Abstract
Background
Cognitive behavioral therapy (CBT) is a first-line psychotherapeutic treatment that has been recommended for psychiatric disorders. Prior neuroimaging studies have provided preliminary evidence suggesting that CBT can have an impact on the activity of brain regions and functional integration between regions. However, the results are far from conclusive. The present article aimed to detect characteristic changes in brain activation following CBT across psychiatric disorders.
Method
Web of Science, Cochrane Library, Scopus, and PubMed databases were searched to identify whole-brain functional neuroimaging studies of CBT through 4 August 2021. To be included in the meta-analysis, studies were required to examine functional activation changes between pre-and post-CBT. The included studies were then divided into subgroups according to different task paradigms. Then, an activation likelihood estimation algorithm (ALE) was performed in the different meta-analyses to identify whether brain regions showed consistent effects. Finally, brain regions identified from the meta-analysis were categorized into eight functional networks according to the spatial correlation values between independent components and the template.
Results
In total, 13 studies met inclusion criteria. Three different meta-analyses were performed separately for total tasks, emotion tasks, and cognition tasks. In the total task ALE meta-analysis, the left precuneus was found to have decreased activation. For the cognition task ALE meta-analysis, left anterior cingulate (ACC) and left middle frontal gyrus (MFG) were found to have decreased activation following CBT. However, the emotion task ALE meta-analysis did not find any specific brain regions showing consistent effects. A review of included studies revealed default mode network (DMN), executive control network (ECN), and salience network (SN) were the most relevant among the eight functional networks.
Conclusion
The results revealed that the altered activation in the prefrontal cortex and precuneus were key regions related to the effects of CBT. Therefore, CBT may modulate the neural circuitry of emotion regulation. This finding provides recommendations for the rapidly developing literature.
Keywords: cognitive behavioral therapy (CBT), psychiatric disorder, neuroimaging, meta-analysis, brain network
Introduction
Cognitive-behavioral therapy (CBT) is a first-line psychotherapeutic treatment that has been proven effective in treating a variety of psychiatric disorders such as major depressive disorder (MDD), anxiety disorder (AD), and obsessive-compulsive disorder (OCD) (Butler et al., 2006; Beck and Dozois, 2011; Hofmann et al., 2012; McMain et al., 2015). This psychotherapy, pioneered by Ellis (1962) and Beck (1970), focuses on identifying maladaptive cognitions and modifying behavioral patterns to alleviate clinical symptoms and improve function (Beck and Dozois, 2011; Hofmann et al., 2012). Moreover, CBT may promote change in conflict and inflexible appraisals of emotional, cognitive, physiological, and social states by reducing avoidance in experience and behavior (McMain et al., 2015).
Many studies have demonstrated the efficacy of CBT in psychiatric disorders (Butler et al., 2006; Hofmann et al., 2012; Van Dis et al., 2020; Matsumoto et al., 2021). 269 quantitative meta-analyses were identified in a review to verify the effectiveness of CBT for psychiatric disorders. And a subsample of 11 meta-analyses was compared response rates between CBT and other treatments. The result showed CBT was highly effective for depression, anxiety disorders, cannabis, and nicotine dependence. And CBT demonstrated superior efficacy as compared to other forms of psychotherapies in personality disorders, bulimia, positive symptoms in schizophrenia (Hofmann et al., 2012). A study showed that a pooled effect size (Hedge g) post-treatment of −0.49 (95% CI −0.68 to −0.29), which indicated CBT had a significant effect on psychiatric disorders (Matsumoto et al., 2021). Moreover, much evidence addressed that CBT produced long-term persistence of therapeutic effects following the termination of treatment (Butler et al., 2006; Van Dis et al., 2020). A meta-analysis suggested CBT was related to symptom improvement in social anxiety disorder (Hedges g, 0.42; k = 3), generalized anxiety disorder [Hedges g, 0.22; the number of studies (k) = 10], posttraumatic stress disorder (Hedges g, 0.84; k = 5), after 2-month follow-up (Van Dis et al., 2020). Similar findings were observed in depression. A meta-analysis found that there was the same effect between antidepressant medication and CBT at follow-up (Cuijpers et al., 2013).
With the development of neuroimaging technology, many non-invasive techniques such as magnetic resonance imaging (MRI), diffusion tensor imaging (DTI), functional magnetic resonance imaging (fMRI), single-photon emission computed tomography (SPECT), and positron emission tomography (PET) are being utilized to identify structural and functional brain alterations in psychiatric disorders. Whether the subject was in the resting state or in performing a specific task, changes in blood oxygenation level-dependent can be monitored through fMRI (Chen and Glover, 2015). Thus, task-based fMRI was employed to identify functional neuroanatomical networks associated with specific task states (Baggio and Junqué, 2019).
Previous neuroimaging studies have reported abnormalities in brain regions and networks in psychiatric disorders (Goodkind et al., 2015; McTeague et al., 2020). However, most studies focused on structural and functional brain alterations of a specific diagnosis. Although different psychiatric disorders had characteristic clinical presentations, there have common functional impairments in cognition, emotion, behavior, and socio-occupational impairment. Previous transdiagnostic studies had evidenced that the abnormalities in the insula, the dorsal anterior cingulate (dACC), the dorsolateral prefrontal cortex (dlPFC) were associated with cognitive dysfunction (Goodkind et al., 2015). And hyperconnectivity was identified between the salience network (SN), the default mode network (DMN), and the frontoparietal network (FPN). These network alterations were related to cognitive deficits (Sha et al., 2019). Furthermore, the disrupted emotional processing was associated with abnormal activation in the prefrontal regions, the amygdala, the hippocampal/parahippocampal gyri, the thalamus, and the fusiform gyrus (McTeague et al., 2020).
Neuroimaging of CBT has increased enormously over the past several decades and increasing evidence has already documented the relationship between neural changes and symptomatic improvement following CBT for psychiatric disorders.
There exists fairly robust evidence documenting alterations in prefrontal cortical regions and functionally related structures following CBT. A systematic review showed that the most common regions altered by CBT included ACC, posterior cingulate cortex (PCC), and orbitofrontal cortex/dorsomedial prefrontal cortex (OFC/VLPFC). Furthermore, the decreased activity in dorsal anterior cingulate (dACC) following CBT was in line with a model of information processing that described a ventral affective circuit and a dorsal cognitive circuit (Franklin et al., 2016). Another review identified that CBT was related to bilateral deactivation of ACC for specific phobia (Ipser et al., 2013). In addition, a meta-analysis found that compared with healthy controls, activation of ACC/PFC was significantly decreased in negative valence disorders, though activity was normalized in patients following CBT (La Buissonniere-Ariza et al., 2021). Furthermore, a study identified that activation of PFC and posterior cingulate cortex (PCC) were decreased after CBT in insomnia. This study suggested that PFC and PCC were associated with sleep-related attention (Kim et al., 2017). Perhaps CBT affects cognitive and emotional processes through ACC (La Buissonniere-Ariza et al., 2021).
Similarly, altered activation was also reported in the insula and amygdala. For example, a meta-analysis identified that the insula and amygdala were consistently responsive to phobic stimuli, but insula activity normalized following CBT (Ipser et al., 2013). This suggests that CBT could improve threat-safety discrimination (La Buissonniere-Ariza et al., 2021). In the same line, post-treatment reductions in the insula and amygdala response to emotion perception were related to greater clinical improvement in adults with anxiety and/or depression (Gorka et al., 2019). It appears that reductions in insula and amygdala activity are a sign of successful CBT intervention in MDD (Zhou et al., 2021). In anxiety disorders, deactivation of the amygdala has also been observed following CBT. In other negative valence disorders, greater pre-treatment activation to emotional stimuli predicted greater reductions in clinical symptoms at post-treatment (La Buissonniere-Ariza et al., 2021).
In addition to the aforementioned brain regions, there are other cortical regions (hippocampus, thalamus, precentral and postcentral gyri) that may potentially be associated with treatment response (Ipser et al., 2013; La Buissonniere-Ariza et al., 2021). However, studies involving these regions are inconsistent.
As evidence mounts, it is clear that information related to affective and cognitive processes is integrated between brain regions. CBT responses have been linked to functional connectivity in the default mode, cognitive control, salience, and frontoparietal networks in psychiatric disorders (Mason et al., 2016; La Buissonniere-Ariza et al., 2021). Besides, previous studies have shown that the dysfunctional connectivity in prefrontal-limbic regions led to conflicts in cognitive processes during the experience of negative affect (Mason et al., 2016). Thus, not only does CBT alter the activity of individual brain regions, but it can also have an effect on functional integration between regions.
Despite the increased interest sparked by neuroimaging studies that assessed brain modifications after CBT, most of them focused on specific disorders or populations with common symptom characteristics. Few neuroimaging studies directly explored neural mechanisms underlying CBT across psychiatric disorders. The impact of CBT on the brains of patients with psychiatric disorders is not clear. The present study aimed to use systematic reviews and meta-analyses to identify neural changes associated with CBT that are common across psychiatric disorders. Furthermore, we selected studies that used task paradigms that focused on the relationship between cognition, emotion, and behavior since disrupted emotional processing and cognitive deficits are common features of multiple psychiatric disorders. In the present study, two coordinate-based ALE meta-analyses were conducted to investigate the changes in cognitive or emotion-related brain regions following CBT. The present systematic review and meta-analysis will provide a clearer view of the current state of research and will identify areas for further investigation.
Methods
Search and Inclusion of Studies
A comprehensive and systematic search was conducted using the Pubmed, Cochrane Library, Scopus, and Web of Science electronic databases before 4 August 2021. This search was based on the following keywords and combinations: (“cognitive behavior* OR cognitive therapy OR behavior therapy OR CBT”) AND (“MRI or magnetic resonance imaging or fMRI or functional magnetic resonance imaging or PET or positron emission tomography or SPECT or single photon emission computed/tomography”).
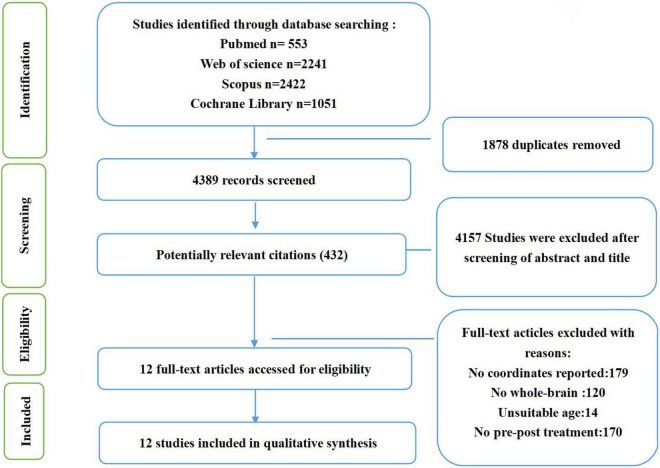
Inclusion criteria required studies to (1) be part of a clinical trial examining cognitive behavioral therapy in psychiatric disorders; (2) compare the neural changes between pre-treatment and post-treatment using fMRI during a task; (3) conduct whole-brain voxel-wise analyses; (4) report results as coordinates in standard Talairach or MNI space; (4) publish in English. Some studies were excluded due to: (1) presenting only baseline coordinates; (2) reporting resting-state fMRI, functional connectivity, voxel-based morphometry, or region-of-interest analyses. If different studies or tasks used the same subjects, we included the study or task which had the largest sample. And Studies that found no significant differences were excluded. The entire search process is shown in the flowchart below (Figure 1).
FIGURE 1.
Flowchart of the searching strategy and study selection process, based on PRISMA template (Liberati et al., 2009; Moher et al., 2009).
Activation Likelihood Estimation Methodology
For the resulting articles, all eligible experiments were entered into the BrainMap database according to paradigm and direction of effect. Eligible coordinates were entered into the database in MNI (Montreal Neuroimaging Institute) space. Coordinates presented in Talairach space were transformed to MNI space using GingerALE. The meta-analysis was performed in GingerALE 3.0.2 software downloaded from the BrainMap website to examine whether there was a significant overlap across multiple studies. The ALE algorithm converted the reported foci into spatial centers of 3-dimensional Gaussian probability distributions to model a modeled activation (MA) map (Eickhoff et al., 2009, 2012). The union of activation probabilities for each focus in the MA map was calculated to obtain ALE scores. And the included studies had been weighted by ALE algorithms. ALE algorithms weighted the localizing power of individual studies by building the proposed uncertainty model.
The 13 studies identified in the literature search included emotion or cognition task paradigms. According to the differences of paradigms, three coordinate-based ALE meta-analyses were separately performed to determine consistency across neuroimaging studies. The ALE meta-analyses were performed on brain areas displaying altered activation with P-value = 0.05, cluster-level = 0.05 and threshold permutations = 1,000.
Then, brain regions displaying alterations were categorized into eight functional networks according to the spatial correlation values between independent components and the template. These eight brain networks were defined mainly according to Shirer’s definition of 90 ROI functions (Shirer et al., 2012).
Results
Studies Included
According to searching procedures, thirteen studies were assessed for eligibility. In these retained studies, 9 used paradigms classified as emotion, and 4 used paradigms classified as cognition, as shown in Table 1. The 13 articles included 1 study for patients with psychosis, 1 for the cocaine-dependent disorder, 1 for eating disorder, 4 for major depressive disorder, and 6 for anxiety disorder. Patients in these studies were aged from 11 to 60, including patient groups and control groups.
TABLE 1.
Characteristics of 13 included studies.
| Author | Disorder | Patients/n | Control patients/n | Healthy/n | Mean age | Gender (male/female) | Task |
Sessions | Contrasts | |
| Cognition | Emotion | |||||||||
| Rubin-Falcone et al., 2020 | MDD | 32 | – | 19 | 34.8 | 15/16 | – | Emotional reactivity and emotion regulation | 14 sessions over 12 weeks | Pre > Post |
| Hauner et al., 2012 | SP | 12 | – | – | 22.3 | 3/9 | – | Emotion photogenic image | 2 h | Pre < Post Pre > Post |
| Fonzo et al., 2014 | GAD | 21 | – | 11 | 34.29 | 16/5 | – | Facial emotion processing | 10 weeks | Pre > Post |
| Fu et al., 2008 | MDD | 16 | – | 16 | 40 | 6/2 | – | Affect recognition | 16 weeks | Pre < Post Pre > Post |
| Sankar et al., 2015 | MDD | 16 | – | 16 | 39.9 | 13/3 | – | Dysfunctional attitudes | 16 weeks | Pre > Post |
| Månsson et al., 2013 | SAD | 13 | 13 | – | 32.46 | 2/11 | – | Affective face processing | 12 weeks | Pre < Post Pre > Post |
| Reinecke et al., 2018 | AD | 14 | 14 | – | 34.8 | 5/9 | – | Emotion regulation | 4 weeks | Pre > Post |
| Kumari et al., 2011 | Psychosis | 28 | 28 | – | 35.68 | 9/11 | – | affect processing | 6–8 months | Pre > Post |
| Kircher et al., 2013 | PD | 21 | 21 | 42 | 35.42 | 29/42 | – | Fear conditioning | 12 weeks | Pre > Post |
| Yoshimura et al., 2014 | MDD | 23 | 15 | 30.5 | 6/2 | Self-referential | – | 12 weeks | Pre > Post | |
| Devito et al., 2012 | SUD | 12 | – | 12 | 37.2 | 7/5 | Stroop | – | 8 weeks | Pre > Post |
| Vocks et al., 2011 | ED | 32 | – | 17 | 26.12 | 9/8 | Body image | – | 10 weeks | Pre > Post |
| Bomyea et al., 2020 | AD | 30 | – | – | 36.6 | 11/15 | Reappraisal-based emotion regulation | – | 10 sessions over 12 weeks | Pre > Post |
Activation Likelihood Estimation Analysis
Activation Likelihood Estimation Meta-Analysis of Cognition Task Following Cognitive Behavioral Therapy
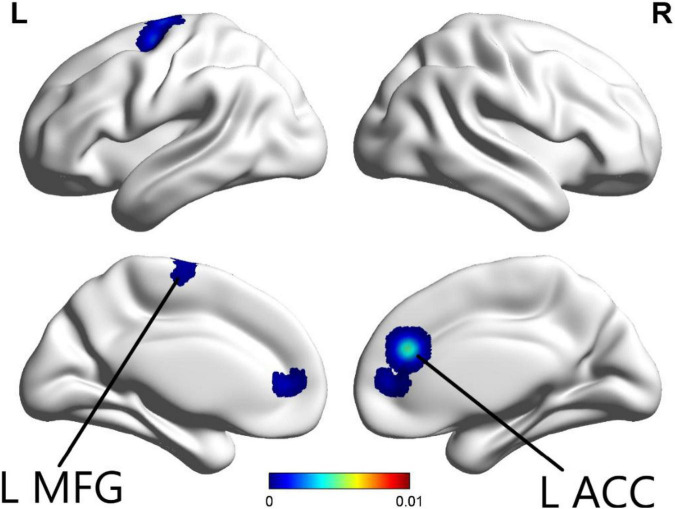
The ALE meta-analysis was performed on brain areas displaying altered activation during cognition tasks from pre to post-CBT with P-value = 0.05, cluster-level = 0.05 and threshold permutations = 1,000. The results showed that the left anterior cingulate (L ACC) and left middle frontal gyrus (L MFG) demonstrated significantly decreased activation (Figure 2 and Table 2).
FIGURE 2.
In the cognition paradigm, significantly decreased activations across the left anterior cingulate (L ACC) and left middle frontal gyrus (L MFG) were found in patients with psychiatric disorders after CBT (p < 0.05).
TABLE 2.
Activation areas resulting from meta-analysis, peak voxels, ALE values, and cluster sizes are included.
| Cluster size (mm3) | ALE | MNI-coordinates |
Brain region | ||
| X | V | z | |||
| Cognition task | |||||
| 1,549 | 0.008694564 | 0 | 46 | 6 | Left anterior cingulate |
| 1,066 | 0.005506158 | −18 | −12 | 61 | Left middle frontal gyrus |
| All task | |||||
| 1,393 | 0.009742522 | −16 | −72 | 12 | Left cuneus |
Activation Likelihood Estimation Meta-Analysis of Emotion Task Following Cognitive Behavioral Therapy
For emotion tasks, we performed the same procedure. No clusters showed significant overlap in ALE maps.
Activation Likelihood Estimation Meta-Analysis of All Tasks Following Cognitive Behavioral Therapy
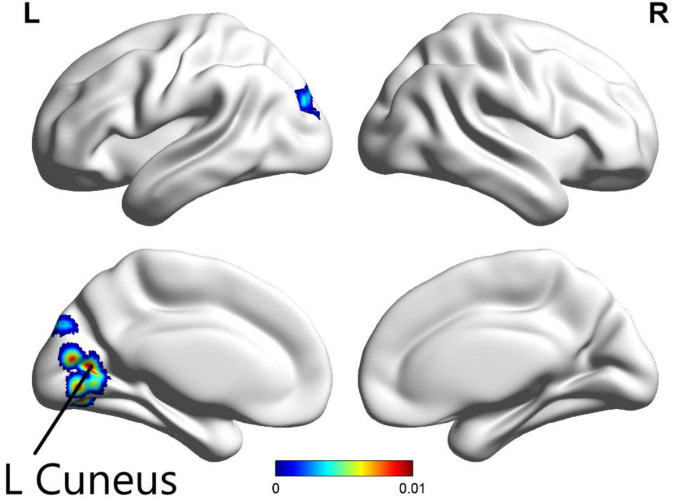
The ALE meta-analysis was performed on brain areas displaying altered activation during all tasks from pre to post-CBT with P-value = 0.05, cluster-level = 0.05 and threshold permutations = 1,000. The results revealed that the left cuneus displayed significantly decreased activation (Figure 3 and Table 2).
FIGURE 3.
In all paradigms, significantly decreased activation in the left cuneus was found in patients with psychiatric disorders after CBT (p < 0.05).
Brain Functional Alterations and Related Brain Networks Following Cognitive Behavioral Therapy
Based on the 13 studies reviewed, the most consistent findings were altered activation between the prefrontal cortex and other limbic regions following CBT. To better understand the effects of CBT on psychiatric patients, brain regions from included studies were categorized into eight functional networks according to the spatial correlation values between independent components and the template. The results showed that 9 brain areas reported reduced activation in DMN including the medial prefrontal cortex (mPFC), ACC, precuneus, PCC, left angular gyrus (AG), left middle occipital gyrus (MOG), right superior frontal gyrus (SFG), right parahippocampal gyrus, and right angular gyrus. 11 brain areas reported reduced activation in ECN including mPFC, ACC, PCC, L AG, precuneus, R SFG, L SPG, L IPG, left inferior temporal gyrus (L ITG), and middle temporal gyrus (MTG). 6 brain areas reported reduced activation in SN including mPFC, precuneus, left insula, ACC, IPG, and right midcingulate gyrus (MGC). The most common outcome across studies was decreased activation in mPFC, ACC, and precuneus (Figure 4).
FIGURE 4.
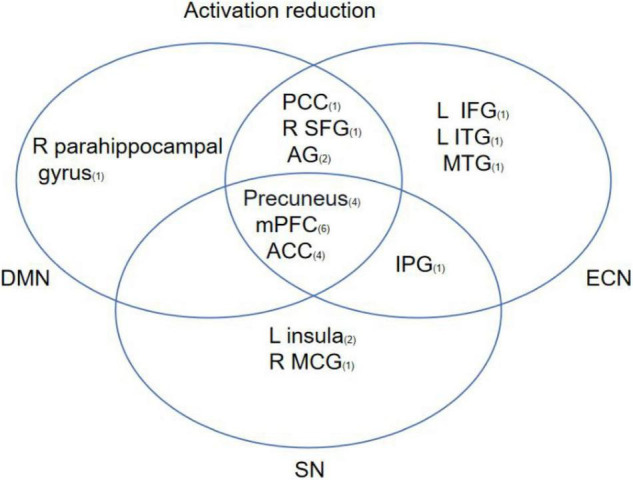
This figure shows regions that have been reported to show CBT-related changes and the overlap between regions involved in different brain networks. The subscript number next to the brain region represents the number of articles related to that brain region.
Discussion
In the present study, a systematic review and meta-analysis were conducted to identify brain regions and related brain networks associated with the effects of CBT in patients with psychiatric disorders. And to our knowledge, this review is the first to discover altered brain activation associated with CBT in task-fMRI. First, the most commonly reported finding was decreased activation in mPFC, ACC, and precuneus from pre to post-treatment. After reviewing all included studies, connectivity within DMN, ECN, and SN was connected with the effects of CBT. Second, the ALE meta-analysis of cognition fMRI studies revealed that the left ACC and left MFG showed significant overlap across studies. Third, the ALE meta-analysis of all fMRI studies revealed that left precuneus showed significant overlap across studies.
For the ALE-meta-analysis of cognition tasks, significantly decreased activation across left ACC and left MFG was found. After referencing previous studies and reviewing the literature, this was an expected finding following CBT. The mPFC is a region that consists ACC, MFG, prelimbic cortex (PL), infralimbic cortex, and medial precentral area. It plays a critical role in emotion regulation, cognitive processing, motivation, and social interaction (Riga et al., 2014; Xu et al., 2019). The dysfunction of mPFC found in psychiatric disorders is related to loss of social skills, motivation deficiency, and dysregulation of cognition and emotion (Xu et al., 2019). Moreover, ACC, as one of the core regions of mPFC, is also involved in emotional processing, higher cognition, motivation, and motor control (Monosov et al., 2020). There was a multitude of evidence in functional imaging studies reporting altered activation of mPFC/ACC following CBT. In one study, from pre- to post-treatment, decreased activation in ACC during fear/angry faces was observed in generalized anxiety disorder (Fonzo et al., 2014). In another study, compared with control groups, depressive patients displayed increased activation in the MPFC during the self/negative condition. In a third study, MPFC and ventral anterior cingulate cortex (vACC) displayed attenuated activation during self-referential processing of negatively valenced words after 12 sessions of CBT (Yoshimura et al., 2014). A recent review examined the involvement of ACC and mPFC in appraisal and expression of negative emotion and found that ACC/mPFC exhibited a modulating effect on limbic regions involved in producing emotional responses (Etkin et al., 2011). The evidence suggests that CBT may participate in emotional processing by acting on mPFC and ACC (Fonzo et al., 2014; Yoshimura et al., 2014).
In the present systematic review and meta-analysis, we also found altered activation of the precuneus. According to the ALE meta-analysis results for all tasks, significantly reduced activation was found in the left cuneus. The precuneus and mPFC are identified as core parts of DMN, which is implicated in various psychiatric disorders (Whitfield-Gabrieli and Ford, 2012). Previous studies have shown that the precuneus performs an important role in a wide range of higher-order cognitive functions including visuospatial imagery, episodic memory retrieval, self-processing, and consciousness (Cavanna and Trimble, 2006). One study reported that greater BOLD deactivation during emotion regulation from pre- to post-treatment in the precuneus was associated with better treatment outcomes (Rubin-Falcone et al., 2020). Similarly, post-treatment increases in precuneus activity were also observed in specific phobia, suggesting that CBT may effectively improve threat-safety discrimination. The abnormal increases in this parietal region suggest a compensatory mechanism of cognitive function. It may be that the precuneus reorganizes the process of emotion stimuli (Álvarez-Pérez et al., 2021). On the other hand, a previous study has shown that self-referential processing is regulated by emotion (Qian et al., 2020). Given the role of the precuneus in self-referential processing, this may reflect greater disengagement in self-referential processing as a function of successful CBT (Herold et al., 2016). Furthermore, effects of CBT were reported across several other brain regions including parietal, occipital, temporal, and limbic regions, suggesting that many brain regions are important for cognition and emotion and may be sensitive to the effects of CBT. However, studies involving these regions are inconsistent.
In the present study, DMN, ECN, and SN were the most relevant networks after reviewing eligible neuroimaging data. The DMN is involved in emotional processing, self-referential processing, and the recollection of prior experiences (Whitfield-Gabrieli and Ford, 2012; Mohan et al., 2016; Zhang and Volkow, 2019; Yeshurun et al., 2021). DMN exhibits hyperactivity during resting states and deactivation during the performance of cognitive tasks when attention is directed externally (Broyd et al., 2009; Whitfield-Gabrieli and Ford, 2012; Andrews-Hanna et al., 2014; Simon and Engström, 2015). Additionally, previous studies showed that abnormal intra-network connectivity of the DMN was associated with abnormalities in cognitive function including self-referential and introspective mental activity, attention, and working memory across psychiatric disorders (Broyd et al., 2009; Vuper et al., 2021). From pre- to post-CBT, normalization of intra-functional connectivity in DMN was positively correlated with clinical improvement in OCD. The evidence suggests that CBT affects the intra-functional connectivity in DMN to modulate cognitive function. In addition, connectivity changes in ECN and SN have also been observed following CBT. The ECN is engaged in a broad spectrum of high-level cognitive functions including planning, decision making, attention regulation, and working memory (Menon, 2011; Xu et al., 2020). SN is associated with regulating emotional and sensory stimuli, adjusting cognitive directivity, and allocating attention (Seeley, 2019). Previous studies have shown that the connectivity between the DMN, the SN, and the CEN impacts cognitive functions and dysfunction of intrinsic wiring, and connectivity in the three core neurocognitive networks has been evidenced in psychiatric disorders (Menon, 2011; Vuper et al., 2021). For instance, in post-traumatic stress disorder (PTSD), altered DMN and SN connectivity was related to the reduction of clinical symptoms following CBT (La Buissonniere-Ariza et al., 2021; Vuper et al., 2021). This result suggests that CBT improves cognitive function by affecting the internal connection of the brain network and the interaction among brain networks. In the present meta-analysis, we chose to include all available studies due to the lack of available CBT fMRI studies in the literature. Although emotion-task ALE meta-analysis did not find any specific brain regions showing consistent effects, the results indicated that CBT had broad rather than specific effects on task-evoked BOLD response across the brain.
Limitations
The present study has several limitations. First, the number of studies in the present ALE meta-analysis was small. It limited our ability to detect more accurate ALE results. To meet the challenges of research heterogeneity and ALE methods, a comprehensive literature search with relatively strict inclusion criteria and strict correction procedures was used to improve the internal effectiveness of the study, which is consistent with previous ale studies. Second, due to the scant amount of literature and uneven types of diseases, our data is not representative of psychiatric disorders as a whole. Third, confounding factors, such as age and gender, are unavoidable limitations.
Conclusion
The present systematic review and ALE meta-analysis was the first summary of the available literature on core neural regions that are related to CBT in task-fMRI. Findings from the present study indicated that CBT was associated with significantly decreased activity in mPFC/ACC and precuneus. Significant changes in neural activity were also identified in intrinsic wiring and connectivity in DMN, ECN, and SN, suggesting that these effects may mediate cognitive improvements and emotion regulation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
CW, SY, HW, NZ, and YW contributed to initiating, designing the study, and collecting the data. SY, QX, JY, HX, and JL made substantial contributions in writing the manuscript. YZ provided editing and writing assistance. All authors reviewed and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Nanjing Brain Hospital, affiliated with the Nanjing Medical University of China for their funding and support. We would grateful for the comments provided by YZ (School of Psychology, Nanjing Normal University, Nanjing, China).
Abbreviations
- CBT
cognitive training
- ALE
activation likelihood estimation
- DMN
default mode network
- ECN
executive control network
- SN
salience network
- ACC
anterior cingulate
- MFG
middle frontal gyrus
- DMN
default mode network
- ECN
executive control network
- SN
salience network
- MDD
major depressive disorder
- AD
anxiety disorder
- OCD
obsessive-compulsive disorder
- FAR
Facial Affect Recognition
- MRI
magnetic resonance imaging
- fMRI
functional magnetic resonance imaging
- PET
positron emission tomography
- PCC
posterior cingulate cortex
- OFC/VLPFC
orbitofrontal cortex/dorsomedial prefrontal cortex
- PTSD
post-traumatic stress disorder.
Funding
This study was supported by the National Natural Science Foundation of China (81971289 and 81871344); Jiangsu Provincial key research and development program (BE2019609); Natural Science Foundation of Jiangsu Province (BK20191369); the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province, China (18KJB190003); Medical Science and Technology Major Project of Nanjing (ZKX19028).
References
- Álvarez-Pérez Y. Rivero F. Herrero M. Viña C. Fumero A. Betancort M. et al. (2021). Changes in brain activation through cognitive-behavioral therapy with exposure to virtual reality: a neuroimaging study of specific phobia. J. Clin. Med. 10. 10.3390/jcm10163505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Smallwood J., Spreng R. N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N.Y. Acad. Sci. 1316 29–52. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio H. C., Junqué C. (2019). Functional MRI in parkinson’s disease cognitive impairment. Int. Rev. Neurobiol. 144, 29–58. 10.1016/bs.irn.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Beck A. T., Dozois D. J. (2011). Cognitive therapy: current status and future directions. Annu. Rev. Med. 62 397–409. 10.1146/annurev-med-052209-100032 [DOI] [PubMed] [Google Scholar]
- Bomyea J., Ball T. M., Simmons A. N., Campbell-Sills L., Paulus M. P., Stein M. B. (2020). Change in neural response during emotion regulation is associated with symptom reduction in cognitive behavioral therapy for anxiety disorders. J. Affect. Dis. 271 207–214. 10.1016/j.jad.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd S. J., Demanuele C., Debener S., Helps S. K., James C. J., Sonuga-Barke E. J. (2009). Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 33 279–296. 10.1016/j.neubiorev.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Butler A. C., Chapman J. E., Forman E. M., Beck A. T. (2006). The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin. Psychol. Rev. 26 17–31. 10.1016/j.cpr.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Cavanna A. E., Trimble M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129 564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Chen J. E., Glover G. H. (2015). Functional magnetic resonance imaging methods. Neuropsychol. Rev. 25, 289–313. 10.1007/s11065-015-9294-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P., Hollon S. D., Van Straten A., Bockting C., Berking M., Andersson G. (2013). Does cognitive behavior therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open 3:e002542. 10.1136/bmjopen-2012-002542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devito E. E., Worhunsky P. D., Carroll K. M., Rounsaville B. J., Kober H., Potenza M. N. (2012). A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug Alcohol Depend 122 228–235. 10.1016/j.drugalcdep.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S. B., Bzdok D., Laird A. R., Kurth F., Fox P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage 59 2349–2361. 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S. B., Laird A. R., Grefkes C., Wang L. E., Zilles K., Fox P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30 2907–2926. 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15, 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo G. A., Ramsawh H. J., Flagan T. M., Sullivan S. G., Simmons A. N., Paulus M. P., et al. (2014). Cognitive-behavioral therapy for generalized anxiety disorder is associated with attenuation of limbic activation to threat-related facial emotions. J. Affect. Dis. 169 76–85. 10.1016/j.jad.2014.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin G., Carson A. J., Welch K. A. (2016). Cognitive behavioural therapy for depression: systematic review of imaging studies. Acta Neuropsychiatr. 28 61–74. 10.1017/neu.2015.41 [DOI] [PubMed] [Google Scholar]
- Fu C. H., Williams S. C., Cleare A. J., Scott J., Mitterschiffthaler M. T., Walsh N. D., et al. (2008). Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol. Psychiatr. 64 505–512. 10.1016/j.biopsych.2008.04.033 [DOI] [PubMed] [Google Scholar]
- Goodkind M., Eickhoff S. B., Oathes D. J., Jiang Y., Chang A., Jones-Hagata L. B., et al. (2015). Identification of a common neurobiological substrate for mental illness. JAMA Psychiatr. 72 305–315. 10.1001/jamapsychiatry.2014.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka S. M., Young C. B., Klumpp H., Kennedy A. E., Francis J., Ajilore O., et al. (2019). Emotion-based brain mechanisms and predictors for SSRI and CBT treatment of anxiety and depression: a randomized trial. Neuropsychopharmacology 44 1639–1648. 10.1038/s41386-019-0407-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner K. K., Mineka S., Voss J. L., Paller K. A. (2012). Exposure therapy triggers lasting reorganization of neural fear processing. Proc. Natl. Acad. Sci. U.S.A. 109 9203–9208. 10.1073/pnas.1205242109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold D., Spengler S., Sajonz B., Usnich T., Bermpohl F. (2016). Common and distinct networks for self-referential and social stimulus processing in the human brain. Brain Struct. Funct. 221 3475–3485. 10.1007/s00429-015-1113-9 [DOI] [PubMed] [Google Scholar]
- Hofmann S. G., Asnaani A., Vonk I. J., Sawyer A. T., Fang A. (2012). The Efficacy of Cognitive Behavioral Therapy: A Review of Meta-analyses. Cognit. Ther. Res. 36 427–440. 10.1007/s10608-012-9476-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser J. C., Singh L., Stein D. J. (2013). Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin. Neurosci. 67 311–322. 10.1111/pcn.12055 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Lee Y. J., Kim N., Kim S., Choi J. W., Park J., et al. (2017). Exploration of changes in the brain response to sleep-related pictures after cognitive-behavioral therapy for psychophysiological insomnia. Sci. Rep. 7:12528. 10.1038/s41598-017-13065-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher T., Arolt V., Jansen A., Pyka M., Reinhardt I., Kellermann T., et al. (2013). Effect of cognitive-behavioral therapy on neural correlates of fear conditioning in panic disorder. Biol. Psychiatr. 73 93–101. 10.1016/j.biopsych.2012.07.026 [DOI] [PubMed] [Google Scholar]
- Kumari V., Fannon D., Peters E. R., Ffytche D. H., Sumich A. L., Premkumar P., et al. (2011). Neural changes following cognitive behaviour therapy for psychosis: a longitudinal study. Brain 134 2396–2407. 10.1093/brain/awr154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Buissonniere-Ariza V., Fitzgerald K., Meoded A., Williams L. L., Liu G., Goodman W. K., et al. (2021). Neural correlates of cognitive behavioral therapy response in youth with negative valence disorders: A systematic review of the literature. J. Affect. Dis. 282 1288–1307. 10.1016/j.jad.2020.12.182 [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson K. N., Carlbring P., Frick A., Engman J., Olsson C. J., Bodlund O., et al. (2013). Altered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder. Psychiatry Res. 214 229–237. 10.1016/j.pscychresns.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Mason L., Peters E., Kumari V. (2016). Functional connectivity predictors and mechanisms of cognitive behavioural therapies: A systematic review with recommendations. Aust. N.Z. J. Psychiatr. 50 311–321. 10.1177/0004867415624970 [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Hamatani S., Shimizu E. (2021). Effectiveness of videoconference-delivered cognitive behavioral therapy for adults with psychiatric disorders: systematic and meta-analytic review. J. Med. Internet Res. 23:e31293. 10.2196/31293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMain S., Newman M. G., Segal Z. V., Derubeis R. J. (2015). Cognitive behavioral therapy: current status and future research directions. Psychother. Res. 25 321–329. 10.1080/10503307.2014.1002440 [DOI] [PubMed] [Google Scholar]
- McTeague L. M., Rosenberg B. M., Lopez J. W., Carreon D. M., Huemer J., Jiang Y., et al. (2020). Identification of Common Neural Circuit Disruptions in Emotional Processing Across Psychiatric Disorders. Am. J. Psychiatr. 177 411–421. 10.1176/appi.ajp.2019.18111271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Mohan A., Roberto A. J., Mohan A., Lorenzo A., Jones K., Carney M. J., et al. (2016). The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review. Yale J. Biol. Med. 89 49–57. [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monosov I. E., Haber S. N., Leuthardt E. C., Jezzini A. (2020). Anterior Cingulate Cortex and the Control of Dynamic Behavior in Primates. Curr. Biol. 30 R1442–R1454. 10.1016/j.cub.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Wang Z., Li C., Gao X. (2020). Prioritised self-referential processing is modulated by emotional arousal. Q. J. Exp. Psychol. 73 688–697. 10.1177/1747021819892158 [DOI] [PubMed] [Google Scholar]
- Reinecke A., Thilo K. V., Croft A., Harmer C. J. (2018). Early effects of exposure-based cognitive behaviour therapy on the neural correlates of anxiety. Transl. Psychiatr. 8:225. 10.1038/s41398-018-0277-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga D., Matos M. R., Glas A., Smit A. B., Spijker S., Van Den Oever M. C. (2014). Optogenetic dissection of medial prefrontal cortex circuitry. Front. Syst. Neurosci. 8:230. 10.3389/fnsys.2014.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin-Falcone H., Weber J., Kishon R., Ochsner K., Delaparte L., Doré B., et al. (2020). Neural predictors and effects of cognitive behavioral therapy for depression: the role of emotional reactivity and regulation. Psychol. Med. 50 146–160. 10.1017/s0033291718004154 [DOI] [PubMed] [Google Scholar]
- Sankar A., Scott J., Paszkiewicz A., Giampietro V. P., Steiner H., Fu C. H. (2015). Neural effects of cognitive-behavioural therapy on dysfunctional attitudes in depression. Psychol. Med. 45 1425–1433. 10.1017/S0033291714002529 [DOI] [PubMed] [Google Scholar]
- Seeley W. W. (2019). The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 39 9878–9882. 10.1523/JNEUROSCI.1138-17.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z., Wager T. D., Mechelli A., He Y. (2019). Common Dysfunction of Large-Scale Neurocognitive Networks Across Psychiatric Disorders. Biol. Psychiatr. 85 379–388. 10.1016/j.biopsych.2018.11.011 [DOI] [PubMed] [Google Scholar]
- Shirer W. R., Ryali S., Rykhlevskaia E., Menon V., Greicius M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex 22 158–165. 10.1093/cercor/bhr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R., Engström M. (2015). The default mode network as a biomarker for monitoring the therapeutic effects of meditation. Front. Psychol. 6:776. 10.3389/fpsyg.2015.00776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dis E. A. M., Van Veen S. C., Hagenaars M. A., Batelaan N. M., Bockting C. L. H., Van Den Heuvel R. M., et al. (2020). Long-term Outcomes of Cognitive Behavioral Therapy for Anxiety-Related Disorders: A Systematic Review and Meta-analysis. JAMA Psychiatry 77 265–273. 10.1001/jamapsychiatry.2019.3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocks S., Schulte D., Busch M., Grönemeyer D., Herpertz S., Suchan B. (2011). Changes in neuronal correlates of body image processing by means of cognitive-behavioural body image therapy for eating disorders: a randomized controlled fMRI study. Psychol. Med. 41 1651–1663. 10.1017/S0033291710002382 [DOI] [PubMed] [Google Scholar]
- Vuper T. C., Philippi C. L., Bruce S. E. (2021). Altered resting-state functional connectivity of the default mode and central executive networks following cognitive processing therapy for PTSD. Behav. Brain Res. 409:113312. 10.1016/j.bbr.2021.113312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J. M. (2012). Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 8 49–76. 10.1146/annurev-clinpsy-032511-143049 [DOI] [PubMed] [Google Scholar]
- Xu P., Chen A., Li Y., Xing X., Lu H. (2019). Medial prefrontal cortex in neurological diseases. Physiol. Genom. 51 432–442. 10.1152/physiolgenomics.00006.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Chen S., Xue C., Hu G., Ma W., Qi W., et al. (2020). Functional MRI-Specific Alterations in Executive Control Network in Mild Cognitive Impairment: An ALE Meta-Analysis. Front. Aging Neurosci. 12:578863. 10.3389/fnagi.2020.578863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y., Nguyen M., Hasson U. (2021). The default mode network: where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 22 181–192. 10.1038/s41583-020-00420-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S., Okamoto Y., Onoda K., Matsunaga M., Okada G., Kunisato Y., et al. (2014). Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Soc. Cogn. Affect. Neurosci. 9 487–493. 10.1093/scan/nst009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Volkow N. D. (2019). Brain default-mode network dysfunction in addiction. Neuroimage 200 313–331. 10.1016/j.neuroimage.2019.06.036 [DOI] [PubMed] [Google Scholar]
- Zhou W., Yuan Z., Yingliang D., Chaoyong X., Ning Z., Chun W. (2021). Differential patterns of dynamic functional connectivity variability in major depressive disorder treated with cognitive behavioral therapy. J. Affect. Dis. 291 322–328. 10.1016/j.jad.2021.05.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.