Abstract
Background
In the field of nanotechnology, the metallic nanoparticles are of remarkable interest because of their unique electronic, magnetic, chemical, and mechanical properties.
Purpose:
In the present work, silver nanoparticles (AgNPs) were synthesized using bio-reduction method.
Research Design:
Silver nitrate was used as metallic precursor and the extract of Moringa oleifera leaves with different concentrations was used as reducing as well capping agent. The extract exhibited strong potential in rapid reduction of silver ions for the synthesis of silver nanoparticles. The synthesized silver nanoparticles were characterized by UV-visible spectroscopy, X-ray diffraction (XRD), and scanning electron microscopy (SEM) techniques.
Results:
The absorption SPR peaks appeared in the range of 415 to 439 nm. SEM analysis exhibited that particles were spherical in shape with size distribution range from 10 nm to 25 nm. The synthesized silver nanoparticles were pure crystalline in nature as confirmed by the XRD spectra with average crystallite size 7 nm. In vitro antibacterial activity of the prepared silver nanoparticles colloidal samples as well the extract was studied using different concentrations of AgNPs (C1 = 100 μg/ml, C2 = 50 μg/ml, C3 = 25 μg/ml) by well diffusion method against Gram negative Escherichia coli. The antibacterial performance was assessed by measuring the zone of inhibition (ZOI).
Conclusions
The results suggested that AgNPs prepared by green approach can be considered as an alternative antibacterial agent.
Keywords: Moringa oleifera, AgNPs, antibacterial agent, Escherichia coli, green synthesis
Introduction
Nano-science deals with the materials manipulation and miracles at nanometer scale where materials properties behave significantly different from bulk material. 1 Nanotechnology has wide area of research, development, and industrial activity which has grown very quickly all over the world from the last decade. 2 The integration of biotechnology and nanotechnology has come up as bio-nanotechnology to grow eco-friendly and biosynthetic technology to synthesize nanomaterial. The assembly of atoms with size ranging 1–100 nm is known as nanoparticles. Metallic nanoparticles are extensively studied nowadays as they possess unique electrical, chemical, optical, catalytic properties and show good antibacterial activity due their large surface area to volume ratio. 3 Different kinds of nanomaterial like alginate 4 gold, magnesium 5 titanium, copper, zinc, 6 and silver are found. Sliver nanoparticles are reported to have strong antimicrobial activity among the most commonly used nanoparticles. 7
Nanotechnology is one of the major applications of nano-science. Nano scale material fabrication provides the basis of integration and control on molecules as well as atoms. For instance, DNA silicon chips are wonderful application of nanotechnology. Nanotechnology can be applied in almost every field like in military defense for missile system, medicine, astronomy, and computing. 8 There are number of latest devices and materials such as eyeliner, suntan lotion, sterile socks, and self-cleaning glass have been synthesized and fabricated with the help of nanotechnology. The injection of drug inside the human body with greater precision and accuracy is very strong application of the nanotechnology. According to scientists, nanofood will be effective for our stomach and prevents us from over eating. 9
Different methods like electrochemical reduction, photochemical reduction, heat evaporation and biological are used for the synthesis of silver nanoparticles. As reported in literature, these methods are very expensive and also involve in the use of hazardous chemicals which may cause the biological and environmental risks. Currently, silver nanoparticles synthesis from plants extract is receiving a greater attention because of environment friendly behavior. 10 The plants extract works as reducing agent as well as capping agent in synthesis of nanoparticles. Different plants extract such as M. oleifera (Horseradish), A. indica (Neeem), T. foenum-graecum (Fenugreek) and C. colocynthis (Bitter Apple) etc. have been successfully used for the synthesis of metal nanoparticles. 11
Recently, it has become a need of time to develop new bactericidal materials to resist the bacterial strains because antibiotics are not sufficient to overcome this. To handle these problems, it is very essential to fabricate these agents. Many antimicrobial agents have been created with the help of the nanotechnology although; the size of antitoxin is less than any other nanoparticle. The particles size range is from 5 to 10 nm and some particles size is in the range from 1 to 10 nm. Nanotechnology reduces and gives a compact shape which helps to increase the surface to volume ratio. Microscopic scale is 50 to 5000 times greater than the nanometer scale. 12 Metal nanoparticles have been shown their tremendous applications as antimicrobial agents and also play a vital role against various types of pathogenic viruses. So, nanomaterial provides a plate form for the invention of these antimicrobial reagents. Antimicrobial nanoparticles kill the bacteria by damaging cell membrane and in this way act as 1 of most important weapon against antibiotics resistant bacteria. 13
Different green synthesis methods such as polyoxometalates, pollens, polysaccharide, and irradiations are used for the synthesis of silver nanoparticles. Green synthesis defined as the practice of biologically friendly elements like plants, bacteria and fungi for nanoparticles synthesis. These striking green approaches are free of the short falls as per conventional synthetic approaches.14,15 One of them is the bio-organism in which plant extract is being used. It is environment friendly, having no toxicity effects as compared to chemical reduction methods. The plants extract works as reducing agent as well as capping agents making steady and exact shape of the AgNPs. Bio molecules in the plant extract such as amino acids, vitamins, enzymes, and proteins work to reduce the concentration of silver ions Ag+. Different plant extracts are used for this purpose to reduce the concentration of silver ions for instance moringa, bitter apple, fenugreek, neem, and many more.16,17
Food borne diseases are a major health issue in all over the world. According to WHO (2014) about 30% of the residents in industrial countries are infected by foodborne illness each year. The main source of these food borne diseases is the intake of contaminated food infected by bacteria, virus and fungal toxins in human. The food can be contaminated during pre or post harvesting due to minimal processing, transportation, handling procedures and during preparation. Commonly, foodborne pathogens that are found in different foods are Escherichia coli, Salmonella sp., Campylobacter sp., Listeria sp. and Clostridia sp. 18
The prevalence of infectious diseases has increased globally as the excessive and misuse of drugs result antibiotic resistance to the pathogens known as multidrug resistant pathogens. 19 These multidrug resistant pathogens lead to numerous health issues and economic damage. The multidrug resistant pathogens may cause cross contamination of foodstuffs during transport of food. Therefore, new and natural antibacterial agents are required to condemn these multidrug resistant food-borne pathogens. 20
M. oleifera is 1 of the magic plants that it preferably used as a medicine in traditional system. Because of its therapeutic properties, it has been used for many years. It is mostly reported as an antibacterial, antipyretic, antifungal, antiepileptic, antihypertensive, antiulcer, antitumor, diuretic, anti-inflammatory, antispasmodic, antidiabetic, and antioxidant. Different parts of M. oleifera such as leaves, seeds, peels, flowers and barks can be used for medicinal applications. Especially flowers are a rich source of phenolic acid, methionine, flavonoid, cysteine, antioxidant vitamin, tocopherol, potassium, calcium, β-carotene, and several different proteins.21-23 In the present study, M. oleifera leave extract was used for the synthesis of AgNPs by reducing the silver ions present in the silver nitrate. The main purpose of current study was the synthesis, optimization, characterization, and evaluation of antibacterial potential of these silver nanoparticles against E coli.
Materials and Methods
Sample Collection
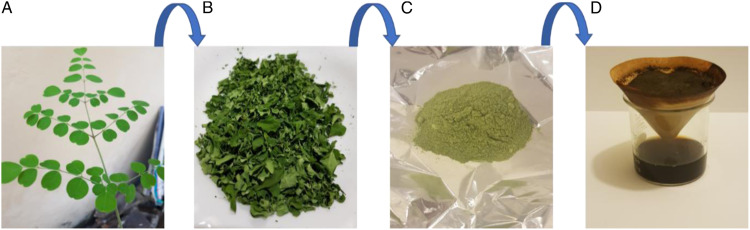
The M. oleifera leaves were collected from the botanical garden of university of the Punjab, Lahore, Pakistan and authenticated from the research center of the corresponding university. The leaves were washed with tap water four to five times before detached from the shoots. Then leaves were shade dried and fine powder was made using grinder. Silver nitrate (AgNO3) was obtained from chemistry department, University of the Punjab, Lahore, Pakistan.
M. oleifera Leaf Extract Preparation
For extract preparation 10 g fine powder of M. oleifera leaves was added into 100 mL deionized water. Then it was heated at 100°C for 20 to 30 minutes. The obtain extract was filtered using filter paper (Whatman no. 1) and stored at 4°C for further use.
Precursor Preparation
Silver nitrate (AgNO3) was used as precursor for the synthesis of silver nanoparticles from the M. oleifera leaves extract. 1 mM solution of silver nitrate was prepared using double distilled water and stored at 4°C in refrigerator.
Biosynthesis of Silver Nanoparticles
The green synthesis method was followed for the synthesis of silver nanoparticles. For the reduction of Ag+ ions, 1 mL M. oleifera leaves extract was added drop wise into 100 mL of 1 mM aqueous solution of AgNO3 and heated at 60–80°C for 1 hour. The change in color was observed from dark brown to reddish brown which indicated the formation of silver nanoparticles. Similarly, .5, 1, 2 and 2.5 mL of M oleifera extract was suspended for the preparation of S1, S2, S3, and S4 respectively (Table 1).
Table 1.
The detailed summary of the experimental processes for the synthesis of silver nanoparticles using the green synthesis method.
| Sample (AgNPs) | Precursor AgNO3 (1 mM) | Reducing agent (MLE) | R, Mlatio (Precursor/Reducing agent) | Reaction Time | Stirring | Temperature |
|---|---|---|---|---|---|---|
| S 1 | 50 mL | 0.5 | 1/100 | 60 mint | Constant | 60–80°C |
| S 2 | 50 mL | 01 | 1/50 | 60 mint | Constant | 60–80°C |
| S 3 | 50 mL | 02 | 1/25 | 60 mint | Constant | 60–80°C |
| S 4 | 50 mL | 2.5 | 1/20 | 30 mint | Constant | 60–80°C |
Substrate Cleaning and Sample Preparation
The glass slides were washed with double tap water using detergent. The slides were washed with acetone and IPA for 15 minutes repeatedly and dried with nitrogen gun. After cleaning the glass slides were placed on petri dishes and AgNPs solution was put on the glass slides drop by drop.
Characterization of Silver Nanoparticles
UV-VIS Absorption Spectroscopy
UV-Vis spectroscopy (Shimadzu, UV-1800, Japan) was used to measure the absorption in ultraviolet and visible range of electromagnetic spectrum. It can also confirm the quality of material. The formation of leaves extract mediated silver nanoparticles was confirmed by the spectral analysis. The UV-VIS spectra of the synthesized silver nanoparticles were recorded using Spectrometer by continuous scanning from 200 nm to 800 nm and for baseline correction; the distilled water was used as reference. 24
Scanning Electron Microscopy (SEM)
Scanning Electron Microscope (FEI Nova NanoSEM 450, USA) was used for the morphological analysis. The shape, size, and crystalline nature of silver nanoparticles were confirmed by the SEM. 25
X-Ray Diffraction Analysis
For structural analysis of the synthesized silver nanoparticles X-ray powder diffractometer (D-max IIA, Rigaku, Japan) was used. The data were collected in 2θ range. The crystalline domain size was calculated using D. Scherrer’s equation (
Where, D = average crystalline domain size, β denotes Full Width Half Maximum (FWHM);
K = .94, λ = wavelength of X-ray (1.54 A0) and θ is the Brag’s angle).26-28
Antibacterial Analysis
Antibacterial activity was evaluated by the method of Jain and Mehata. 29 The bacterial culture of Gram negative E coli strain was equipped by the standard procedures.
Growth of Bacteria
The E coli strain was grown in nutrient broth medium supplemented with 1 mM concentration of MLE-AgNPs, incubated at 30°C (constant pH 8.0) for 72 hours at 150 r/min to give aerobic conditions to the strain. After that interval the broth was centrifuged at 10,000 r/min for 10 minutes. OD was measured at different time intervals (6, 12, 18, 24, 30, 36, 42, 48, 54, 60 and 72 hours). Growth curves of E coli exposed and unexposed to MLE-AgNPs were evaluated by the absorbing value of OD600.
Well Diffusion Method
M.oleifera leaf extract and three different concentrations of silver nanoparticles were sterilized by exposure to ultraviolet radiations for 1 hour to eliminate any undesirable bacterial contamination. After sterilization of petri dishes, agar was poured into the petri plates. After solidification bacterial culture was evenly spread on nutrient agar plates with L-rod. The media was punched with 8 mm diameter holes and 20 μL aqueous samples of water, plant extract and three different concentrations of AgNPs were added in these wells by using micropipette. After 24 hrs of incubation at 37°C, the plates were observed for bacterial growth.
Determination of Minimum Inhibitory Concentration (MIC) of MLE-AgNPs
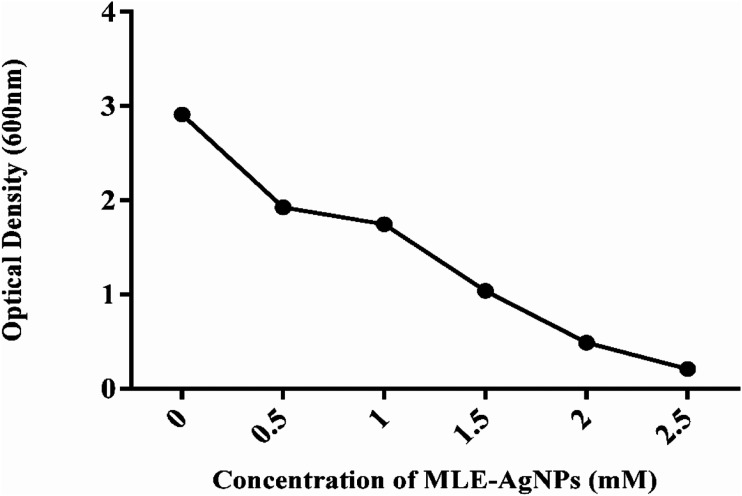
Pure bacterial culture was turbid in broth, to perceive the minimum inhibitory concentration of AgNPs at which the turbidity was totally eradicated and a clear solution was found. 30 The minimum inhibitory concentration (MIC) of MLE-AgNPs against E coli was evaluated by exposing the bacterium with MLE-AgNPs, with different volumes of growth medium. E coli cells were supplemented individually to 10 mL broth culture in 20 mL volume conical flasks. The final concentration of MLE-AgNPs was .5, 1.0, 1.5, 2, and 2.5 mM and concentration of E coli cells was 106 cfu/ml. These flasks were placed for 24 hours at 37°C at 150 r/min on shaking. After 24 hours growth rate and bacterial concentration were examined by determining optical density (OD) at 600 nm. (.1 OD600 corresponds to108 cell/ml).
Statistical Analysis
All statistical analysis was performed using graph pad prism version 6.01. All results in this paper were presented as the mean with standard error. Significance of differences was determined by one way and two-way analysis of variance (ANOVA) with (P < .05).
Results
M.oleifera leaf extract was prepared after washing, dried, and grinded leaves into powder form. The powder was mixed with deionized water to get the final extract. AgNO3 was mixed with different concentrations of M oleifera leaf extract to form MLE-AgNPs. The main sign of silver nanoparticle formation was the change in color from light yellow to dark brown within 60 min of reaction time. MLE works as a reducing agent as well as a capping agent and controls the agglomeration. Under the necessary conditions such as constant stirring and temperature, free silver atoms agglomerated and provided the basis for the formation of colloidal silver nanoparticles. Figures 1, 2, and 3 represented the M. oleifera leaf extract, synthesis of silver nanoparticles (MLE-AgNPs) and different levels of MLE-AgNPs respectively.
Figure 1.
Extract from M. oleifera leaves.
Figure 2.
Synthesis of silver nanoparticles using M. oleifera leaves extract.
Figure 3.
Four different types of AgNPs shown different colors along with extract and AgNO3.
UV-VIS Absorption Spectra Analysis
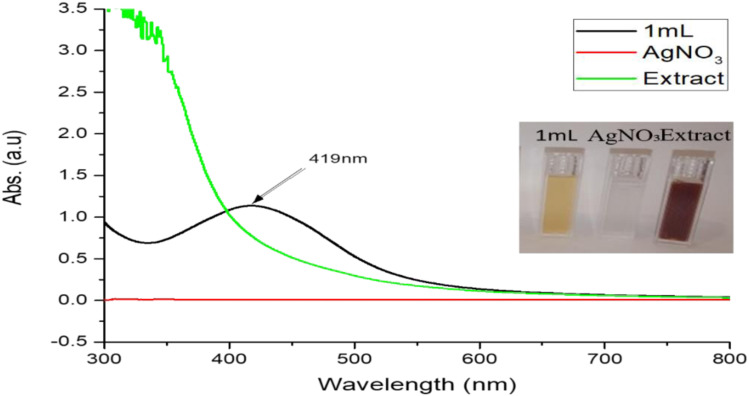
UV-VIS spectrophotometer was used for the determination of optical properties of M. oleifera leaves mediated AgNPs. The formation of the AgNPs was followed by measuring the SPR over the range of 300–800 nm. Prior studies displayed the silver nanoparticles (AgNPs) contributed to the absorption at around the 390–470 nm in the UV-VIS spectra. While in current study, Figure 4 shows the absorption band of the green synthesized Ag NPs. The UV-VIS spectra of AgNPs showed intense surface plasmon resonance (SPR) peak at around the 419 nm. The shape, size, morphology, and composition of the synthesized silver nanoparticles directly influenced the SPR bands.
Figure 4.
UV-Vis absorption spectra of silver nanoparticles AgNO3 and extract.
Effect of Extract Concentration
Concentration effect of MLE was also an interesting observation on the synthesis of silver nanoparticles. The concentration of the MLE on the preparation of the AgNPs was inspected by the UV-VIS spectroscopy. A significant effect was observed by the concentration on the morphology during UV-VIS analysis. The concentration of MLE varied from 1 mL to 10 mL in 90 mL AgNO3 (1 mM) other condition keeping constant. The shape and position of the SPR vary with concentration variation.
X-Ray Diffraction (XRD) Analysis
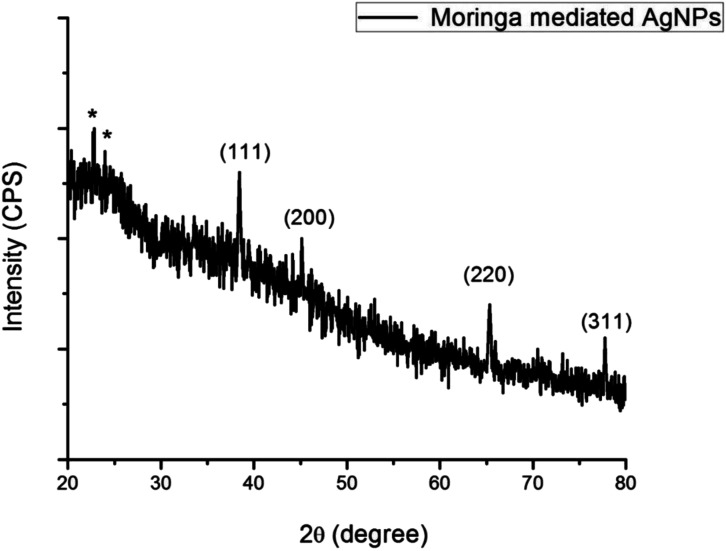
X-ray diffraction method was used for the investigation of the crystalline structure along with lattice characteristics of the synthesized AgNPs. The angle 2θ values ranging from 20° to 100° using Cu Kα whose wavelength range 1.5406 A°. Four sharp peaks can be seen clearly in the Figure 5 that appeared at 2θ values 38.4°, 44.5°, 64.90°, and 77.4°. These 2θ values are the representation of the following diffraction planes (111), (200), (220), and (311) of the FCC structure of the synthesized silver nanoparticles. The maximum intensity peak found at (111) plane that is the ideal orientation of the structure. There are some extra star peaks observed in the graph because of the biological components in extract. Crystallite size is 8.05 nm (Table 2) that can be calculated by using Debye Scherrer’s formula
Figure 5.
X-ray diffraction spectra of M. oleifera leaf extract.
Table 2.
A brief summary of silver nanoparticle obtained from XRD results.
| Sample | Peak Position (2θ) | Diffraction Plane (hkl) | FWHM, (β) radians | Lattice Parameter (a), nm | Crystalline Size (D),nm |
|---|---|---|---|---|---|
| S | 38.4° | 111 | .0221 | .404 | 8.05 |
Scanning Electron Microscopy Analysis
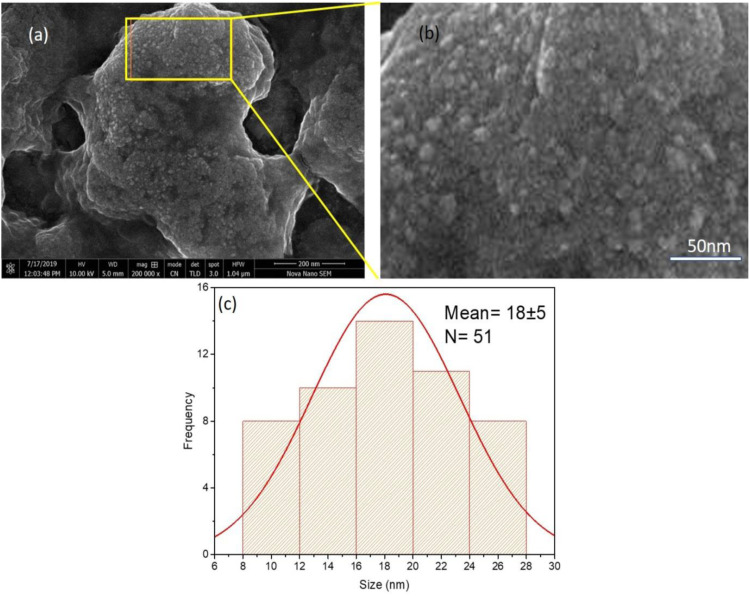
SEM (scanning electron microscope) was used to determine the size and morphology of the synthesized AgNPs. Structural studies done by SEM showed the presence of phenolic compounds, amino acids, waxes, antioxidants vitamins, and flavonoids. SEM image and its particular magnified image along with histogram are shown in Figure 6. The magnified SEM image of sample S showed the agglomeration of the silver nanoparticles. Observation shown that mostly particles are of spherical in shape, the particles size distribution is in the range of 10 to 25 nm and their average size is 18 nm revealed by the histogram (Table 3).
Figure 6.
(a) SEM image of AgNPs, (b) Particular section magnified image (c) Particle size distribution histogram.
Table 3.
Results obtained from UV-VIS and SEM analysis.
| Sample | Color | Absorbance Peak | Shape | Average size (nm) |
|---|---|---|---|---|
| AgNPs | Red | 439 | Spherical | 18 |
| AgNPs | Black | 419 | Spherical | 18 |
Antibacterial Activity
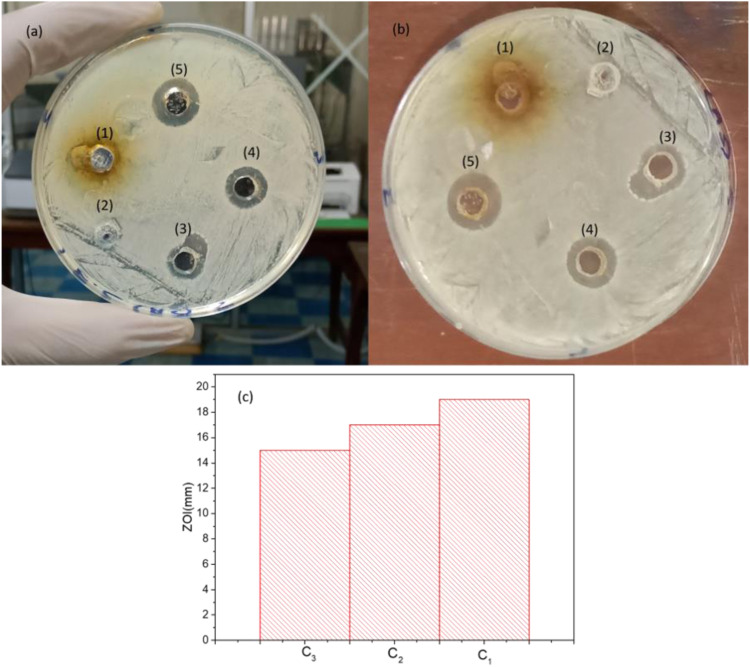
Agar well diffusion method was used to investigate the antibacterial activity of the synthesized silver nanoparticles against the E coli bacterium. Different concentrations of the silver nanoparticles of sample ‘s’ along with leaves extract and pure water as negative control were tested to diminish E coli. The zone of inhibition around the wells was the representation of antibacterial activity of silver nanoparticles (Figure 7). The zone of inhibition were 19 mm, 17 mm, and 15 mm for the concentration C1 (100 µg/ml), C2 (50 µg/ml), and C3 (25 µg/ml), no zone of inhibition appeared for pure water and MOF extract. The antibacterial activity of the synthesized AgNPs increases steadily as the stack solution increases.
Figure 7.
(a and b) Antibacterial activities of AgNPs against E. coli. (c) ZOI histogram.
Minimum Inhibitory Concentration
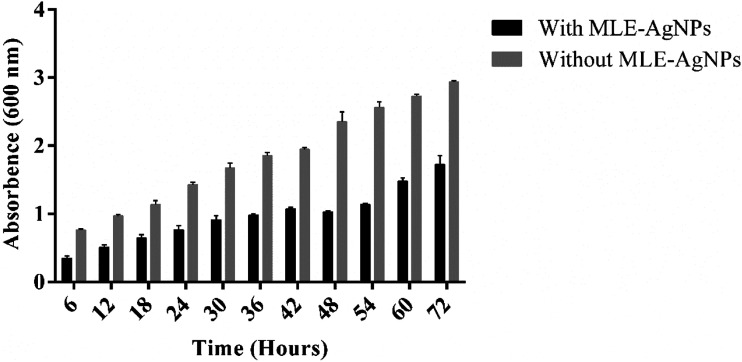
The growth curve of E coli by measuring the OD600 with different concentrations of MLE-AgNPs is shown in Figure 8. The concentrations .5, 1.0, 1.5, 2 and 2.5 mM of MLE-AgNPs, the growth curve of E coli includes three phases: lag, exponential, and stabilization phase. Though, decline phase could not be exposed since we evaluated the total number of E coli, (live and dead) based on OD600. E coli reached exponential phase promptly when there was no MLE-AgNPs. E coli growth with different time intervals was also measured and maximum inhibition was observed in silver nanoparticles compared to without silver nanoparticles as shown in Figure 9. While increasing the concentration of MLE-AgNPs more delay takes place in growth and vice versa. When concentration was 5 mM, there was no growth of E coli within one week, representing the minimum inhibitory concentration (MIC) of MLE-AgNPs to E coli was 5 mM.
Figure 8.
Effect of different concentrations of (MLE-AgNPs) on the growth of E. coli.
Figure 9.
Effect of (MLE-AgNPs) on the growth of E. coli at different time interval.
Discussion
Previously, numerous studies were performed for synthesize AgNPs with significant antimicrobial activities from plant extracts like leaf extracts of Acalypha indica, 17 Solidago altissima, 16 Xanthium strumerium L, 22 Murraya koenigii (curry leaf), 23 Ocimum sanctum (Tulsi leaf)8,15 seed extracts of Acacia farnesiana, 24 Macrotyloma uniflorum, 26 root extracts of Trianthema decandra, 27 stem extracts of Ocimum sanctum, 8 and even fruit extracts of Musa paradisiaca (banana) peels 28 and Carica papaya. 31 In these studies, phytocompounds in the plant extract serve as reducing and/or capping agent in the reaction with silver nitrate (AgNO3), a commonly used precursor in silver nanoparticle synthesis. 32 . According to Šileikaitė et al., 33 during the synthesis of silver nanoparticles by green synthesis method, the original mechanism happened can be explained by liquefying the metallic silver nitrate into deionized water. Initially, silver nitrate changes into silver ions. As the M. oleifera leaves extract added into AgNO3 reduction process occurred and silver ions reduced into free metallic atoms (Ag). MLE works as a reducing agent as well as a capping agent and controls the agglomeration. Under the necessary conditions such as constant stirring and temperature, free silver atoms agglomerated and provided the basis for the formation of colloidal silver nanoparticles.
The surface Plasmon resonance shifted toward red shift by increasing concentration and resulted stable particles because larger functional ligand as reported by Bar et al. 13 But our prepared extract was too much thick and on large concentration there was no peak observed in UV-VIS spectra. As we reduced the concentration, the SPR shifted towards blue correspondingly that resulted smaller size particles which are more stable because of large surface area. By diluting the extract, clear peak was obtained but peak position remained same. We used to optimize AgNPs for further characterization.
The strength of MLE-AgNPs might be as a consequence of capping agents in M. oleifera leaves, like proteins, polysaccharides or phenolic composites as attributed by Khan et al. 34 The results are in accordance to Sur et al. 35 where the absorption peaks were arising at 401 nm due to the excitation of local plasmon and the fluctuations of the transmission electrons of gold and silver nanoparticles which resembled to the distinct transverse plasmon that was a spherical silver nanoparticle.
According to Öztürk et al., 36 XRD showed the angle (2θ) peak at 37.8° which indicated the pure silver nanoparticles. Furthermore, X-ray diffraction investigations specified that these MOS-AgNPs (Nanoparticles) were crystalline in nature. There were also (2θ) angle peaks at 42°, 65°, and 78° companionable to 200, 220, and 311 silver planes respectively. Besides, the dispersed peaks in the XRD configuration might be due to the presence of biological levels that happened on the exterior of MOS-AgNPs. 37 investigated that the diffraction crests obtained were wide might be due to small crystalline sizes.
A similar study was conducted by Mehwish et al. 38 in which SEM image of the MOS-AgNPs (Silver nanoparticles with M. oleifera seeds) shown a uniform shape and globular exterior structure. Chemical investigations of MOS-AgNPs showed the existence of silver and other components with silver masses. Ag was there as a result of the development of MOS-AgNPs, although the further elements were from M. oleifera extract. In the current learning, the average size of MOL-AgNPs was 10 to 25 nm which is in agreement with the results of 39 who found MOS-AgNPs size 10.2 nm and Enicostemma axillare (Lam.) leaf extract with an average size of 18.12 nm respectively. According to Sur et al., 35 the size of nanoparticles varies between 20 and 40 nm, while the average size of silver nanoparticles was typically about 30 nm with virtually globular in shape and nearly monodispersed in nature.
These results are in comparison with Masurkar et al. 40 where the antimicrobial activity of silver nanoparticles contrary to E coli was extensively described. According to the results, the rumors on the antibacterial action in contrast to S. aureus were inconstant where some claiming exceptional actions of AgNPs against S aureus, however, others reported nothing or insignificant activity against the same S aureus strain. These different results might be due to the dissimilar shapes of AgNPs acquiescent from variable synthetic procedures. 41 The results as suggested by Premanathan et al. 42 antibacterial activity of silver nanoparticles contrary to various bacteria was shape dependent. According to Khan et al. 43 who experimented silver nanoparticles on different Gram negative and Gram positive bacterial strains, the following order was observed E. coli > P. aeruginosa > M. luteus > S. aureus with the toxicity of silver nanoparticles. Therefore, it can be quantified from several results that the synthesized AgNPs are more operational against Gram negative bacteria than Gram positive bacteria. Similar to our results where E coli is more prone to even less concentration of MLE-AgNPs. Similar results were also found from green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity against E. coli, P. aeruginosa, and S. aureus. 44 In short, results represented that it is possible to synthesize silver nanoparticles from leaves extract of M oleifera which is efficient, simple and quick techniques with good antibacterial properties.
Conclusion
Conclusively, M. leifera leaves extract produce extracellular AgNPs with good stability power in the solution. The synthesized silver nanoparticles were operated as an active substrate against E coli which is recognized resistant against maximum commercially available drugs. This green synthesis of silver nanoparticles may prove a quick, cost free and suitable alternative of synthetic antibiotics against multi drugs resistance bacteria. Finally, biosynthesis of silver nanoparticles proved to be an ideal potential candidate for the medical application where antimicrobial activity is essential.
ORCID iDs
Riffat Yasmin https://orcid.org/0000-0002-5754-571X
Rizwan Asif https://orcid.org/0000-0002-0061-7233
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Whitesides GM. Nanoscience, nanotechnology, and chemistry. Small. 2005;1(2):172-179. [DOI] [PubMed] [Google Scholar]
- 2.Filipponi L, Sutherland D. Interdisciplinary Nanoscience Center (iNANO). Denmark: Aarhus University; 2010. [Google Scholar]
- 3.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76-83. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad Z, Pandey R, Sharma S, Khuller GK. Alginate nanoparticles as antituberculosis drug carriers: Formulation development, pharmacokinetics and therapeutic potential. Indian J Chest Dis Allied Sci. 2006;48(3):171-176. [PubMed] [Google Scholar]
- 5.Gu H, Ho PL, Tong E, Wang L, Xu B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Letters. 2003;3(9):1261-1263. [Google Scholar]
- 6.Schabes-Retchkiman PS, Canizal G, Herrera-Becerra R, Zorrilla C, Liu HB, Ascencio JA. Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles. Opt Mater. 2006;29(1):95-99. [Google Scholar]
- 7.Gong P. Preparation and antibacterial activity of Fe3O4 Ag nanoparticles. Nanotechnology. 2007;18(28):285-604. [Google Scholar]
- 8.Anand K, Tiloke C, Phulukdaree A, et al. Biosynthesis of palladium nanoparticles by using Moringa oleifera flower extract and their catalytic and biological properties. J Photochem Photobiol B Biol. 2016;165:87-95. [DOI] [PubMed] [Google Scholar]
- 9.Silva GA. Introduction to nanotechnology and its applications to medicine. Surg Neurol. 2004;61(3):216-220. [DOI] [PubMed] [Google Scholar]
- 10.Verma A, Mehata MS. Controllable synthesis of silver nanoparticles using neem leaves and their antimicrobial activity. J Radiation Res Applied Sci. 2016;9(1):109-115. [Google Scholar]
- 11.Velusamy P, Das J, Pachaiappan R, Vaseeharan B, Pandian K. Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L.). Ind Crop Prod. 2015;66:103-109. [Google Scholar]
- 12.Namratha N, Monica P. Synthesis of silver nanoparticles using Azadirachta indica (Neem) extract and usage in water purification. AJP Tech. 2013;3(4):170-174. [Google Scholar]
- 13.Bar H. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A Physicochem Eng Asp. 2009;339(1-3):134-139. [Google Scholar]
- 14.Patra JK, Baek KH. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J Nanomater. 2014. [Google Scholar]
- 15.Varthini T, Bai GCV, Mani RJ. Green Synthesis of Silver Nanoparticles Using Seed Extract of Moringa Oleifera Medicinal Plant, 2018. [Google Scholar]
- 16.Satyavani K, Ramanathan T, Gurudeeban S. Green synthesis of silver nanoparticles by using stem derived callus extract of bitter apple (Citrullus colocynthis). Dig J Nanomater Biostruct. 2011;6(3):1019-1024. [Google Scholar]
- 17.Angelina EDR, Bavyaa R, Rajagopal R. Green synthesis and characterization of silver nanoparticles using Fenugreek seed extract. Int J Sci Res Pub. 2013;3(7):1-3. [Google Scholar]
- 18.Das SK, Khan MMR, Guha AK, Das AR, Mandal AB. Silver-nano biohybride material: synthesis, characterization and application in water purification. Bioresour Technol. 2012;124:495-499. [DOI] [PubMed] [Google Scholar]
- 19.Tanwar M, Meena J, Meena LS. Nanoparticles: Scope in drug delivery. Adv Biomat Biodevices. 2014;487. [Google Scholar]
- 20.Woh PY, Thong KL, Lim YAL, Behnke JM, Lewis JW, Mohd Zain SN. Microorganisms as an indicator of hygiene status among migrant food handlers in Peninsular Malaysia. Asia Pac J Publ Health. 2017;29(7):599-607. [DOI] [PubMed] [Google Scholar]
- 21.Bindhu MR, Sathe V, Umadevi M. Synthesis, characterization and SERS activity of biosynthesized silver nanoparticles. Spectrochim Acta Mol Biomol Spectrosc. 2013;115:409-415. [DOI] [PubMed] [Google Scholar]
- 22.Surendra TV, Roopan SM. Photocatalytic and antibacterial properties of phytosynthesized CeO2 NPs using Moringa oleifera peel extract. J Photochem Photobiol B Biol. 2016;161:122-128. [DOI] [PubMed] [Google Scholar]
- 23.Vasanth K, Ilango K, MohanKumar R, Agrawal A, Dubey GP. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf B Biointerfaces. 2014;117:354-359. [DOI] [PubMed] [Google Scholar]
- 24.Perkampus HH. UV-VIS Spectroscopy and its Applications. SSBM; 2013. [Google Scholar]
- 25.Nasiriboroumand M, Montazer M, Barani H. Preparation and characterization of biocompatible silver nanoparticles using pomegranate peel extract. J Photochem Photobiol B Biol. 2018;179:98-104. [DOI] [PubMed] [Google Scholar]
- 26.Warren BE. X-Ray diffraction methods. J Appl Phys. 1941;12(5):375-384. [Google Scholar]
- 27.Pecharsky V, Zavalij P. Fundamentals of Powder Diffraction and Structural Characterization of Materials. SSBM; 2008. [Google Scholar]
- 28.Suryanarayana C, Norton M. X-ray Diffraction: A Practical Approach. New York: SSBM; 2013. [Google Scholar]
- 29.Jain S, Mehata MS. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep. 2017;7(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Qu F, Xu H, et al. Role of reactive oxygen species in the antibacterial mechanism of silver nanoparticles on Escherichia coli O157:H7. E. coli O157: H7Biometals. 2012;25(1):45-53. [DOI] [PubMed] [Google Scholar]
- 31.Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9(5):2673-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moodley JS, Krishna SBN, Pillay K, Sershen fnm, Govender P. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv Nat Sci Nanosci Nanotechnol. 2018;9(1):015011. [Google Scholar]
- 33.Šileikaitė A. Analysis of silver nanoparticles produced by chemical reduction of silver salt solution. Mater Sci. 2006;12(4):1320-1392. [Google Scholar]
- 34.Khan MA, Khan T, Nadhman A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv Colloid Interface Sci. 2016;234:132-141. [DOI] [PubMed] [Google Scholar]
- 35.Kumar Sur U, Ankamwar B, Karmakar S, Halder A, Das P. Green synthesis of silver nanoparticles using the plant extract of Shikakai and Reetha. Mater Today Proc. 2018;5(1):2321-2329. [Google Scholar]
- 36.Öztürk BY, Gürsu BY, Dağ İ. Antibiofilm and antimicrobial activities of green synthesized silver nanoparticles using marine red algae. Gelidium corneum. Process Biochem. 2020;89:208-219. [Google Scholar]
- 37.Nayak D, Ashe S, Rauta PR, Kumari M, Nayak B. Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater Sci Eng C. 2016;58:44-52. [DOI] [PubMed] [Google Scholar]
- 38.Mehwish HM. Green synthesis of a silver nanoparticle using Moringa oleifera seed and its applications for antimicrobial and sun-light mediated photocatalytic water detoxification. J Environ Chem Eng. 2021;9(4):1-13. [Google Scholar]
- 39.Raj S, Chand Mali S, Trivedi R. Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem Biophys Res Commun. 2018;503(4):2814-2819. [DOI] [PubMed] [Google Scholar]
- 40.Masurkar SA, Chaudhari PR, Shidore VB, Kamble SP. Effect of biologically synthesised silver nanoparticles on Staphylococcus aureus biofilm quenching and prevention of biofilm formation. IET Nanobiotechnology. 2012;6(3):110-114. [DOI] [PubMed] [Google Scholar]
- 41.Liu HL, Dai SA, Fu KY, Hsu SH. Antibacterial properties ofsilver nanoparticles in three different sizes and their nanocomposites with a new waterborne polyurethane. Int J Nanomed. 2010;5(1):1017-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed Nanotechnol Biol Med. 2011;7(2):184-192. [DOI] [PubMed] [Google Scholar]
- 43.Khan M, Khan ST, Khan M, et al. Antibacterial properties of silver nanoparticles synthesized using Pulicaria glutinosa plant extract as a green bioreductant. Int J Nanomed. 2014;9(1):3551-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garibo D, Borbón-Nuñez HA, Díaz de León JN, et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci Rep. 2020;10(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]