to produce HTRs as determined by visual and computer counts. ED50 potency values were 0.14 and 0.15 mg/kg s.c. for computer and visual counts, respectively. (F) Correlation between average visual and computer scores for HTRs produced in studies evaluating the ability of M100907 (0.01 or 0.1 mg/kg s.c.) administered 30 min prior to DOI (1 mg/kg s.c.) to block HTRs. Pearson r and p-values are shown in the figures for correlation analyses (C and F) or are reported in the associated text in the Results and Discussion Section for two-way ANOVAs (B and E).
Computer scores and visual scores for the dose–response studies were not statistically different (scoring method F1,60 = 0.74, p = 0.39; scoring method x dose interaction F5,60 = 0.03, p = 0.99), but a significant main effect of DOI dose was observed (F5,60 = 17.29, p < 0.0001). Post-hoc tests revealed that both scoring methods detected significant increases in HTR after 0.3, 1, and 3 mg/kg s.c. DOI when compared to vehicle controls (p < 0.05 Tukey’s test; Figure 2B; Table 1). Computer scores and visual scores for the DOI dose–response results were strongly and positively correlated (Figure 2C), indicating results from both scoring methods are similar. Finally, the ED50 potency values for both the computer (0.14 mg.kg s.c.) and visual (0.15 mg/kg s.c.) scores were nearly identical, again supporting the similarity of the counts for both scoring methods (Figure 2D).
Table 1. Dose–Response Effects of DOI to Produce HTR as Determined by Visual and Computer Scoring Methods.
| dose DOI (mg/kg s.c.) | visual score (mean ± SEM) | computer score (mean ± SEM) | n |
|---|---|---|---|
| 0 | 6.2 ± 1.5 | 4.8 ± 1.1 | 6 |
| 0.03 | 12.3 ± 1.9 | 9.5 ± 1.9 | 6 |
| 0.1 | 21.8 ± 2.3 | 18.3 ± 1.1 | 6 |
| 0.3 | 55.2 ± 3.3 | 50.7 ± 3.9 | 6 |
| 1 | 60.5 ± 9.5 | 53.2 ± 10.2 | 6 |
| 3 | 48.5 ± 14.7 | 45.0 ± 16.0 | 6 |
In antagonist studies, testing the ability of M100907 to block HTRs produced by DOI, no differences were found between visual and computer counts (scoring method F1,50 = 1.29, p = 0.26; scoring method × treatment interaction F4,50 = 0.28, p = 0.89; Figure 2E). Overall treatment effects were observed (F4,50 = 68.37, p < 0.0001), which revealed that the only group to produce significant HTR vs vehicle for both scoring methods was the M100907 vehicle + DOI group (p < 0.05 Tukey’s test). M100907 at both doses blocked HTRs produced by DOI (Figure 2E; Table 2). As observed for computer and visual scores from DOI dose–response studies, scores for the antagonist studies were strongly and positively correlated (Figure 2F), again suggesting a similarity between scores from both HTR scoring methods.
Table 2. Blockade of DOI-Induced HTR by M100907 as Determined by Visual and Computer Scoring Methods.
| dose M100907, DOI (mg/kg s.c.) | visual score (mean ± SEM) | computer score (mean ± SEM) | n |
|---|---|---|---|
| 0, 0 | 4.0 ± 1.6 | 2.2 ± 1.0 | 6 |
| 0.1, 0 | 1.2 ± 0.9 | 0.8 ± 0.8 | 6 |
| 0.01, 1 | 9.8 ± 2.9 | 7.0 ± 2.0 | 6 |
| 0.1, 1 | 1.3 ± 0.6 | 0.8 ± 0.4 | 6 |
| 0, 1 | 49.0 ± 6.8 | 42.7 ± 6.6 | 6 |
Importantly, our results with DOI dose–response and antagonist pharmacological studies are consistent with the previous literature, which demonstrate that DOI produces HTRs via actions at 5-HT2A receptors.1,6 The present results also support the conclusion that the counts detected by the software analysis and direct visual observation are very similar and statistically indistinguishable. Out of all of the HTRs identified by direct visual observation, the software identified 86% and only 2–3% of events were removed as false positives prior to data analyses when visually confirming events designated HTRs by the software (Figure 1F; Supporting Information Video 3). Altogether, these results suggest that the software was able to reliably detect a high number of HTRs identified by trained observers, and no statistical differences were observed between scoring methods.
Effects of Increased Lighting on Computer Software vs Visual Detection of DOI-Induced HTRs in Mice
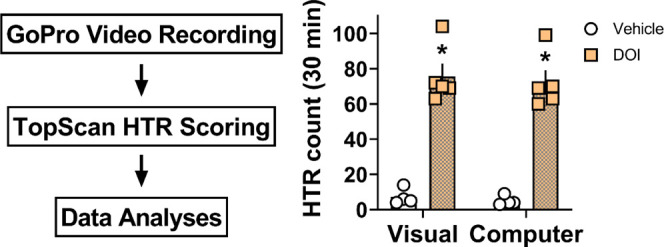
Based on the initial results, it was suggested by the software developers that increasing light levels and dispersion may improve the accuracy of the software counts vs visual counts for mouse HTR events. The developers noticed that in some parts of the videos, slight shadows and lighting inconsistencies throughout the testing arena may have hindered the detection of some HTRs by the software. To test this, experiments examining the ability of 1 mg/kg s.c. DOI to produce HTRs were conducted under conditions of increased illumination with GoPro Zeus Mini magnetic swivel clip accessory lights (GoPro) placed above the open field arenas (Figure 3A). The lights were set to 125 lumens, which provided additional light at the level of the mouse and increased the dispersion of light in the testing arena.
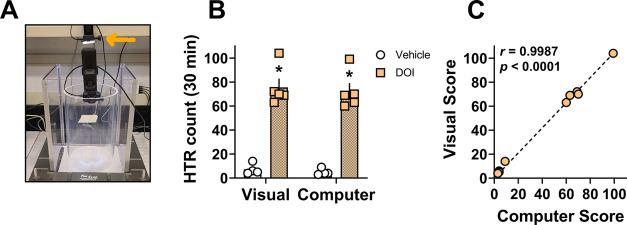
Figure 3.
Effects of increased lighting on the similarity of HTR scores from visual vs computer software-based detection methods. (A) Added GoPro light accessory to increase dispersed lighting in open field test arenas during experimental recordings. (B) Two-way ANOVA comparison of visual vs computer scores for experiments testing DOI vs saline to produce HTRs over the 30 min testing session with Tukey’s post-test. (C) Correlation between average visual scores and computer scores. Bars with asterisks represent statistically significant within scoring method treatment differences (p < 0.05) versus saline vehicle. Pearson r and p values are shown in the figure for the correlation analysis (C) or are reported in the associated text in the Results and Discussion Section for the two-way ANOVA comparison (B).
As expected, overall treatment differences were detected (F1,14 = 135.8, p < 0.0001) and DOI produced significantly more HTRs compared to vehicle controls for both the computer as well as visual scoring methods under increased lighting conditions (p < 0.05 Tukey’s test; Figure 3B; Table 3). Again, no statistical differences were found between scoring methods (scoring method F1,14 = 0.24, p = 0.63; scoring method x treatment interaction F1,14 = 0.009, p = 0.92). Visual scores from two trained raters were strongly and positively correlated for experiments examining the effects of increased light on the HTR scoring accuracy of the computer software-based scoring method (data not shown; Pearson r = 0.9978, p < 0.0001), indicating similarity of scores. Further, the average rater score and the software-generated scores for this experiment were also strongly and positively correlated, again supporting the similarity of visual vs computer scoring methods (Figure 3C). The false positive discovery rate was again low (2–3%), and all of these events were removed prior to data analyses in the data review stage to confirm HTRs (Figure 1F; Supporting Information Video 3). Finally, software scores in this set of experiments detected 94% of total HTRs identified by direct visual observation, supporting improved accuracy with increased illumination in the testing arenas.
Table 3. Effects of DOI vs Vehicle for Producing HTRs as Determined for Visual vs Computer Scoring Methods under Increased Lighting Conditions.
| dose DOI (mg/kg s.c.) | visual score (mean ± SEM) | computer score (mean ± SEM) | n |
|---|---|---|---|
| 0 | 7.3 ± 2.3 | 5.0 ± 1.4 | 4 |
| 1 | 75.6 ± 7.3 | 72.2 ± 6.9 | 5 |
Testing the Performance of Computer Software-Based HTR Detection for Potential False Positive Events
Amphetamine-Induced Hyperlocomotion and Stereotypy-like behavior
Since many psychedelic compounds can interact with non-5-HT2A sites and can induce complex behavioral effects,2,16,23−25 we sought to test whether the computer software-based system would generate false positive HTR events associated with other types of drug-induced behavioral responses. First, to test whether an increase in locomotor activity or repetitive movements (i.e., stereotypy-like behavior) can elicit false positive HTR events in the computer software-based system, we examined the dose-related effects of the known psychostimulant d-amphetamine (AMPH) in mice. As expected,26−29 the time-course data in Figure 4A illustrate that AMPH produced dose- and time-dependent effects on distance traveled over the 45 min testing session. The highest dose of 10 mg/kg produced an initial significant increase in total distance traveled over the first 5–15 min post injection (Figure 4B, F3,12 = 6.92 p = 0.0059, p < 0.01 vs vehicle) that dropped from 15 to 45 min back to the saline vehicle control level (Figure 4C, n.s. p > 0.05). AMPH (3 mg/kg) increased the distance traveled starting at approximately 15–20 min post injection (Figure 4A), which was significantly higher (F3,12 = 13.20 p = 0.0004) than vehicle controls for total distance traveled over the last 30 min of the testing session (Figure 4C, p < 0.001 vs vehicle).
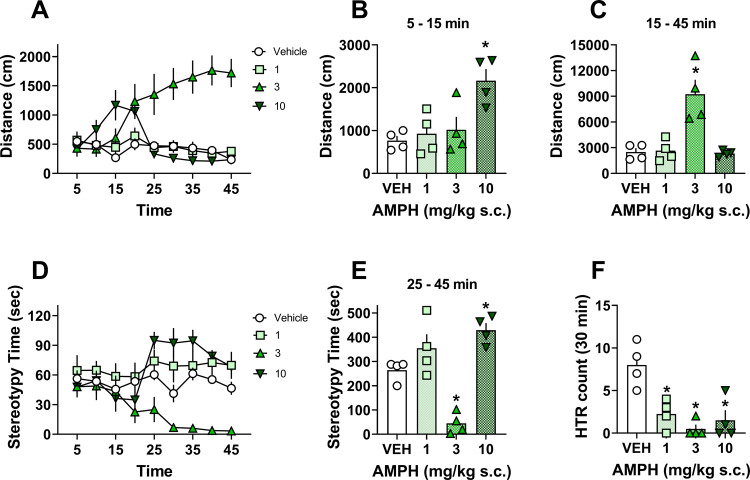
Figure 4.
Dose–response effects of amphetamine on locomotor activity, stereotypy-like behavior, and HTR in mice. (A) Time course of distance traveled after s.c. administration of saline vehicle, 1, 3, or 10 mg/kg amphetamine (AMPH). (B, C) One-way ANOVA comparisons of dose-related effects of AMPH vs vehicle on total distance traveled from 5 to 15 (B) or 15 to 45 (C) min post injection. (D) Time-course of time spent engaged in stereotypy-like repetitive locomotor behaviors. (E) One-way ANOVA comparison of total stereotypy time induced by AMPH vs vehicle. (F) One-way ANOVA results from software-based detection of HTR responses induced by AMPH vs vehicle controls. Bars with asterisks represent statistically significant treatment differences (Dunnett’s post-test, p < 0.05) versus saline vehicles. p-values are shown in the associated text in the Results and Discussion Section.
The time-course data also illustrate that AMPH produced dose- and time-dependent effects on time spent engaged in stereotypy-like behavior during the testing session (Figure 4D). Specifically, 10 mg/kg produced a significant increase (p < 0.05 vs vehicle), while 3 mg/kg AMPH exhibited a significant decrease (p < 0.01 vs vehicle) in total time spent engaged in stereotypy-like behavior for the last 20 min of the testing session (Figure 4E, F3,12 = 21.78 p < 0.0001). The increased stereotypy noted at 10 mg/kg likely explains the decrease in distance traveled starting at 20–25 min for this dose (Figure 4A), as mice were engaged in stereotypy-like behaviors instead of locomotor activity (Figure 4D,E). Conversely, the 3 mg/kg dose of AMPH produced a significant decrease in time spent engaged in stereotypy-like behavior that corresponded with an increase in distance traveled for the last 30 min of the testing session (Figure 4A,C). AMPH (1 mg/kg) produced no significant changes in behavior vs vehicle controls under these conditions. Ultimately, the AMPH data highlight that the mice were engaged in hyperlocomotion and stereotypy-like behaviors, which allowed the assessment of potential false positive HTR events associated with these behaviors.
The total number of HTRs was assessed over the last 30 min of the testing session using the computer software-based detection system. All doses of AMPH produced significant decreases in the total number of HTRs scored across the testing session vs vehicle controls (Figure 4F, F3,12 = 11.17 p = 0.0009). There were 11 false positive events discovered that were removed from the final HTR analysis. Ten of 11 false positive events were in the 10 mg/kg dose condition and seemed related to abnormal ear posture induced by stereotypy-like behavior at this dose. The other false positive count was triggered by an atypical quick head movement of a mouse treated with 3 mg/kg of AMPH. The discovery of a small number of false positive events associated with hyperlocomotion and stereotypy-like behavior reinforces the requirement of reviewing HTR events detected by the computer software prior to data analysis. Overall, AMPH-induced behavioral responses seemed to mask the basal level of HTRs seen in control mice, probably due to hyperlocomotion and engagement in stereotypy-like behavior. In summary, the computer software-based HTR detection system does not introduce false positive HTR counts due to hyperlocomotion per se, but some false positives are generated from stereotypy-like behaviors that alter canonical ear posture and head movements of rodents. Importantly, these false positives are easily identified and removed by reviewing specific HTR events prior to data analysis.
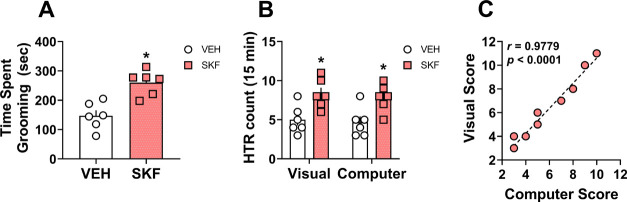
SKF-38393-Induced Grooming
Next, we tested the ability of drug-induced grooming events to elicit false positive HTR counts using the computer software-based HTR detection system. A dose of 10 mg/kg of the selective dopamine D1-like receptor partial agonist, SKF-38393, is known to reliably increase grooming time in mice and has been used in validation studies for magnetometer-based detection systems.19,20,30 Thus, we administered 10 mg/kg of SKF-38393 to mice and 15 min experimental sessions were captured on video, beginning 15 min after drug administration, and used to detect possible false positive HTR events using computer software scoring. The videos were assessed for HTRs and duration of grooming sequences via blinded visual scoring. As anticipated,19,30 SKF-38393 produced significantly more total time spent grooming over the testing session compared to vehicle controls (Figure 5A, t = 4.42 df = 10 p = 0.0013). SKF-38393 treatment (F1,20 = 19.35 p = 0.0003) also produced a slight increase in the total number of HTRs over the testing session, significantly higher than saline vehicle controls for both computer and visual counts (p = 0.03 and 0.02, respectively, Figure 5B). There was no statistically significant difference between scoring methods (F1,20 = 0.31 p = 0.58) and no scoring method x treatment interaction (F1,20 = 0.01 p = 0.91), and scores from both detection methods were robustly correlated (Figure 5C). Furthermore, the software picked up 75 out of 80 HTRs visually scored (94%). There were 3 grooming events in SKF-38393-treated mice incorrectly scored as HTR events by the software; however, this is in line with our reported false positive rate of ∼3% and again highlights the importance of reviewing events scored prior to data analysis. The overall data from SKF-38393 experiments suggest that the computer software-based HTR detection system is relatively insensitive to most potential false positive events evoked by normal or excessive grooming events.
Figure 5.
Effects of SKF-38393 on grooming duration and HTR scores from visual vs computer software-based detection methods. (A) Comparison of total time spent grooming in SKF-38393 (SKF) and saline vehicle-treated mice. (B) Two-way ANOVA comparison of visual vs computer scores for experiments testing SKF vs saline to produce HTRs with Tukey’s post-test. (C) Correlation between visual scores and computer scores for SKF experiments. Filled values and bars with asterisks represent statistically significant within scoring method treatment differences (p < 0.05) versus saline vehicle. Pearson r- and p-values are shown in the figures for correlation or are reported in the associated text in the Results and Discussion Section for two-way ANOVA analyses.
Advantages and Limitations of Using Computer Software-Based Scoring for HTR Detection
The data described in this report demonstrate that the software-based scoring of HTRs can be a useful tool for studying 5-HT2A receptor activity, with some advantages and limitations when compared to existing methods. Computer software-based scoring of HTR events offers several advantages over traditional visual scoring. Visual scoring either from video recordings13−17 or at several intervals during the testing session8−12 is cumbersome, time-intensive, subject to potentially biased scoring, and requires trained raters, all of which can be mitigated with the software scoring method described here or by use of magnetometer systems. Scoring with the software-based system is automated, guarding against biased scoring. Scoring with this system is also much less cumbersome and time-intensive as a result of automated computer software scoring. One can set up a batch of analyses and let the software run while completing other tasks, freeing up much of the time needed for traditional visual scoring from video recordings or being present during experiments to observe behavioral responses.
Magnetometer-based HTR scoring systems share many of the same advantages of software-based scoring relative to traditional visual scoring, due to the development of semi-19 as well as fully automated20,21 systems. However, all modern automated magnetometer-based methods require intracranial surgery and head magnet placement or anesthesia for proper ear-tag placement.22 Further, using head-mounted magnets or ear-tags has limitations in that they can detach from the skull, cause ulcerations or deformation of the ear, or become attached by magnetic attraction with tags of cage mates if group-housed.22,31 The software-based HTR scoring system is noninvasive by utilizing color contrast ear tracking and requires no objects to be placed on subjects for the recording of HTR events. Color contrast animal models for video analyses have also been used to detect other body parts for behavioral scoring, such as the paw for gait analyses,32 and this strategy may be amenable for use in detecting other features of animal behavior in various contexts.
Another benefit of the software-based HTR detection described here is that each event can be visually reviewed for each recording to remove any false positive events and confirm HTR events prior to data analysis (see Supporting Information Video 3). Magnetometer-based systems also have effective methods for filtering out and detecting false positive signals in data generated20−22 but lack any way to visually confirm these events as described for the software-based scoring. The ability to visually review video clips of HTR events, and have full video recordings of experimental sessions to conduct additional analyses or interrogate for other behavioral effects, is another advantage of the software-based detection system. Indoleamine psychedelics, like tryptamines (i.e., psilocybin) and lysergamides (i.e., LSD), are nonselective receptor agonists when compared to phenylalkylamine psychedelics (i.e., DOI), often leading to more complex behavioral effects in rodents.16,24,25,33,34 Therefore, having the ability to not only assess HTR behavior, but also other behavioral effects is an important experimental consideration that can be addressed with video-based behavioral analysis and optimized with automated software scoring.
Further comparing magnetometer-based vs the computer software-based system for scoring of HTRs, the software-based system is limited by the lack of ability to measure the dynamics of the head movement as in magnetometer-based systems.20−22 Magnetometer-based systems can discriminate between low-frequency, high-frequency, low-amplitude, and long-duration HTRs to study differences in dynamics between drug treatments or other experimental manipulations.21 The software-based HTR scoring system from Clever Sys Inc. identifies features of each response such as average velocity values for all head movements recorded for each HTR event scored in analyzed videos, but these parameters have not been explicitly validated for studying dynamics of HTR-induced head movements. Having the ability to assess the dynamics of the head movement, as in the magnetometer-based HTR detection systems, can be an important feature for filtering out false positive data and other non-HTR events.19,21 It may also be useful to study differences in the dynamics of HTR head movements between different classes of psychedelics or other novel compounds emerging from drug discovery efforts.7,35 Future studies could address whether software-based scoring is capable of capturing any information about head dynamics by comparing parameters scored in magnetometer and software-based HTR detections systems. Currently though, measuring dynamics of HTR head movements is only available by using magnetometer-based detection systems.
Accuracy for software-based HTR detection was found to be slightly lower (94%) than reported for magnetometer-based systems (96–99%) vs visually identified HTRs. However, no statistically significant differences were observed between visual scores and computer scores of HTRs, and scores from both methods were highly correlated in all of the present studies, supporting sufficient accuracy for use as another tool to examine HTR in drug discovery and neuroscience. Further, since comparisons to visual scoring are the standard for assessing the accuracy of most modern HTR detection systems19,20,22 and visual scores are known to show inter-rater variability,19,36 the differences between accuracies of magnetometer- and software-based systems may be negligible. Supporting this is the fact that the potency of (±)DOI in our studies (0.14 mg/kg s.c. for computer counts) is comparable to the potency of (−)DOI as detected in a magnetometer-based detection system.5 Further, the HTR counts at 1 mg/kg s.c. (±)DOI in our study (∼72 counts/30 min) were also comparable to the counts at 1 mg/kg i.p. (±)DOI (∼75 counts/30 min) as determined in a magnetometer-based detection system.22 This suggests that potency and maximal effects of DOI for HTR in mice were similar when determined in computer software- and magnetometer-based HTR detection systems.
Additionally, the noninvasive and “plug and play” nature of software-based detection may make it more easily adopted across research laboratories. Since the advent of semi- and fully automated magnetometer HTR detection systems, a few laboratories have adopted this method for studying HTR in mice,19−22,37 but many investigators still rely on visual scoring.7,11,13,25,38−42 Software-based HTR detection systems offer another option for research groups wishing to automate the scoring of HTR events with comparable reliability to magnetometer methods. False positives were generally low and can be easily removed, similar to event and signal filtering described for magnetometer-based detection systems. Additionally, false positives from hyperlocomotion and grooming were rare. There were a small number of false positive HTR events scored by the software from a stereotypy-induced change in ear posture that should be noted, but again these events are easily spotted for removal when reviewing prior to data analysis. Admittedly, the current studies did not assess the potential for false positive jumping events, which has been identified to be important in validation studies for magnetometer-based HTR detection systems.20−22 Despite this limitation and based on unpublished observations testing other psychedelics in HTR studies that sometimes induce jumping at high doses, we report that these events can be easily visualized and removed prior to data analysis as described herein.
One other disadvantage of software-based HTR detection vs modern magnetometer detection systems is the time for data acquisition. While the software-based HTR detection method described here greatly decreases the time spent scoring events vs visual scoring, the software-based method falls short of modern magnetometer methods in this regard. More specifically, software-based analyses of a single video lasting 30 min would take approximately 1 h due to the slower video speeds used. By comparison, the modern magnetometer-based HTR detection systems are capable of providing data in near real-time fashion after an experiment. Future improvements of the software-based detection platform should evaluate the potential for analyzing videos at higher speeds in real time to assess whether this limitation could be mitigated to provide data accurately under these conditions. Having multiple computers running HTR analysis software may also speed up total analysis times for software-based HTR detection to be more in line with the relative speed of magnetometer HTR systems. The software-based HTR detection system is dynamic in this sense, making it potentially adaptable across varying needs for both occasional and high-throughput use.
Finally, the strain of mice and rodent species used can influence the effects of DOI on HTR (see Canal and Morgan Table 1 for examples).1 Rats generally have a lower-frequency HTR compared to mice, and across strains of mice, there is variability in the frequency of HTR observed at 1 mg/kg DOI. C57BL/6J mice are most regularly used and produce a reliable HTR at this dose of DOI as well as across many different psychedelic agents,5 whereas other strains such as DBA-2J or C57BL/6N are more and less sensitive at the same dose of DOI.1 This highlights the importance of strain, substrain, and species when studying HTR in rodents. One potential issue is whether software-based scoring of HTR events can generalize to other strains of mice that differ in color from C57BL/6J mice or to other rodent species used to study 5-HT2A activity. We predict that the color contrast model should be able to accommodate scoring in other strains of mice and rodent species, but this may require the use of different background contrast colors, altered software color model settings, different arenas, and altered lighting conditions depending on the desired set up conditions. It is our view that pilot studies necessary for setting up the computer-based system in each specific laboratory could be optimized to measure HTR in other strains of mice and other rodent species.
Conclusions
Our results show that computer software-based scoring of HTRs is strongly correlated with direct visual scoring of HTRs from video recordings. The number of HTRs obtained by both scoring methods was statistically indistinguishable for dose response effects of DOI as well as in antagonist pretreatment studies administering the 5-HT2A antagonist, M100907, prior to DOI. The data further demonstrate that increasing light levels in the test area can improve the accuracy of computer software counts to more closely mimic counts obtained by direct visual observation. Few false positives were detected, and these events were easily identified and removed prior to data analyses. Overall, the present findings support the utility of this novel noninvasive computer software-based scoring method to study drug-induced HTRs in mice. This method provides another tool for the assessment of HTRs in mice that is reliable, noninvasive, and unbiased.
Methods
Animals
All experiments were performed using adult (2–4 months old) male C57BL/6J mice (The Jackson Laboratory) weighing 20–30 g. Mice were housed in the vivarium at the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA) in Baltimore MD. The animal facilities are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Mice were single-housed with ad libitum access to food and water, under standard 12 h light–dark conditions (lights on from 0700 to 1900 h). The NIDA IRP Animal Care and Use Committee approved all procedures described in the present studies.
Drugs
(±)-DOI hydrochloride (DOI) was purchased from Cayman Chemical and dissolved in a sterile 0.9% saline vehicle for drug administration. (+)-M100907 freebase, generously provided by Drs. A. Sulima and K. Rice, was initially dissolved in 100% DMSO and diluted to 1% DMSO/99% sterile saline (0.9%) vehicle for drug administration. d-amphetamine sulfate (NIDA IRP pharmacy) and SKF-38393 hydrochloride (Sigma) were both dissolved in a 0.9% sterile saline vehicle. All drug doses and their respective vehicles were administered subcutaneously (s.c.) at a volume of 0.01 mL/g body weight.
Experimental Design and Video Recordings
Hero Black 7 GoPro cameras (GoPro) were used to record high frame rate (120 frames per sec) overhead videos (960p resolution) of male C57BL/6J mice (Figure 1B) that received injections of the known 5-HT2 receptor agonist DOI or its saline vehicle. For dose–response experiments, DOI was administered s.c. at doses ranging from 0.03 to 3 mg/kg. For antagonist experiments, 1.0 mg/kg of DOI was administered 30 min after pretreatment with the 5-HT2A antagonist M100907 (0.01 or 0.1 mg/kg s.c.). On the day of an experiment, mice in their home cages were transported from the vivarium to the experimental test room and were given 1 h for acclimation. For the experimental sessions, mice received drug or vehicle injections and were placed into cylindrical acrylic arenas (7.5 in diameter) housed inside of TruScan mouse locomotor boxes (Coulbourn Instruments). The arenas had transparent floor panels with white bench paper underneath to provide a light background for contrast. GoPro cameras were mounted ∼ 10 inches above the arena floor, and experimental test sessions were recorded for 30 min post injection. All experiments occurred during the light phase of the light–dark cycle between 0900 and 1700 h local time. Subjects were randomized to treatment conditions and were repeat tested once per 1–2 weeks to avoid tolerance to the effects of DOI on HTR.1,43−45 After videos of each experiment were recorded, the video files were transferred to an external hard drive for storage until subsequent computer analysis as described below.
Computer Software-Based HTR Scoring
A commercially available software package (TopScan, Clever Sys Inc.) was adapted by the developer for use in measuring HTR. A custom feature of the TopScan software package monitors the ears of the mice to detect head movements that are classified as HTRs. All videos were analyzed for HTRs according to the developer’s instructions. Briefly, experimental details (mouse ID, treatment, date, experiment #, etc.) were entered into the software and the following three procedures were carried out for each video: (1) a background image file was generated to distinguish the arena and its background from the mouse, as shown in Figure 1C, (2) an arena file was generated that can be customized to the shape and area of any arena contained in each video, as shown in Figure 1D, and (3) an animal color model file used to track the ears was checked to ensure there was clear visualization of the ears for each set of videos, as shown in Figure 1E (See Supporting Information Videos 1 and 2). The animal color model is dynamic and can be adapted for different lighting conditions or to detect different color contrasts. In the present case, the animal color model was optimized for tracking lightly colored ears of C57BL/6J mice versus their dark-colored body under the lighting conditions of our recording setup. All of the experiments used the same animal color model except experiments that tested increased lighting conditions. In this case, the animal color model had to be adjusted to accommodate the increase in illumination. An ideal animal color model image to track the ears is shown in Figure 1E.
Using all three of the aforementioned parameters created for each video in the software, and the experimental information entered into the software database, we ran automated software scoring of experimental videos. The automated analysis can be done for each video one by one or in larger batches of videos scored one after the other, called “batch analyses.” The advantage of batch analyses is that once the software finishes scoring a given video, it will automatically move on to score the next video in a series designated by the user. The automated scoring of each video takes about twice the amount of time required for the actual video recording (for example, it takes 20 min to score a 10 min video). After videos are scored, all HTRs identified for each video can be quickly reviewed in the software as a list of short video segments that can be viewed and used to remove any potential non-HTR events or false positives as well as confirm HTRs (Figure 1F and Supporting Information Video 3). Finally, the data can be exported in several different formats for further processing and statistical analyses.
Trained Observer HTR Visual Scoring
Two trained observers watched the videos and visually scored the number of HTRs in 5 min bins for each 30 min experimental session. The total number of HTRs observed for each 30 min video was tallied. The visual scoring was carried out blind to treatment conditions, and HTRs from the two trained observers were averaged to determine the total number of HTRs per video as well as to assess the relationship to the software-based scoring method.
Amphetamine-Induced Locomotor Activity and Stereotypy-like Behavior
Mice (n = 4/dose) were injected subcutaneously with 0 (vehicle), 1, 3, or 10 mg/kg amphetamine and placed in the same arenas used for HTR detection. The testing session lasted for 45 min post injection and videos for HTR analyses were captured for the last 30 min of the session. Locomotor activity (distance traveled in cm) in the horizontal plane was recorded throughout the testing using TruScan photobeam arrays (Coulbourn Instruments). Stereotypy-like repetitive episodes (comprised of at least 3 movements less than 1.499 beam spaces occurring 2 sec or less apart) were also recorded with this system.
SKF-38393-Induced Grooming
SKF-38393 (10 mg/kg) or vehicle was administered s.c. and mice (n = 6/treatment) were placed in arenas used for HTR recordings for 30 min. The last 15 min was recorded for HTR analysis and for blinded visual scoring of the number of HTRs and duration of grooming events for each video.
Data Analysis and Statistics
Visual scores from the trained observers and computer scores from the software analyses were compared by two-way ANOVA (scoring method × treatment) with Tukey’s post-hoc multiple comparisons test to assess differences between scoring methods and effects of drug treatment. Pearson r correlations were computed to assess relationships between visual scores from trained observers and computer software scores. Potency (ED50) values were determined from dose–response studies using nonlinear regression of the rising phase of the curve. One-way ANOVA with Dunnett’s post-hoc multiple comparison test vs vehicle controls was used to evaluate treatment effects of AMPH on distance traveled, stereotypy-like repetitive locomotor behavior, and HTR. Unpaired Student’s t-test was used to compare the grooming time of SKF-38393 treatment vs vehicle controls. α was set at p < 0.05 for all analyses, which were conducted using GraphPad Prism 9 software.
Acknowledgments
This work was supported generously by the National Institute on Drug Abuse Intramural Research Program grant number DA-000523-13 (MHB). The authors would like to thank Clever Sys Inc. (Reston, VA) for allowing them to test out the software feature and for helpful feedback regarding the methods described as well as the initial setup of their system. The authors also thank Drs. A. Sulima and K. Rice (NIH) for providing M100907 for antagonist studies
Glossary
Abbreviations Used
- HTR
head twitch response
- 5-HT2A
serotonin 2A receptor
- DOI
2,5-dimethoxy-4-iodoamphetamine
- M100907
MDL 100,907
- LSD
lysergic acid diethylamide
- AMPH
d-amphetamine
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.1c00237.
Author Contributions
G.C.G. designed the study, conducted all experiments, analyzed the data, and wrote the manuscript; M.R.C. and S.A.M. scored experimental videos and contributed to the final version of the manuscript; T.W. designed the new feature of the commercially available software, contributed advice for set up of initial recordings, and provided comments on the final version of the manuscript; M.H.B. assisted with data interpretation and critically reviewed the manuscript.
The authors declare the following competing financial interest(s): G.C.G., S.A.M., M.C., and M.H.B. report no conflicts of interest related to the present work. T.W. works for Clever Sys Inc. and developed the head twitch detector feature for the commercially available TopScan platform.
Supplementary Material
References
- Canal C. E.; Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012, 4, 556–576. 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLER D. L.; UMBREIT W. W. “Permanent” Alteration of Behavior in Mice by Chemical and Psychological Means. Science 1956, 124, 723–724. 10.1126/science.124.3225.723. [DOI] [PubMed] [Google Scholar]
- Corne S. J.; Pickering R. W. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 1967, 11, 65–78. 10.1007/bf00401509. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Chatha M.; Klein A. K.; Wallach J.; Brandt S. D. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 2020, 167, 107933 10.1016/j.neuropharm.2019.107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J.; Weisstaub N. V.; Zhou M.; Chan P.; Ivic L.; Ang R.; Lira A.; Bradley-Moore M.; Ge Y.; Zhou Q.; Sealfon S. C.; Gingrich J. A. Hallucinogens Recruit Specific Cortical 5-HT2A Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 2007, 53, 439–452. 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Cameron L. P.; Tombari R. J.; Lu J.; Pell A. J.; Hurley Z. Q.; Ehinger Y.; Vargas M. V.; McCarroll M. N.; Taylor J. C.; Myers-Turnbull D.; Liu T.; Yaghoobi B.; Laskowski L. J.; Anderson E. I.; Zhang G.; Viswanathan J.; Brown B. M.; Tjia M.; Dunlap L. E.; Rabow Z. T.; Fiehn O.; Wulff H.; McCorvy J. D.; Lein P. J.; Kokel D.; Ron D.; Peters J.; Zuo Y.; Olson D. E. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021, 589, 474–479. 10.1038/s41586-020-3008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. L.; Raehal K. M.; Bohn L. M. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 1079–1084. 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek G. J. Activation of adenosine(1) (A(1)) receptors suppresses head shakes induced by a serotonergic hallucinogen in rats. Neuropharmacology 2009, 56, 1082–1087. 10.1016/j.neuropharm.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. A.; Stein A. R.; French H. T.; Murphy D. L. Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT). Br. J. Pharmacol. 2010, 159, 879–887. 10.1111/j.1476-5381.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal C. E.; Olaghere da Silva U. B.; Gresch P. J.; Watt E. E.; Sanders-Bush E.; Airey D. C. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology 2010, 209, 163–174. 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore J. S.; Decker A. M.; Sulima A.; Rice K. C.; Partilla J. S.; Blough B. E.; Baumann M. H. Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats. Neuropharmacology 2018, 142, 240–250. 10.1016/j.neuropharm.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Simoneau J.; Cohen M. S.; Zimmerman S. M.; Henson C. M.; Rice K. C.; Woods J. H. Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J. Pharmacol. Exp. Ther. 2010, 335, 728–734. 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. B.; Santini M. A.; Aznar S.; Knudsen G. M.; Rios M. Changes in 5-HT2A-mediated behavior and 5-HT2A- and 5-HT1A receptor binding and expression in conditional brain-derived neurotrophic factor knock-out mice. Neuroscience 2010, 169, 1007–1016. 10.1016/j.neuroscience.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. L.; Kurita M.; Holloway T.; López J.; Cadagan R.; Martínez-Sobrido L.; García-Sastre A.; González-Maeso J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J. Neurosci. 2011, 31, 1863–1872. 10.1523/jneurosci.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Koedood L.; Powell S. B.; Geyer M. A. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 2011, 25, 1548–1561. 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway T.; Moreno J. L.; González-Maeso J. HSV-Mediated Transgene Expression of Chimeric Constructs to Study Behavioral Function of GPCR Heteromers in Mice. J. Visualized Exp. 2016, e53717 10.3791/53717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. K.; Lee M. A.; Jarvik M. E. A device for analyzing drug-induced responses in freely moving mice. J. Exp. Anal. Behav. 1972, 18, 415–418. 10.1901/jeab.1972.18-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A. Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology 2013, 227, 727–739. 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M.; Shin J. M.; Vohra H. Z.; Hideshima K. S.; Schneck M.; Poklis J. L.; González-Maeso J. Fully automated head-twitch detection system for the study of 5-HT(2A) receptor pharmacology in vivo. Sci. Rep. 2019, 9, 14247 10.1038/s41598-019-49913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L. Automated detection of the head-twitch response using wavelet scalograms and a deep convolutional neural network. Sci. Rep. 2020, 10, 8344 10.1038/s41598-020-65264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M.; Vohra H. Z.; González-Maeso J. Automated quantification of head-twitch response in mice via ear tag reporter coupled with biphasic detection. J. Neurosci. Methods 2020, 334, 108595 10.1016/j.jneumeth.2020.108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 2011, 61, 364–381. 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J. C. Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology 2009, 203, 251–263. 10.1007/s00213-008-1356-8. [DOI] [PubMed] [Google Scholar]
- Rodriguiz R. M.; Nadkarni V.; Means C. R.; Pogorelov V. M.; Chiu Y.-T.; Roth B. L.; Wetsel W. C. LSD-stimulated behaviors in mice require β-arrestin 2 but not β-arrestin 1. Sci. Rep. 2021, 11, 17690 10.1038/s41598-021-96736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley S. L.; Thomas K. V. Influence of catecholamines on dexamphetamine-induced changes in locomotor activity. Psychopharmacology 1978, 58, 283–288. 10.1007/bf00427392. [DOI] [PubMed] [Google Scholar]
- Young J. W.; Goey A. K.; Minassian A.; Perry W.; Paulus M. P.; Geyer M. A. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology 2010, 208, 443–454. 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph R. J.; Paulus M. P.; Geyer M. A. Strain-specific effects of amphetamine on prepulse inhibition and patterns of locomotor behavior in mice. J. Pharmacol. Exp. Ther. 2001, 298, 148–155. [PubMed] [Google Scholar]
- Schindler C. W.; Thorndike E. B.; Partilla J. S.; Rice K. C.; Baumann M. H. Amphetamine-like Neurochemical and Cardiovascular Effects of α-Ethylphenethylamine Analogs Found in Dietary Supplements. J. Pharmacol. Exp. Ther. 2021, 376, 118–126. 10.1124/jpet.120.000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr B. S.; Starr M. S. Grooming in the mouse is stimulated by the dopamine D1 agonist SKF 38393 and by low doses of the D1 antagonist SCH 23390, but is inhibited by dopamine D2 agonists, D2 antagonists and high doses of SCH 23390. Pharmacol., Biochem. Behav. 1986, 24, 837–839. 10.1016/0091-3057(86)90421-1. [DOI] [PubMed] [Google Scholar]
- Kitagaki M.; Hirota M. Auricular chondritis caused by metal ear tagging in C57BL/6 mice. Vet. Pathol. 2007, 44, 458–466. 10.1354/vp.44-4-458. [DOI] [PubMed] [Google Scholar]
- Andersson L. S.; Larhammar M.; Memic F.; Wootz H.; Schwochow D.; Rubin C.-J.; Patra K.; Arnason T.; Wellbring L.; Hjälm G.; Imsland F.; Petersen J. L.; McCue M. E.; Mickelson J. R.; Cothran G.; Ahituv N.; Roepstorff L.; Mikko S.; Vallstedt A.; Lindgren G.; Andersson L.; Kullander K. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature 2012, 488, 642–646. 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. D.; Kavanagh P. V.; Twamley B.; Westphal F.; Elliott S. P.; Wallach J.; Stratford A.; Klein L. M.; McCorvy J. D.; Nichols D. E.; Halberstadt A. L. Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775). Drug Test Anal. 2018, 10, 310–322. 10.1002/dta.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav. Brain Res. 2015, 277, 99–120. 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.; Tjia M.; Mullen B.; Cao B.; Lukasiewicz K.; Shah-Morales S.; Weiser S.; Cameron L. P.; Olson D. E.; Chen L.; Zuo Y. An analog of psychedelics restores functional neural circuits disrupted by unpredictable stress. Mol. Psychiatry 2021, 26, 6237. 10.1038/s41380-021-01159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T. A.; Calil H. M. Screening hallucinogenic drugs: Systematic study of three behavioral tests. Psychopharmacologia 1975, 42, 163–171. 10.1007/BF00429548. [DOI] [PubMed] [Google Scholar]
- Cao D.; Yu J.; Wang H.; Luo Z.; Liu X.; He L.; Qi J.; Fan L.; Tang L.; Chen Z.; Li J.; Cheng J.; Wang S. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 2022, 375, 403–411. 10.1126/science.abl8615. [DOI] [PubMed] [Google Scholar]
- Dunlap L. E.; Azinfar A.; Ly C.; Cameron L. P.; Viswanathan J.; Tombari R. J.; Myers-Turnbull D.; Taylor J. C.; Grodzki A. C.; Lein P. J.; Kokel D.; Olson D. E. Identification of Psychoplastogenic N,N-Dimethylaminoisotryptamine (isoDMT) Analogues through Structure-Activity Relationship Studies. J. Med. Chem. 2020, 63, 1142–1155. 10.1021/acs.jmedchem.9b01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C.; Ly C.; Dunlap L. E.; Vargas M. V.; Sun J.; Hwang I. W.; Azinfar A.; Oh W. C.; Wetsel W. C.; Olson D. E.; Tian L. Psychedelic-inspired drug discovery using an engineered biosensor. Cell 2021, 184, 2779–2792.e2718. 10.1016/j.cell.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgrave N.; Troppoli T. A.; Wulff A. B.; Cole A. B.; Thompson S. M. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2022489118 10.1073/pnas.2022489118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraf T. S.; Felsing D. E.; Armstrong J. L.; Booth R. G.; Canal C. E. Evaluation of lorcaserin as an anticonvulsant in juvenile Fmr1 knockout mice. Epilepsy Res. 2021, 175, 106677 10.1016/j.eplepsyres.2021.106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Gray B. W.; Bailey J. M.; Smith D. A.; Hansen M.; Kristensen J. L. Hallucinogen-like effects of 2-([2-(4-cyano-2,5-dimethoxyphenyl) ethylamino]methyl)phenol (25CN-NBOH), a novel N-benzylphenethylamine with 100-fold selectivity for 5-HT2A receptors, in mice. Psychopharmacology 2015, 232, 1039–1047. 10.1007/s00213-014-3739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani N. A.; Martin B. R.; Glennon R. A. Withdrawal from chronic treatment with (+/-)-DOI causes super-sensitivity to 5-HT2 receptor-induced head-twitch behaviour in mice. Eur. J. Pharmacol. 1990, 186, 115–118. 10.1016/0014-2999(90)94066-7. [DOI] [PubMed] [Google Scholar]
- Gewirtz J. C.; Marek G. J. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 2000, 23, 569–576. 10.1016/s0893-133x(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Klein A. K.; Chatha M.; Laskowski L. J.; Anderson E. I.; Brandt S. D.; Chapman S. J.; McCorvy J. D.; Halberstadt A. L. Investigation of the Structure-Activity Relationships of Psilocybin Analogues. ACS Pharmacol. Transl. Sci. 2021, 4, 533–542. 10.1021/acsptsci.0c00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.