OBJECTIVES:
To evaluate the sleep and circadian rest-activity pattern of critical COVID-19 survivors 3 months after hospital discharge.
DESIGN:
Observational, prospective study.
SETTING:
Single-center study.
PATIENTS:
One hundred seventy-two consecutive COVID-19 survivors admitted to the ICU with acute respiratory distress syndrome.
INTERVENTIONS:
Seven days of actigraphy for sleep and circadian rest-activity pattern assessment; validated questionnaires; respiratory tests at the 3-month follow-up.
MEASUREMENTS AND MAIN RESULTS:
The cohort included 172 patients, mostly males (67.4%) with a median (25th–75th percentile) age of 61.0 years (52.8–67.0 yr). The median number of days at the ICU was 11.0 (6.00–24.0), and 51.7% of the patients received invasive mechanical ventilation (IMV). According to the Pittsburgh Sleep Quality Index (PSQI), 60.5% presented poor sleep quality 3 months after hospital discharge, which was further confirmed by actigraphy. Female sex was associated with an increased score in the PSQI (p < 0.05) and IMV during ICU stay was able to predict a higher fragmentation of the rest-activity rhythm at the 3-month follow-up (p < 0.001). Furthermore, compromised mental health measured by the Hospital Anxiety and Depression Scale was associated with poor sleep quality (p < 0.001).
CONCLUSIONS:
Our findings highlight the importance of considering sleep and circadian health after hospital discharge. Within this context, IMV during the ICU stay could aid in predicting an increased fragmentation of the rest-activity rhythm at the 3-month follow-up. Furthermore, compromised mental health could be a marker for sleep disruption at the post-COVID period.
Keywords: actigraphy, acute respiratory distress syndrome, intensive care unit, Pittsburgh Sleep Quality Index, post-COVID, sequelae
COVID-19 affected more than 200 million people worldwide until August 2021. During the acute phase of the disease, approximately 30% of the patients develop severe complications, which increase the risk of hospitalization, ICU admission, and death (1). In addition, recent studies report a set of symptoms observed months after hospital discharge including fatigue, joint pain, dyspnea, cough, anxiety, depression, and cognitive impairment, among others (2, 3).
While studies investigating the different sequelae in COVID-19 patients are rapidly emerging, a comprehensive evaluation of sleep and circadian rhythms in this context is yet to be performed. Complaints of insomnia and disturbed sleep are often observed, reaching a prevalence of up to 31% (4–6). Nevertheless, all the available findings are based on self-reported assessments. Therefore, additional investigations using validated sleep questionnaires and objective measurements are extremely necessary.
The hospital and particularly the ICU are harmful environments for sleep and circadian health. Accordingly, critically ill patients are exposed to excessive noise and interruptions during the night, unusual feeding schedules, and mistimed artificial light at the detriment of sunlight exposure (7–9). In addition, an appropriate amount of activity during the day and the maintenance of a social routine are often hindered by restrictions related to the hospital context and the health condition of the patients. These factors ultimately lead to poor sleep quality, increased fragmentation of the rest-activity pattern, and decreased synchronization between the endogenous rhythms and the external environment (10, 11). Furthermore, other situations often experienced by critical COVID-19 patients such as depression and anxiety may affect sleep and circadian health (12).
The main objective of this study was to evaluate the sleep and circadian rest-activity pattern of critical COVID-19 survivors 3 months after hospital discharge. To accomplish this, subjective and objective evaluations were performed using validated questionnaires and wrist actigraphy. We hypothesized that an important number of patients would have compromised sleep and circadian health. We also performed two additional analyses. First, we investigated whether baseline characteristics and ICU-related procedures could predict sleep and circadian outcomes after hospital discharge. Second, we evaluated whether the other sequelae could be associated with sleep and circadian alterations.
MATERIALS AND METHODS
Study Population
This is a prospective, observational, single-center study designed to evaluate the sleep and circadian rest-activity pattern of critical COVID-19 survivors after hospital discharge (Fig. S1, http://links.lww.com/CCM/H39). Patients were recruited at the Hospital Universitari Arnau de Vilanova-Santa Maria (Lleida, Spain) between March 2020 and April 2021. The inclusion criteria included individuals more than 18 years old who had a confirmed diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection through polymerase chain reaction, developing acute respiratory distress syndrome, and consequently being admitted to the ICU. The exclusion criteria included: 1) patients in palliative care and 2) patients with severe mental and/or physical disability that could prevent the proposed evaluations.
This study was approved by the Medical Ethics Committee of the Hospital Universitari Arnau de Vilanova (Identifier: CEIC-2510) and conducted according to the principles outlined by the Declaration of Helsinki. Informed consent was acquired for all patients.
Study Design
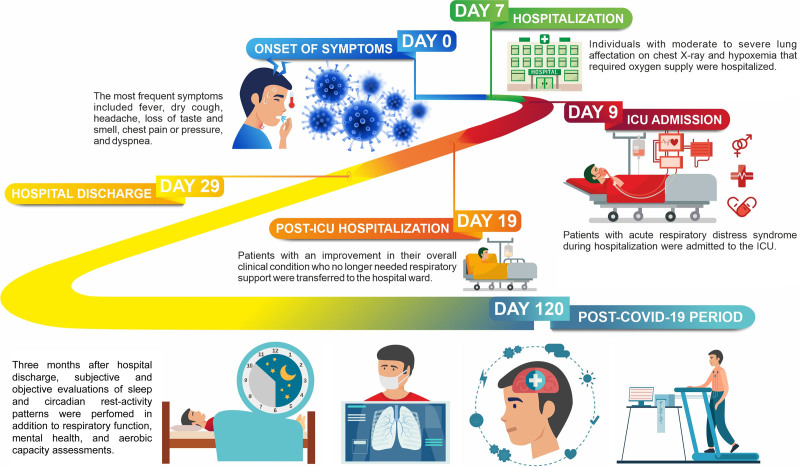
Patients recruited at baseline arrived at the Hospital Universitari de Santa Maria (Lleida, Spain) for the medical appointment 3 months after hospital discharge (Fig. 1). A clinical evaluation was performed followed by subjective and objective assessments of sleep and circadian rest-activity pattern. In the sequence, we evaluated respiratory function, mental health, and aerobic capacity.
Figure 1.
Clinical chronology of COVID-19 patients included in the study. The time of hospitalization and time spent at the ICU are represented as median (percentile 25–75).
Clinical Data
Clinical data were obtained at baseline (ICU stay) and at the clinical evaluation 3 months after hospital discharge. Age, sex, body mass index, comorbidities, alcohol consumption, smoking habits, time spent at the ICU, duration of invasive mechanical ventilation (IMV), duration of noninvasive mechanical ventilation (NIMV), hours in prone position, pharmacotherapy, Pao2 to Fio2 ratio, and peripheral oxygen saturation to Fio2 ratio were collected at baseline. COVID-19–related symptoms were obtained at the clinical evaluation after hospital discharge.
Sleep and Circadian Rest-Activity Pattern
Pittsburgh Sleep Quality Index.
Sleep quality was assessed by the Pittsburgh Sleep Quality Index (PSQI). This questionnaire is composed of 19 questions representing one of the seven components of sleep quality: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, sleep medication intake, and daytime dysfunction. Each component score is rated on a 3-point scale, leading to a sum of up to 21 points. A PSQI score greater than 5 indicates a poor sleep quality, whereas a PSQI score less than or equal to 5 indicates a good sleep quality (13, 14).
Epworth Sleepiness Scale.
Excessive daytime somnolence was assessed by the Epworth Sleepiness Scale (ESS). This questionnaire is composed of eight questions to assess the chance of falling asleep during different daily situations. Each question is rated on a 3-point scale, in which 0 represents no chance of occurrence, and 3 indicates a high chance of occurrence. The overall score ranges from 0 to 24 points. Higher scores represent increased daytime somnolence (15–17).
Satisfaction Alertness Timing Efficiency Duration.
Sleep health was further assessed by the Satisfaction Alertness Timing Efficiency Duration. This questionnaire is composed of five questions representing one of the following sleep-related dimensions each: subjective satisfaction, alertness during waking hours, appropriate timing, efficiency, and duration. Each question is rated on a 2-point scale, leading to a sum of up to 10 points. Higher scores indicate better sleep health (18).
Actigraphy.
Recruited patients who arrived for the medical appointment 3 months after hospital discharge were randomly selected for the objective assessment of sleep and circadian rest-activity pattern through the use of a wrist-mounted actigraph (Actiwatch 2; Philips Respironics, Murrysville, PA) for 7 days. A sleep log was also delivered to be completed during the same period. The following variables were obtained: time in bed (hr), total sleep time (hr), sleep efficiency (%, defined as the ratio between total sleep time and the time spent in bed), latency (min, defined as the time spent awake until the first sleep episode while in bed), and time spent awake after sleep onset (WASO) (min). In addition, activity counts of 60-second epochs were obtained, from which different variables associated with the rest-activity rhythm were calculated. The intradaily variability (IV) represents the fragmentation of the rest-activity rhythm within each 24-hour period, indicating whether there are daytime naps and/or nocturnal activity episodes. The interdaily stability (IS) represents the similarity between one 24-hour period and the next, indicating how synchronized the internal rest-activity rhythm is with the different zeitgebers over 7 days of actigraphy. The mean activity of the 5 consecutive hours with the lowest activity (L5) and the mean activity of the 10 consecutive hours with the highest activity (M10) were used to calculate the relative amplitude (RA) (M10–L5/M10 + L5). The amplitude represents the robustness of the rest-activity rhythm, indicating whether a difference in the magnitude of activity between active and rest phases occurs (19).
Respiratory Function
Airway function was measured and represented as previously described (3). The diffusing lung capacity for carbon monoxide (Dlco) was the variable used to represent the respiratory function. CT of the chest was performed to evaluate the severity of lung affectation. To quantify this, we calculated the total severity score (TSS). A detailed description of the procedure can be found in González et al (3).
Mental Health Evaluation
The Hospital Anxiety and Depression Scale (HADS) were used to assess signs of anxiety and depression. This questionnaire consists of a 7-item anxiety subscale and a 7-item depression subscale. Each item is rated on a 3-point scale, leading to a sum of up to 21 points. A score greater than 8 indicates possible anxiety or depression, whereas a score less than or equal to 8 indicates the opposite (20–22).
Aerobic Capacity
The 6-minute walking test (6MWT) was performed to evaluate the aerobic capacity (23). The traveled distance was compared with reference values. Accordingly, the predicted 6-minute walked distance (6MWD) was calculated based on the following equations: for men, predicted 6MWD = (7.57 × height)–(5.02 × age)–(1.76 × weight)–309 m; for women, predicted 6MWD = (2.11 × height)–(5.78 × age)–(2.29 × weight) + 667 m (24). Percent predicted 6MWD (PP-6MWD) was calculated using the formula: PP-6MWD = 6MWD/Predicted 6MWD × 100.
Statistical Analysis
Descriptive statistics were performed to describe sociodemographic and clinical characteristics as well as ICU-related information and post-COVID sequelae. Absolute and relative frequencies were used for qualitative data. The means (sd) and medians (25th–75th percentile) were estimated for quantitative variables with normal and non-normal distributions, respectively. The normality of the distribution was assessed by the Shapiro-Wilk test.
We evaluated possible associations between baseline characteristics (data obtained during the ICU stay) and sleep/circadian-related data (PSQI score, IV, IS, and RA) collected 3 months after hospital discharge. PSQI score was chosen based on the observed outcomes and in the clinical relevance of this questionnaire (25). IV, IS, and RA are variables that represent important dimensions of the circadian rest-activity pattern (19). The selection of baseline variables was performed using a relaxed least absolute shrinkage and selection operator (LASSO) model (26, 27). Tenfold cross-validation was carried out to determine the lambda parameter of the LASSO model (28). Lambda was selected as the value that minimized the mean square error. To perform the LASSO analysis, missing values were replaced by means of the nonmissing values.
We investigated possible associations between sleep/circadian-related data (PSQI score, IV, IS, and RA) and other sequelae collected 3 months after hospital discharge. The chosen variables represented different physiologic domains that could be associated with sleep and circadian rhythms within this context. These included the depression score (HADS), anxiety score (HADS), Dlco, TSS, PP-6MWD, and other COVID-19–related symptoms (only those with a higher prevalence were included in the analysis). Pearson coefficient tests were performed to assess correlations between variables.
The p value threshold defining statistical significance was set at less than 0.05. All statistical analyses were performed using R software, Version 4.0.2 (R Core Team; Vienna, Austria).
RESULTS
Baseline Characteristics of the Cohort
The cohort included 172 COVID-19 patients admitted to the ICU. Most of them were males (67.4%) with a median (25th–75th percentile) age of 61.0 years (52.8–67.0 yr) (Table 1). Different comorbidities were present, including obesity (48.5%), hypertension (47.1%), and diabetes mellitus (22.7%). The patients spent a median number of 23 days (14.0–38.2 d) at the hospital and 11 days (6.00–24.0 d) at the ICU, where 51.7% received IMV and 70.9% needed NIMV. Similar characteristics were observed considering only the individuals who performed the actigraphy (Table S1, http://links.lww.com/CCM/H39).
TABLE 1.
Baseline Characteristics of the Cohort
| Characteristics | Global, n = 172 |
|---|---|
| Sociodemographic data | |
| Sex, male | 116 (67.4%) |
| Age, yr | 61.0 (52.8–67.0) |
| Body mass index, kg/m2 | 29.8 (26.8–34.2) |
| Habits | |
| Tobacco | |
| Current smoker | 7 (4.07%) |
| Former smoker | 72 (41.9%) |
| Nonsmoker | 93 (54.1%) |
| Chronic alcohol abuse | 6 (3.49%) |
| Comorbidities | |
| Obesity | 83 (48.5%) |
| Hypertension | 81 (47.1%) |
| Diabetes mellitus | 39 (22.7%) |
| Chronic lung disease | 10 (5.81%) |
| Hospital stay | |
| Days | 23.0 (14.0–38.2) |
| ICU stay | |
| Days | 11.0 (6.00–24.0) |
| Minimum Pao2 to Fio2 ratio | 110 (84.0–168) |
| Procedures | |
| Mechanical ventilation | |
| Invasive | 89 (51.7%) |
| Days | 16.0 (9.00–26.0) |
| Noninvasive | 122 (70.9%) |
| Days | 3.00 (1.00–5.00) |
| Prone position | 84 (48.8%) |
| Prone position, hr | 41.0 (24.0–73.0) |
| Pharmacotherapy | |
| Antibiotics | 142 (82.6%) |
| Corticosteroids | 153 (89.0%) |
| Tocilizumab | 108 (63.2%) |
| Hydroxychloroquine | 55 (32.0%) |
| Remdesivir | 25 (14.5%) |
Qualitative and quantitative data are represented as n (%) and median (25th–75th percentile), respectively.
Missings: obesity, 1; minimum Pao2/Fio2, 6; prone position, hr, 7; tocilizumab, 1.
Sleep and Circadian Rest-Activity Pattern
According to the PSQI, most of the patients presented poor sleep quality (60.5%) with a mean (sd) score of 7.09 (4.41) (Table 2). Sleep duration and sleep efficiency were the most affected domains with 73.9% of the patients sleeping less than the recommended hours and 57.5% with a sleep efficiency lower than 85% (29). The objective evaluation of sleep through actigraphy further confirmed these findings, demonstrating that 50.8% of the patients slept less than 7 hours per night, which was possibly associated with the WASO (Table 3). Accordingly, sleep efficiency was decreased in 55.4% of the patients. The circadian rest-activity pattern presented substantial variability within our sample (Fig. S2, http://links.lww.com/CCM/H39).
TABLE 2.
Sleep Subjective Evaluation of Sleep (Questionnaires)
| Questionnaires | Global, n = 172 |
|---|---|
| Pittsburgh Sleep Quality Index | 7.09 (4.41) |
| Good sleep quality | 68 (39.5%) |
| Poor sleep quality | 104 (60.5%) |
| Subjective sleep quality | 1.12 (0.79) |
| Very good | 33 (19.2%) |
| Fairly good | 97 (56.4%) |
| Fairly bad | 31 (18.0%) |
| Very bad | 11 (6.40%) |
| Sleep latency | 1.14 (1.10) |
| ≤ 15 min | 64 (37.2%) |
| 16–30 min | 49 (28.5%) |
| 31–60 min | 30 (17.4%) |
| > 60 min | 29 (16.9%) |
| Sleep duration | 1.41 (1.10) |
| > 7 hr | 45 (26.2%) |
| 6–7 hr | 50 (29.1%) |
| 5–6 hr | 39 (22.7%) |
| < 5 hr | 38 (22.1%) |
| Sleep efficiency | 1.08 (1.15) |
| ≥ 85% | 73 (42.4%) |
| 75–84% | 46 (26.7%) |
| 65–74% | 19 (11.0%) |
| < 65% | 34 (19.8%) |
| Sleep disturbance | 0.97 (0.62) |
| Not during past month | 32 (18.6%) |
| Less than once a week | 116 (67.4%) |
| Once or twice a week | 21 (12.2%) |
| Three or more times a week | 3 (1.74%) |
| Sleep medication intake | 0.78 (1.29) |
| Not during past month | 125 (72.7%) |
| Less than once a week | 1 (0.58%) |
| Once or twice a week | 5 (2.91%) |
| Three or more times a week | 41 (23.8%) |
| Daytime dysfunction | 0.60 (0.86) |
| Never | 104 (60.5%) |
| A few times | 40 (23.3%) |
| Sometimes | 21 (12.2%) |
| A lot of times | 7 (4.07%) |
| Epworth Sleepiness Scale | 6.12 (3.77) |
| Satisfaction Alertness Timing Efficiency Duration | 7.54 (2.16) |
Qualitative data are presented as n (%). Quantitative data are presented as mean (sd).
TABLE 3.
Objective Evaluation of Sleep (Actigraphy)
| Variables | Global, n = 65 |
|---|---|
| Sleep | |
| Total sleep time, hr | 6.98 (6.33–7.67) |
| > 9 | 3 (4.62%) |
| 7–9 | 29 (44.6%) |
| < 7 | 33 (50.8%) |
| Time in bed, hr | 8.38 (7.73–9.10) |
| Sleep efficiency, % | 84.6 (81.0–88.3) |
| ≥ 85 | 29 (44.6%) |
| 75–84 | 29 (44.6%) |
| < 75 | 7 (10.8%) |
| Latency, min | 10.0 (5.00–18.0) |
| ≤ 30 | 57 (87.7%) |
| 31–45 | 6 (9.23%) |
| > 45 | 2 (3.08%) |
| Arousals, number | 25.5 (7.07) |
| Wake after sleep onset, min | 51.0 (39.0–66.0) |
| 0–20 | 1 (1.54%) |
| 21–40 | 17 (26.2%) |
| ≥ 40 | 47 (72.3%) |
| Rest-activity rhythm | |
| Interdaily stability | 0.59 (0.13) |
| Intradaily variability | 0.81 (0.19) |
| Relative amplitude | 0.89 (0.85–0.93) |
| The mean activity of the 10 consecutive hr with more activity | 238 (170–315) |
| The mean activity of the 5 consecutive hr with less activity | 12.2 (8.46–19.6) |
Qualitative data are represented as n (%). The means (sd) and medians (25th–75th percentile) were estimated for variables with normal and non-normal distributions, respectively.
Other Sequelae After Hospital Discharge
We evaluated other sequelae including those related to respiratory function, mental health, and aerobic capacity (Table S2, http://links.lww.com/CCM/H39). Similar to previous findings (3), 75.4% of patients presented an abnormal Dlco (< 80%) and the mean (sd) distance in the 6MWT was 408 meters (90.5 m). Such distance was 87.8% (23.8) of that predicted for healthy individuals after adjusting for sex, age, height, and weight. In relation to mental health, 5.92% of the patients presented abnormal scores for depression and 14.2% for anxiety. Also, the most prevalent symptoms after hospital discharge were muscular fatigue (21.7%) and cough (18.4%). Similar findings were observed considering only the individuals who performed the actigraphy.
Predictive Factors for Sleep and Circadian Outcomes After Hospital Discharge
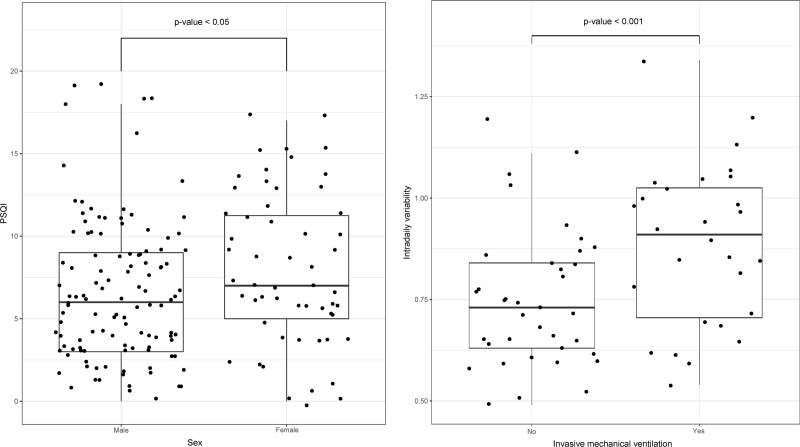
We investigated whether baseline characteristics and ICU-related procedures could predict poor sleep quality and alterations in the circadian rest-activity pattern 3 months after hospital discharge. We observed that the female sex was associated with an increased score in the PSQI (p < 0.05) (Fig. 2). Also, the patients that received IMV during the ICU stay presented increased IV at the 3-month follow-up compared with those who did not need this procedure (p < 0.001).
Figure 2.
Predictive factors for poor sleep quality and increased fragmentation of the circadian rest-activity rhythm after hospital discharge. t test was performed to assess correlations between the variables. The p value threshold defining statistical significance was set at less than 0.05. PSQI = Pittsburgh Sleep Quality Index.
Associations Between Sequelae After Hospital Discharge
We investigated whether sleep and circadian rest-activity pattern were associated with other important sequelae within this context. The analysis demonstrated a positive correlation between the PSQI score and both anxiety (rho = 0.51; p < 0.001) and depression (rho = 0.47; p < 0.001) scores obtained with the HADS (Fig. S3, http://links.lww.com/CCM/H39). No significant correlations were found in relation to the IV, IS, and RA (Figs. S4–S6, http://links.lww.com/CCM/H39).
DISCUSSION
In the current study, we performed a comprehensive analysis of the sleep and circadian rest-activity pattern of critical COVID-19 survivors 3 months after hospital discharge. Subjective evaluation using the PSQI revealed a poor sleep quality, which was further confirmed by objective evaluation using actigraphy. In addition, female sex was associated with an increased score in the PSQI while IMV during the ICU stay were able to predict an increased fragmentation of the rest-activity rhythm 3 months after hospital discharge. Furthermore, we observed that poor sleep quality was strongly associated with anxiety and depression at the 3-month follow-up.
Recent studies suggest the presence of sleep alterations in COVID-19 patients months after hospitalization. Arnold et al (4) observed that 24% of 110 consecutive COVID-19 patients reported insomnia 3 months after hospital admission. This number appeared to be higher in another cohort of patients, reaching 31% at 3–4 months postadmission (5). In addition, a recent meta-analysis including 31 studies demonstrated a pooled prevalence of sleeping disturbances in 34% of 5,153 COVID-19 patients (31). Nevertheless, all of these findings are based on self-reported assessments. For the first time, through validated questionnaires and an objective evaluation, we demonstrated that a great percentage of COVID-19 patients present poor sleep quality, particularly related to insufficient sleep duration and inappropriate sleep efficiency. The prevalence of individuals with compromised sleep quality herein observed was higher than that in previous studies, which could be related to differences in the methods used to assess sleep as well as differences among the populations. In fact, our cohort was exclusively composed of critical COVID-19 patients admitted to the ICU, while the referred studies included patients with different severities. Regardless, our findings reinforce the importance of considering the compromised sleep quality as a component of the post-COVID period, which is of particular interest given the influence of sleep on respiratory and immune function (31–33).
The hospital and particularly the ICU are known for excessive noise and interruptions during the night, unusual feeding schedules, and mistimed artificial light at the detriment of sunlight exposure (7–9). In addition, habitual procedures performed in this environment may account for poor sleep quality and circadian disruption. We observed that IMV during the ICU stay predicted increased fragmentation of the circadian rest-activity rhythm at 3 months after hospital discharge. This procedure requires the administration of sedative agents that usually affect the sleep structure and circadian rhythms (34, 35). IMV-associated events such as the irregular noradrenergic response and the so-called “biotrauma” may have contributed to altered sleep and circadian rhythms as well (36, 37). Furthermore, given the complex relationship between respiratory function and circadian rhythms (38–40), the respiratory condition of patients submitted to IMV may have been related to increased fragmentation of the rhythm at the 3-month follow-up. Further studies will be necessary to evaluate whether causality between the aforementioned variables is present.
In addition to the characteristics collected at baseline and factors related to the ICU stay, other sequelae may have contributed to alterations in sleep and circadian health at the 3-month follow-up. We observed an association between poor sleep quality, anxiety, and depression. Such an expected relationship was previously demonstrated in several contexts, including those related to the COVID-19 outbreak (41). However, to our knowledge, the current investigation is the first to confirm this association in COVID-19 patients.
It is important to address some limitations of this study. First, the objective assessment of sleep through actigraphy included a subset of patients randomly selected from the global population. Nevertheless, both groups were similar in relation to the baseline characteristics. Also, the subjective analysis of sleep was corroborated by the actigraphy data. Second, the COVID-19 context prevented a baseline assessment of sleep and circadian health, and therefore it was not possible to evaluate whether the patients already had circadian or sleep alterations prior to being hospitalized or infected. Third, it was not possible to establish whether sleep and circadian alterations were consequences of ICU stay or SARS-CoV-2 infection itself. In fact, given the observational design of this study, relationships of causality cannot be confirmed. However, this was beyond the objective of this study. We aimed to evaluate the sleep and circadian health of critical COVID-19 patients after hospital discharge, to highlight the importance of considering these factors in clinical practice. This is the first report presenting a proper characterization of sleep and circadian rest-activity pattern of critical COVID-19 patients through validated questionnaires and an objective assessment. Previous studies were mainly based on self-reported assessments of sleep health and without any evaluation related to circadian function. In addition, with the prospective design and a well-characterized cohort, it was possible to establish potential predictive factors at baseline, including those related to ICU stay, for the adverse outcomes observed after hospital discharge.
CONCLUSIONS
Critical COVID-19 survivors may present poor sleep quality and alterations in the circadian rest-activity pattern 3 months after hospital discharge. Within this context, female sex could aid in predicting a worse sleep quality while IMV during the ICU stay could predict an increased fragmentation of the rest-activity rhythm at the post-COVID period. Furthermore, compromised mental health could be a marker for sleep disruption at the 3-month follow-up.
ACKNOWLEDGMENTS
We would like to express our sincere gratitude to all of the patients.
Supplementary Material
Footnotes
*See also p. 1021.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Mr. Benítez, Dr. Labarca, Dr. Torres, Dr. González, Dr. de Gonzalo-Calvo, Dr. Barbé, and Dr. Targa were involved in conceptualization. Ms. Santisteve, Ms. Carmona, and Dr. González were involved in recruitment, clinical evaluation, and assessment of respiratory function. Ms. Vaca and Ms. Minguez were involved in assessment of sleep, circadian rest-activity pattern, anxiety, and depression. Ms. Moncusí-Moix, Ms. Gort-Paniello, and Dr. Targa were involved in data management and analyses of actigraphy data. Mr. Benítez was involved in statistical analysis. Mr. Benítez, Dr. Torres, Dr. Fagotti, Dr. de Gonzalo-Calvo, Dr. Barbé, and Dr. Targa were involved in data interpretation. Dr. Targa was involved in writing. All authors were involved in revision.
Funding was provided by Instituto de Salud Carlos III (CIBERESUCICOVID, COV20/00110), cofunded by European Regional Development Fund, “Una manera de hacer Europa”, and by the Donation program “estar preparados”, UNESPA, Madrid, Spain. Dr. Labarca has received financial support from Agencia Nacional de Investigacion y Desarrollo, Chile, grant COVID 1005. Dr. de Gonzalo-Calvo has received financial support from Instituto de Salud Carlos III (Miguel Servet 2020: CP20/00041), co-funded by the European Social Fund/“Investing in your future”.
Dr. Labarca has received financial support from Agencia Nacional de Investigacion y Desarrollo, Chile, grant COVID 1005. Dr. de Gonzalo-Calvo has received financial support from Instituto de Salud Carlos III (Miguel Servet 2020: CP20/00041), co-funded by the European Social Fund/“Investing in your future.” Drs. de Gonzalo-Calvo and Barbé disclosed work for hire. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. ; Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19): Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020; 34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalbandian A, Sehgal K, Gupta A, et al. : Post-acute COVID-19 syndrome. Nat Med 2021; 27:601–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González J, Benítez ID, Carmona P, et al. : Pulmonary function and radiologic features in survivors of critical Covid-19: A 3-month prospective cohort. Chest 2021; 160:187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold DT, Hamilton FW, Milne A, et al. : Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021; 76:399–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrigues E, Janvier P, Kherabi Y, et al. : Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81:e4–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Huang L, Wang Y, et al. : 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021; 397:220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman NS, Gazendam J, Levan L, et al. : Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med 2001; 163:451–457 [DOI] [PubMed] [Google Scholar]
- 8.Gazendam JAC, Van Dongen HPA, Grant DA, et al. : Altered circadian rhythmicity in patients in the ICU. Chest 2013; 144:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korompeli A, Muurlink O, Kavrochorianou N, et al. : Circadian disruption of ICU patients: A review of pathways, expression, and interventions. J Crit Care 2017; 38:269–277 [DOI] [PubMed] [Google Scholar]
- 10.Cho Y, Ryu SH, Lee BR, et al. : Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int 2015; 32:1294–1310 [DOI] [PubMed] [Google Scholar]
- 11.Moss TG, Carney CE, Haynes P, et al. : Is daily routine important for sleep? An investigation of social rhythms in a clinical insomnia population. Chronobiol Int 2015; 32:92–102 [DOI] [PubMed] [Google Scholar]
- 12.Wulff K, Gatti S, Wettstein JG, et al. : Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 2010; 11:589–599 [DOI] [PubMed] [Google Scholar]
- 13.Royuela Rico A, Macías Fernández JA: Propiedades clinimétricas de la versión castellana del cuestionario de Pittsburgh. Vigilia-Sueño 1997; 9:81–94 [Google Scholar]
- 14.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. : The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989; 28:193–213 [DOI] [PubMed] [Google Scholar]
- 15.Johns MW: Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 1992; 15:376–381 [DOI] [PubMed] [Google Scholar]
- 16.Chiner E, Arriero JM, Signes-Costa J, et al. : [Validation of the Spanish version of the Epworth Sleepiness Scale in patients with a sleep apnea syndrome]. Arch Bronconeumol 1999; 35:422–427 [DOI] [PubMed] [Google Scholar]
- 17.Ferrer M, Vilagut G, Monasterio C, et al. : [Measurement of the perceived impact of sleep problems: The Spanish version of the functional outcomes sleep questionnaire and the Epworth sleepiness scale]. Med Clin (Barc) 1999; 113:250–255 [PubMed] [Google Scholar]
- 18.Benítez I, Roure N, Pinilla L, et al. : Validation of the Satisfaction, Alertness, Timing, Efficiency and Duration (SATED) questionnaire for sleep health measurement. Ann Am Thorac Soc 2020; 17:338–343 [DOI] [PubMed] [Google Scholar]
- 19.Gonçalves BS, Adamowicz T, Louzada FM, et al. : A fresh look at the use of nonparametric analysis in actimetry. Sleep Med Rev 2015; 20:84–91 [DOI] [PubMed] [Google Scholar]
- 20.Bjelland I, Dahl AA, Haug TT, et al. : The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52:69–77 [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP: The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67:361–370 [DOI] [PubMed] [Google Scholar]
- 22.Caro I, Ibáñez E: La Escala Hospitalaria de Ansiedad y Depresión. Bol Psicol 1992; 36:43–69 [Google Scholar]
- 23.Crapo RO, Casaburi R, Coates AL, et al. : ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117 [DOI] [PubMed] [Google Scholar]
- 24.Enright PL, Sherrill DL: Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158:1384–1387 [DOI] [PubMed] [Google Scholar]
- 25.Mollayeva T, Thurairajah P, Burton K, et al. : The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev 2016; 25:52–73 [DOI] [PubMed] [Google Scholar]
- 26.Leisman DE, Harhay MO, Lederer DJ, et al. : Development and reporting of prediction models. Crit Care Med 2020; 48:623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hastie T, Tibshirani R, Tibshirani RJ: Extended comparisons of best subset selection, forward stepwise selection, and the lasso. arXiv:1707.08692v2 [Google Scholar]
- 28.Zhang Y, Yang Y: Cross-validation for selecting a model selection procedure. J Econom 2015; 187:95–112 [Google Scholar]
- 29.Ohayon M, Wickwire EM, Hirshkowitz M, et al. : National Sleep Foundation’s sleep quality recommendations: First report. Sleep Heal 2017; 3:6–19 [DOI] [PubMed] [Google Scholar]
- 30.Deng J, Zhou F, Hou W, et al. : The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann N Y Acad Sci 2021; 1486:90–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sehirli AÖ, Chukwunyere U, Aksoy U, et al. : The circadian clock gene Bmal1: Role in COVID-19 and periodontitis. Chronobiol Int 2021; 38:779–784 [DOI] [PubMed] [Google Scholar]
- 32.Sengupta S, Ince L, Sartor F, et al. : Clocks, viruses, and immunity: Lessons for the COVID-19 pandemic. J Biol Rhythms 2021; 36:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sengupta S, Brooks TG, Grant GR, et al. : Accounting for time: Circadian rhythms in the time of COVID-19. J Biol Rhythms 2021; 36:4–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Telias I, Wilcox ME: Sleep and circadian rhythm in critical illness. Crit Care 2019; 23:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daou M, Telias I, Younes M, et al. : Abnormal sleep, circadian rhythm disruption, and delirium in the ICU: Are they related? Front Neurol 2020; 11:549908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leach S, Suzuki K: Adrenergic signaling in circadian control of immunity. Front Immunol 2020; 11:1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curley GF, Laffey JG, Zhang H, et al. : Biotrauma and ventilator-induced lung injury: Clinical implications. Chest 2016; 150:1109–1117 [DOI] [PubMed] [Google Scholar]
- 38.Nosal C, Ehlers A, Haspel JA: Why lungs keep time: Circadian rhythms and lung immunity. Annu Rev Physiol 2020; 82:391–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamimi F, Abusamak M, Akkanti B, et al. : The case for chronotherapy in Covid-19-induced acute respiratory distress syndrome. Br J Pharmacol 2020; 177:4845–4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang PL, Ward TM, Burr RL, et al. : Sleep and circadian rhythms in survivors of acute respiratory failure. Front Neurol 2020; 11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Targa ADS, Benítez ID, Moncusí-Moix A, et al. : Decrease in sleep quality during COVID-19 outbreak. Sleep Breath 2021; 25:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]