OBJECTIVES:
As the pandemic advances, the interest in the long-lasting consequences of COVID-19 increases. However, a few studies have explored patient-centered outcomes in critical care survivors. We aimed to investigate frailty and disability transitions in COVID-19 patients admitted to ICUs.
DESIGN:
Prospective cohort study.
SETTING:
University hospital in Sao Paulo.
PATIENTS:
Survivors of COVID-19 ICU admissions.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
We assessed frailty using the Clinical Frailty Scale (CFS). We also evaluated 15 basic, instrumental, and mobility activities. Baseline frailty and disability were defined by clinical conditions 2–4 weeks before COVID-19, and post-COVID-19 was characterized 90 days (day 90) after hospital discharge. We used alluvial flow diagrams to visualize transitions in frailty status, Venn diagrams to describe the overlap between frailty and disabilities in activities of daily living, and linear mixed models to explore the occurrence of new disabilities following critical care in COVID-19. We included 428 participants with a mean age of 64 years, 57% males, and a median Simplified Acute Physiology Score-3 score of 59. Overall, 14% were frail at baseline. We found that 124/394 participants (31%) were frail at day 90, 70% of whom were previously non-frail. The number of disabilities also increased (mean difference, 2.46; 95% CI, 2.06–2.86), mainly in participants who were non-frail before COVID-19. Higher pre-COVID-19 CFS scores were independently associated with new-onset disabilities. At day 90, 135 patients (34%) were either frail or disabled.
CONCLUSIONS:
Frailty and disability were more frequent 90 days after hospital discharge compared with baseline in COVID-19 patients admitted to the ICU. Our results show that most COVID-19 critical care survivors transition to poorer health status, highlighting the importance of long-term medical follow-up for this population.
Keywords: activities of daily living, COVID-19, frailty, intensive care units
At the beginning of the COVID-19 pandemic, global efforts were mostly directed to improving patient survival and equitable access to proper healthcare. As the pandemic progressed, management strategies advanced, and hospital mortality rates gradually decreased in many countries (1). Consequently, the pandemic entered a new phase, with a greater number of survivors but also with increased morbidity. Similar to what has been verified in previous critical care cohorts, COVID-19 patients may experience new and persistent disability (i.e., need help from others) on executing routine activities (e.g., bathing, walking, and shopping) after the acute infection (2–5). Furthermore, these new health conditions possibly reflect lower physiologic reserves (i.e., frailty [6]) and increased clinical vulnerability, itself a risk factor for worse outcomes such as readmissions and poorer quality of life (7, 8).
Despite their clinical relevance for patients and societies, long-term outcomes in COVID-ICU survivors remain poorly explored. For example, although several studies have investigated persistent COVID-19 symptoms, under the condition named “long COVID (9),” they have either provided little insight regarding ICU survivors (probably those at higher risk of worse long-term outcomes) or were limited to small subgroup analyses from larger cohorts (4, 10). Some authors have described chest CT alterations or reduced lung function following COVID-ICU admissions (11, 12). They have also observed that COVID-19 survivors may evolve with cardiovascular, musculoskeletal, and neurologic problems that lead to various symptoms, including fatigue, weakness, pain, and cognitive deficits (9–11). Nonetheless, the authors have not explored how their findings on long COVID translate into patient-centered outcomes, including frailty and disability, often associated with such health problems and symptoms. Finally, the clinical course of preexisting frailty after COVID-19, the occurrence of new-onset frailty, and their overlap with new disabilities are still unclear.
Therefore, we aimed to investigate in a cohort of COVID-ICU survivors: 1) the frequency of preexisting and new-onset frailty 90 days after hospital discharge, 2) the occurrence of transitions in frailty status between baseline and post-COVID-19 conditions, 3) the number of new disabilities after critical COVID-19 and their associated risk factors, and 4) the overlap between frailty and disability on performing routine activities.
MATERIALS AND METHODS
Study Design and Patient Population
This is an ancillary study of a longitudinal prospective cohort from Hospital das Clinicas, University of Sao Paulo Medical School (HCFMUSP). The study was completed using a unique hospital identifier to merge the databases from two previously published studies: the COVID-19 and Frailty (CO-FRAIL) Study (13) and the EPIdemiology of Critical COVID-19 (EPICCoV) Study (14). The CO-FRAIL Study included patients greater than or equal to 50 years old consecutively hospitalized between March 30 and July 7, 2020, with confirmed COVID-19 (13). The EPICCoV Study included patients greater than or equal to 14 years old consecutively admitted to the ICU for greater than or equal to 24 hours between March 30 and June 30, 2020 (only the first ICU admission was analyzed when more than one was present), with confirmed or highly suspected COVID-19 (14). Only participants with confirmed COVID-19 in both databases were included in the current analyses. Further details on both studies can be found elsewhere (13–15).
Both CO-FRAIL and EPPICoV studies were reviewed and approved by HCFMUSP’s Research Ethics Committee (approval number 32037120.6.0000.0068 and 31382620.0.0000.0068). Informed consent was obtained in the CO-FRAIL Study (Brazilian Clinical Trials Registry: RBR-7w5zhr) and waived in the EPICCoV Study (Clinicaltrials.gov: NCT04378582).
Data Collection and Definitions
Trained investigators collected the study data using structured electronic case report forms Research Electronic Data Capture (16). These were completed after a detailed review of electronic medical records and laboratory tests. Medical investigators also conducted structured telephone interviews with participants or their proxy to obtain complementary information. Collected data included demographics (e.g., age, sex, and body mass index), previous health conditions and comorbidities (assessed by the Charlson Comorbidity Index [Charlson] [17]), duration of COVID-19 symptoms, and ICU admission characteristics (e.g., Simplified Acute Physiology Score [SAPS-3] [18, 19], presence and severity of organ dysfunction according to the Sequential Organ Failure Assessment [SOFA] score [20], and use of invasive organ support).
Frailty was evaluated using a previously validated tool that has been studied in the context of critical care, the Clinical Frailty Scale (CFS) (8, 21–24). The CFS is a 9-point categorization tool that uses clinical data and visual descriptions to classify patients according to frailty status (8). Baseline frailty was defined according to pre-COVID-19 health conditions, characterized per information regarding the period before the infection (2–4 wk). Geriatrics-trained medical investigators followed existing guidelines to score the CFS (25, 26), using information from the medical records and interviews to characterize physical activity, activity-limiting symptoms (e.g., being “slowed up” or tired), level of independence to complete basic and instrumental activities of daily living (ADL), and cognition (27). Similar to previous ICU studies (8), we combined CFS scores according to four groups: 1–3 (“very fit to “managing well”), 4 (“vulnerable”), 5 (“mildly frail”), and 6–9 (“moderately to terminally ill”). We defined participants as frail using CFS scores greater than or equal to 5 (8, 21, 28).
We assessed patients’ level of independence in 15 routine activities and defined disability as “the need for help from others in executing such activities” (29). The number of disabilities (0–15) at baseline considered the patients’ level of independence 2–4 weeks preceding COVID-19. We assessed five basic ADL (bathing, dressing, eating, grooming, and toileting), five instrumental activities of daily living (IADL: shopping, housekeeping, cooking, taking medications, and using public transportation), and five mobility activities (walking in the neighborhood, walking one block, climbing a flight of stairs, moving from a chair, and walking on flat ground).
Follow-Up Assessments
Trained investigators (blinded to the baseline data) completed follow-up telephone interviews to assess the number and type of disabilities (as defined by the need for help in each of the 15 routine activities described above) and CFS scores at 90 days (day 90) after hospital discharge.
Data Analysis
Quantitative data were presented as the values of mean and sd, or median and interquartile ranges as appropriate, and categorical data were presented as counts and percentages. Paired quantitative data were analyzed using paired t tests.
We explored transitions from pre- to post-COVID-19 frailty statuses according to the four CFS categories (“very fit to managing well,” “vulnerable,” “mildly frail,” and “moderately to terminally ill”) and illustrated them using alluvial flow diagrams (Microsoft Excel 2016 [Microsoft, Redmond, WA] with add-in Power-user for Sankey Diagrams). We considered any transition from a group with better performance to a group with worse performance (e.g., –3 to 4, or 4 to 5) or death as transitions to a worse frailty state. We determined any transition from a group with worse performance to a group with better performance (e.g., –9 to 5, or 5 to 1–3) as transitions to a better frailty state. Those remaining in the same frailty category were considered as not having transitioned (30).
At the end of our follow-up, we used paired t tests to compare the number of disabilities at baseline and day-90, stratifying according to pre-COVID CFS categories. As a sensitivity analysis, we compared paired samples using a bootstrap-t approach with 5,000 iteractions and the 95% CI.
We used mixed linear models to explore the independent association between the number of disabilities at day 90 and possible risk factors, accounting for repeated measures. We defined the total number of disabilities as the dependent variable and baseline CFS categories as the primary independent variable while accounting for age, sex, SOFA scores, Charlson scores, assessment period (pre-COVID-19 or day-90), mechanical ventilation use, and vasoactive drugs during ICU stay as fixed covariates. We did not input missing data in any of our analyses.
Finally, we categorized patients according to the presence of frailty (CFS ≥ 5) and disability on performing ADL (Katz Index of Independence ≥ 1) (30, 31). We then used Venn diagrams to illustrate the overlap between frailty and ADL disability at day 90.
All hypothesis tests were two-tailed with a significance level of 0.05 and performed using IBM SPSS Statistics, Version 20 (Armonk, NY), and MedCalc Statistical Software, Version 20.008 (Ostend, Belgium).
RESULTS
We merged the 1,830-patient database from the CO-FRAIL Study and the 1,503-patient database from the EPICCoV Study, obtaining a sample of 1,028 intersecting participants. During our 90-day follow-up, 585 patients (57%) died in the hospital. Among the 443 discharged participants, 366 (83%) returned directly home, 77 (17%) were transferred to post-acute care facilities, and 15 (1.5%) died after hospital discharge. Therefore, our final sample comprised 428 patients alive at day 90. We did not lose any participants to follow-up (Supplement Fig. S1, http://links.lww.com/CCM/H25).
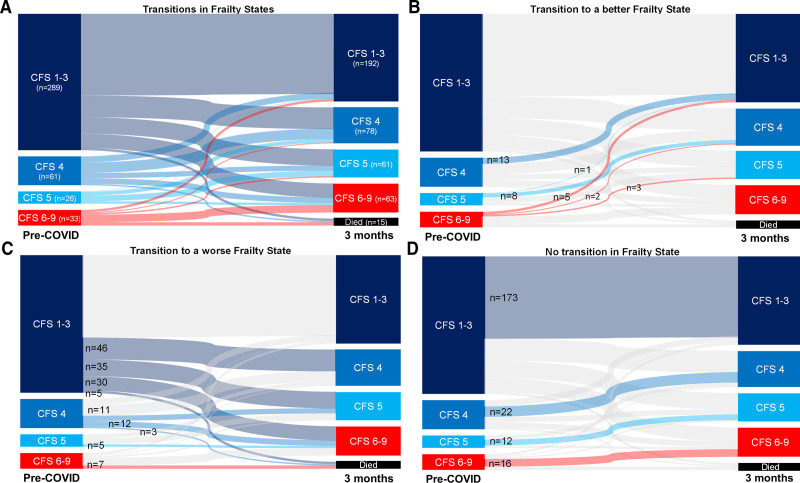
Overall, the mean age was 64 years, 57% were male, the median SAPS-3 score was 59, and 50% were on invasive mechanical ventilation at admission to ICU (demographics are detailed in Table 1; and Supplement Table S1, http://links.lww.com/CCM/H25). Only 60 participants (14%) were frail before COVID-19. We were able to reassess frailty at day 90 in 394 participants (92%) and found that its frequency increased to 31% (124/394). In this group, 71% had not been frail at baseline. As shown in Figure 1, although 223/394 patients (57%) did not change their frailty status, transitions between CFS categories were common in our follow-up. Comparing pre-COVID-19 and postdischarge conditions, transitions to a worse frailty status were observed in 154/394 patients (39%), whereas only 32/394 patients (8%) showed improvement.
TABLE 1.
General Characteristics of the Included Patients
| Characteristic | All Patients |
|---|---|
| n | 428 |
| Age (sd), yr | 63.8 (8.8) |
| Male, n (%) | 244 (57.0) |
| Simplified Acute Physiology Score 3 (IQR) | 59 (49–68) |
| Admission SOFA (IQR)a | 6 (2–9) |
| Admission SOFA, respiratory component (IQR)b | 2 (2–3) |
| Body mass index, kg/m2 (sd) | 29.4 (7.4) |
| Duration of symptoms before ICU (IQR), d | 10 (7–13) |
| Comorbidities, n (%) | |
| Hypertension | 319 (74.5) |
| Chronic obstructive pulmonary disease | 43 (10.0) |
| Heart failure | 52 (12.1) |
| Coronary artery disease | 56 (13.1) |
| Diabetes mellitus | 199 (46.5) |
| Chronic kidney disease | 62 (14.5) |
| Cerebrovascular disease | 31 (7.2) |
| Dementia | 14 (3.3) |
| Cancer | 48 (11.2) |
| Charlson Comorbidity Index (IQR) | 1 (0–3) |
| Frailtyc | |
| Clinical Frailty Scale (1–9), n (%) | |
| Very fit (1) | 30 (7.0) |
| Fit (2) | 89 (20.8) |
| Managing well (3) | 184 (43.0) |
| Vulnerable (4) | 65 (15.2) |
| Mildly frail (5) | 30 (7.0) |
| Moderately frail (6) | 18 (4.2) |
| Severely frail (7) | 12 (2.8) |
| Frailty index (0–1) | 0.15 (0.10–0.21) |
| ICU admission | |
| Glasgow Coma Scale (sd) | 11.6 (5.1) |
| Vasoactive drugs, n (%) | 124 (29.0) |
| Invasive mechanical ventilation, n (%) | 215 (50.2) |
| Length of duration (IQR), d | |
| Invasive mechanical ventilation among those who were ever mechanically ventilated | 8 (5–14) |
| ICU duration of stay | 9 (5–18) |
| Hospital duration of stay | 21 (14–32) |
IQR = interquartile range, SOFA = Sequential Organ Failure Assessment score.
Data were missing for 17 patients (3.9%).
Data were missing for nine patients (2.1%).
Frailty status was assessed in the period 2–4 wk before hospitalization.
Figure 1.
Transitions between frailty states during the 90 d after hospital discharge. A, Changes in frailty states from pre-COVID-19 (2–4 wk before infection) to 3-mo follow-up among survivors of critical COVID-19. B, The number of patients who transitioned to better frailty states. C, The number of patients who transitioned to worse frailty states. D, The number of patients who had no transition in frailty state. CFS = Clinical Frailty Scale.
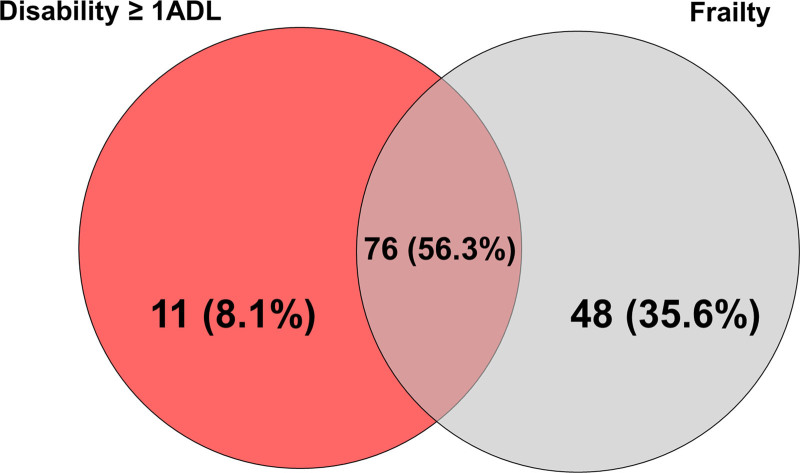
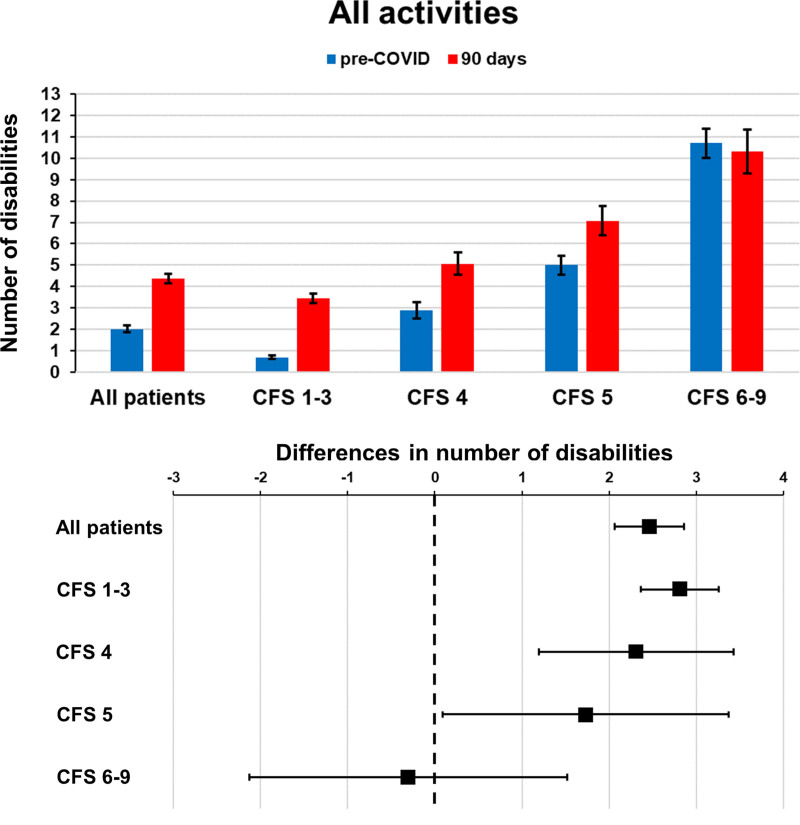
At day 90, 135 patients (34%) presented either frailty or ADL disability. In this group, 76 (56%) had both frailty and ADL disability, 48 (36%) had frail but no ADL disability, and 11 (8%) had ADL disability but not frailty (Fig. 2). We also verified that the number of disabilities at day 90 was significantly higher than baseline in our participants (p < 0.001; Supplement Table S2, http://links.lww.com/CCM/H25; and Fig. 3). Furthermore, we observed that the number of disabilities increased across all three types of activities (ADL, IADL, and mobility) (Supplement Table S3 and Fig. S2, http://links.lww.com/CCM/H25) and that participants with lower pre-COVID-19 CFS scores were more susceptible to such changes (Supplement Table S3, http://links.lww.com/CCM/H25).
Figure 2.
Venn diagram for the overlap of frailty and disability at 3-mo follow-up. Disability was defined as a Katz Activity of Daily Living (ADL) score ≥ 1 and Frailty as a Clinical Frailty Scale ≥ 5. The overlap of frailty with disability in activities of daily living among those who had one of these two syndromes at 3 mo is shown.
Figure 3.
Disabilities in all patients and according to Clinical Frailty Scale (CFS) groups. Top, Mean number of disabilities in all patients and across CFS groups in the pre-COVID-19 period (2–4 wk before infection) and 90 d after hospital discharge. Error bars represent se. Bottom, Mean difference in the number of disabilities in all patients and across CFS groups. Differences were between 90 d after hospital discharge and the pre-COVID-19 period (2–4 wk before infection). Error bars represent the 95% CI.
Sensitivity analysis with bootstrapping did not substantially change our main results (Supplement Table S4, http://links.lww.com/CCM/H25). Conversely, the proportion of patients without disabilities decreased between baseline (preserved ADL: 92%; preserved IADL: 65%; and preserved mobility: 61%) and day 90 (preserved ADL: 79%; preserved IADL: 38%; and preserved mobility: 38%). After controlling for possible confounders, we found that higher pre-COVID-19 CFS scores and assessment at day 90 were independently associated with more disabilities (Supplement Table S5, http://links.lww.com/CCM/H25).
DISCUSSION
We verified in a sample of 428 COVID-19 patients admitted to the ICU that: 1) one in every three participants were frail at the end of a 90-day follow-up, one in five had new-onset frailty, and several previously frail patients transitioned to a worse frailty status, 2) the number of disabilities more than doubled after critical illness, primarily in the previously non-frail subgroup, 3) after controlling for age, comorbidities, and acuity, the number of disabilities at day 90 was independently associated with pre-COVID frailty status, and 4) a considerable number of our participants had frailty but no ADL disability at day 90. Even though frailty was more frequent at the end of our follow-up, one in four patients required assistance to perform self-care (ADL) activities, and two-thirds needed help in mobility and to live independently in the community (IADL). Our findings emphasize the considerable impact critical COVID-19 might have on patients and their families even after hospital discharge.
Our results contribute to understanding the “continuum of critical illness and survivorship” (30) in COVID-19. To the best of our knowledge, this is the first study to demonstrate a high rate of worsening frailty following COVID-19 critical care. Over one-third of our participants transitioned to worse health status 3 months after hospital discharge, most of them developing frailty in this period. Given the association between frailty and long-term adverse outcomes, our findings indicate a new layer of burden for patients and their families and highlight the importance of rehabilitation for post-ICU care. We were unable to address whether transitions to worse frailty status resulted from critical illness, COVID-19, or an interaction between them, but Brummel et al (30) described similar trajectories in non-COVID ICU survivors. In their study, 46% of discharged patients transitioned to worse frailty status, compared with 39% in our cohort. Further research is needed to address if such changes are related to potentially modifiable factors during critical illness or are more specific to severe COVID-19.
We observed a substantial increase in the number of disabilities during our 90-day follow-up. From a patient-centered perspective, these are likely more relatable results than frailty since they reflect the day-to-day perceptions of patients and their families (i.e., if one needs any help to perform a routine activity). New disabilities predominantly affected mobility and IADL, possibly resulting from post-ICU physical and cognitive impairments, which are well-described in patients who experience acute respiratory distress syndrome (ARDS) and delirium (32, 33). ARDS and delirium are ubiquitous in COVID-ICU patients (34, 35). These patients are often treated with neuromuscular blockade agents (36) and deep sedation (37), and are kept in social isolation (34) with limited mobilization (38). Therefore, they are likely more vulnerable to post-ICU weakness and post-delirium cognitive decline. Thorough daily reassessment of the indications and maintenance of each of these practices is essential for high-quality COVID-ICU care.
Previous studies have investigated disabilities following non-COVID critical illness (24, 39). Higgins et al (39) recently reported that over half (59%) of the ICU patients in a heterogeneous cohort had new disabilities 6 months after hospital discharge. Nevertheless, the authors did not find an association between baseline frailty and new disabilities (39). In another multicenter cohort study, Brummel et al (24) observed 3 months after hospital discharge that frailty was associated with a higher risk of death, IADL-disability, and poorer physical quality of life, but not with ADL-disability. Some notable differences between these studies and our results should be acknowledged. First, our cohort evaluated only COVID-19 patients, who might share more homogeneous pathophysiological pathways leading to disabilities than previous heterogeneous cohorts. Second, critical COVID-19 usually requires more extended periods of invasive mechanical ventilation than described in other scenarios (median 8 d in our study compared with 2 d in Brummel et al’s [24] study and 3 d in Higgins et al’s [39] study). Therefore, our patients were submitted to longer periods of sedation and immobilism, which might explain the impact we observed on ADL. Finally, we included participants ≥50 years old who might be at higher risk of adverse outcomes than younger patients included in other cohorts.
Although frailty and disability represent different constructs, there is considerable overlap between these conditions (40). We observed that one in eight COVID-ICU survivors had frailty but no ADL disability 90 days after discharge. Since the presence of frailty is associated with poorer long-term outcomes in ICU survivors (e.g., new disabilities) (7, 40) and multicomponent interventions might mitigate its occurrence/progression and clinical consequences, we underscore the importance of screening for frailty when COVID-19 patients are discharged from the hospital. Even more important is to recognize that the patients’ quality of life has deteriorated in COVID-ICU survivors. Both conditions, frailty and disability, mean life-changing events for patients and families, also pose an extra burden on healthcare systems and societies as the pandemic advances and millions of people recover from the severe acute respiratory syndrome coronavirus-2 infection. Therefore, it is vital to implement a systematic approach to medical care after hospital discharge, not only to capture persistent symptoms and new limitations but to identify characteristics that might guide rehabilitation and recovery interventions (3).
Our study has notable strengths. First, both CO-FRAIL and EPICCoV studies included consecutive patients with minimal missing data. Therefore, we had an extensive database at our disposal that allowed thorough analyses of COVID-19 hospitalizations and postdischarge outcomes. Second, data related to frailty, health status, and pre- and post-ICU disabilities were collected by geriatrics-trained physicians, whereas data related to the ICU stays were obtained by a critical-care-trained team, ensuring high-quality data. Third, we had minimal loss of information in our 90-day postdischarge follow-up. Finally, we obtained novel and relevant disability data in ICU survivors, information previously lacking in the literature (41).
The study also had limitations. First, although our data were built from the largest teaching hospital in Latin America, receiving COVID-19 cases from 85 cities and 278 secondary hospitals, mainly from the metropolitan area of Sao Paulo, our findings are from a single center, which might limit the generalizability of our results. Second, frailty was scored using medical records and telephone interviews, not face-to-face evaluations, and our data were, therefore, susceptible to recall bias. However, due to visitation restrictions and respiratory isolation, the described approach was necessary and even advised by recent guidelines (25). Third, the CFS partially relies on the presence of disabilities (26). Thus, some overlap between frailty and disability would be expected, and their association may have been overestimated by our results. However, the Venn diagram suggests that they are distinct syndromes, as previously demonstrated by Fried et al (40). Finally, the time frame of 90 days might have been insufficient to address post-ICU recovery (32, 33). More conclusive answers on the long-term functional trajectories of COVID-ICU patients require further research and more extended follow-up periods.
CONCLUSIONS
In conclusion, frailty was associated with unfavorable patient-centered outcomes in COVID-19 patients admitted to the ICU. One in three of our critical care patients were frail 3 months after hospital discharge, with frequent transitions to worse health status and the development of new disabilities over the 90-day follow-up. Providers should be aware that assessing frailty can bring valuable information to their clinical decisions and treatment plans before, during, and even after critical illness in COVID-19.
ACKNOWLEDGMENTS
We thank the dedicated work performed by healthcare workers and staff in our hospital during the COVID-19 crisis.
Supplementary Material
Footnotes
*See also p. 1023.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Taniguchi, Avelino-Silva, and Aliberti contributed to the conception and design of the work. All authors performed the acquisition, analysis, and interpretation of data for the work; contributed to the drafting and revised it; and approved the final version before submission.
Supported, in part, by Hospital das Clinicas Hospital das Clinicas da Faculdade de Medicina da Universidade de Sao Paulo (HCFMUSP), Faculdade de Medicina, Universidade de Sao Paulo, Brazil, from donations to the #HCComvida campaign. The #HCComvida fundraising campaign was a joint initiative from ordinary citizens, healthcare professionals, and researchers appealing to the broader society to support HCFMUSP’s frontline work against the COVID-19 pandemic. Donations were directed to the institution’s emergency initiatives related to fighting COVID-19, including research projects. The funders had no role in the study’s design, collection, management, analysis, or interpretation of the data or the preparation, review, or approval of the article.
The authors have disclosed that they do not have any potential conflicts of interest.
A complete list of investigators from the COVID-19 and Frailty Study Group, EPIdemiology of Critical COVID-19 Study Group, and COVID Hospital das Clinicas, University of Sao Paulo Medical School Study Group is provided in the Supplementary Materials (http://links.lww.com/CCM/H25).
Brazilian Clinical Trials Registry: RBR-7w5zhr and Clinicaltrials.gov: NCT04378582.
REFERENCES
- 1.Navaratnam AV, Gray WK, Day J, et al. : Patient factors and temporal trends associated with COVID-19 in-hospital mortality in England: An observational study using administrative data. Lancet Respir Med 2021; 9:397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai SV, Law TJ, Needham DM: Long-term complications of critical care. Crit Care Med 2011; 39:371–379 [DOI] [PubMed] [Google Scholar]
- 3.Denehy L, Puthucheary Z: Surviving COVID-19: A familiar road to recovery? Lancet Respir Med 2021; 9:1211–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans RA, McAuley H, Harrison EM, et al. ; PHOSP-COVID Collaborative Group: Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir Med 2021; 9:1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg A, Young J, Iliffe S, et al. : Frailty in elderly people. Lancet 2013; 381:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagshaw SM, Stelfox HT, Johnson JA, et al. : Long-term association between frailty and health-related quality of life among survivors of critical illness: A prospective multicenter cohort study. Crit Care Med 2015; 43:973–982 [DOI] [PubMed] [Google Scholar]
- 8.Bagshaw SM, Stelfox HT, McDermid RC, et al. : Association between frailty and short- and long-term outcomes among critically ill patients: A multicentre prospective cohort study. CMAJ 2014; 186:E95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crook H, Raza S, Nowell J, et al. : Long covid-mechanisms, risk factors, and management. BMJ 2021; 374:n1648. [DOI] [PubMed] [Google Scholar]
- 10.Morin L, Savale L, Pham T, et al. ; Writing Committee for the COMEBAC Study Group: Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021; 325:1525–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L, Yao Q, Gu X, et al. : 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021; 398:747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Gassel RJJ, Bels J, Remij L, et al. : Functional outcomes and their association with physical performance in mechanically ventilated coronavirus disease 2019 survivors at 3 months following hospital discharge: A cohort study. Crit Care Med 2021; 49:1726–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aliberti MJR, Szlejf C, Avelino-Silva VI, et al. ; COVID HCFMUSP Study Group: COVID-19 is not over and age is not enough: Using frailty for prognostication in hospitalized patients. J Am Geriatr Soc 2021; 69:1116–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira JC, Ho YL, Besen BAMP, et al. ; EPICCoV Study Group: Protective ventilation and outcomes of critically ill patients with COVID-19: A cohort study. Ann Intensive Care 2021; 11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira JC, Ho YL, Besen BAMP, et al. ; EPICCoV Study Group: Characteristics and outcomes of patients with COVID-19 admitted to the ICU in a university hospital in São Paulo, Brazil - study protocol. Clinics (Sao Paulo) 2020; 75:e2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium: The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frenkel WJ, Jongerius EJ, Mandjes-van Uitert MJ, et al. : Validation of the Charlson Comorbidity Index in acutely hospitalized elderly adults: A prospective cohort study. J Am Geriatr Soc 2014; 62:342–346 [DOI] [PubMed] [Google Scholar]
- 18.Metnitz PG, Moreno RP, Almeida E, et al. ; SAPS 3 Investigators: SAPS 3–from evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med 2005; 31:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno RP, Metnitz PG, Almeida E, et al. ; SAPS 3 Investigators: SAPS 3–From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 2005; 31:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent JL, Moreno R, Takala J, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 21.Rockwood K, Song X, MacKnight C, et al. : A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muscedere J, Waters B, Varambally A, et al. : The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intensive Care Med 2017; 43:1105–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utino Taniguchi L, Ibrahim Q, Azevedo LCP, et al. : Comparison of two frailty identification tools for critically ill patients: A post-hoc analysis of a multicenter prospective cohort study. J Crit Care 2020; 59:143–148 [DOI] [PubMed] [Google Scholar]
- 24.Brummel NE, Bell SP, Girard TD, et al. : Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med 2017; 196:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute for Health and Care Excellence: COVID-19 Rapid Guideline: Critical Care in Adults. NICE Guideline [NG159], 2020. Available at: https://www.nice.org.uk/guidance/NG159. Accessed March 21, 2020 [PubMed]
- 26.Rockwood K, Theou O: Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J 2020; 23:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan DC, Tsou HH, Chen CY, et al. : Validation of the Chinese-Canadian study of health and aging clinical frailty scale (CSHA-CFS) telephone version. Arch Gerontol Geriatr 2010; 50:e74–e80 [DOI] [PubMed] [Google Scholar]
- 28.Flaatten H, De Lange DW, Morandi A, et al. ; VIP1 study group: The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥80 years). Intensive Care Med 2017; 43:1820–1828 [DOI] [PubMed] [Google Scholar]
- 29.Ferrante LE, Pisani MA, Murphy TE, et al. : Functional trajectories among older persons before and after critical illness. JAMA Intern Med 2015; 175:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brummel NE, Girard TD, Pandharipande PP, et al. : Prevalence and course of frailty in survivors of critical illness. Crit Care Med 2020; 48:1419–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz S, Ford AB, Moskowitz RW, et al. : Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963; 185:914–919 [DOI] [PubMed] [Google Scholar]
- 32.Herridge MS, Tansey CM, Matté A, et al. ; Canadian Critical Care Trials Group: Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364:1293–1304 [DOI] [PubMed] [Google Scholar]
- 33.Pandharipande PP, Girard TD, Jackson JC, et al. ; BRAIN-ICU Study Investigators: Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369:1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pun BT, Badenes R, Heras La Calle G, et al. ; COVID-19 Intensive Care International Study Group: Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): A multicentre cohort study. Lancet Respir Med 2021; 9:239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berlin DA, Gulick RM, Martinez FJ: Severe Covid-19. N Engl J Med 2020; 383:2451–2460 [DOI] [PubMed] [Google Scholar]
- 36.National Heart Lung, Blood Institute PCTN ; Moss M, Huang DT, Brower RG, et al. : Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med 2019; 380:1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salluh JI, Sharshar T, Kress JP: Does this patient have delirium? Intensive Care Med 2017; 43:693–695 [DOI] [PubMed] [Google Scholar]
- 38.Pun BT, Balas MC, Barnes-Daly MA, et al. : Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019; 47:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins AM, Neto AS, Bailey M, et al. ; PREDICT Study Investigators: Predictors of death and new disability after critical illness: A multicentre prospective cohort study. Intensive Care Med 2021; 47:772–781 [DOI] [PubMed] [Google Scholar]
- 40.Fried LP, Ferrucci L, Darer J, et al. : Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004; 59:255–263 [DOI] [PubMed] [Google Scholar]
- 41.Jung C, Flaatten H, Fjølner J, et al. ; COVIP study group: The impact of frailty on survival in elderly intensive care patients with COVID-19: The COVIP study. Crit Care 2021; 25:149. [DOI] [PMC free article] [PubMed] [Google Scholar]