OBJECTIVES:
To determine whether patients admitted to an ICU during times of unprecedented ICU capacity strain, during the COVID-19 pandemic in the United Kingdom, experienced a higher risk of death.
DESIGN:
Multicenter, observational cohort study using routine clinical audit data.
SETTING:
Adult general ICUs participating the Intensive Care National Audit & Research Centre Case Mix Programme in England, Wales, and Northern Ireland.
PATIENTS:
One-hundred thirty-thousand six-hundred eighty-nine patients admitted to 210 adult general ICUs in 207 hospitals.
INTERVENTIONS:
Multilevel, mixed effects, logistic regression models were used to examine the relationship between levels of ICU capacity strain on the day of admission (typical low, typical, typical high, pandemic high, and pandemic extreme) and risk-adjusted hospital mortality.
MEASUREMENTS AND MAIN RESULTS:
In adjusted analyses, compared with patients admitted during periods of typical ICU capacity strain, we found that COVID-19 patients admitted during periods of pandemic high or pandemic extreme ICU capacity strain during the first wave had no difference in hospital mortality, whereas those admitted during the pandemic high or pandemic extreme ICU capacity strain in the second wave had a 17% (odds ratio [OR], 1.17; 95% CI, 1.05–1.30) and 15% (OR, 1.15; 95% CI, 1.00–1.31) higher odds of hospital mortality, respectively. For non-COVID-19 patients, there was little difference in trend between waves, with those admitted during periods of pandemic high and pandemic extreme ICU capacity strain having 16% (OR, 1.16; 95% CI, 1.08–1.25) and 30% (OR, 1.30; 95% CI, 1.14–1.48) higher overall odds of acute hospital mortality, respectively.
CONCLUSIONS:
For patients admitted to ICU during the pandemic, unprecedented levels of ICU capacity strain were significantly associated with higher acute hospital mortality, after accounting for differences in baseline characteristics. Further study into possible differences in the provision of care and outcome for COVID-19 and non-COVID-19 patients is needed.
Keywords: bed census, COVID-19, critical care, intensive care unit capacity strain, mortality
Outcomes for critically ill patients admitted to ICUs are influenced by a variety of factors, in addition to the therapies received. These factors include: the organization of care (e.g., “closed” compared with “open” ICUs) (1), the experience gained from previously treating similar patients (e.g., volume-outcome relationships) (2, 3), and the numbers/skill mix of available staff (e.g., patient to intensivist ratios) (4). We recently reported that how busy an ICU is on any given day (termed ICU capacity strain) is associated with acute hospital mortality (5). ICU capacity strain can be seen as a mismatch between supply and demand, with availability of beds, staff, and/or other resources (as supply) and the need to admit and provide care for critically ill patients (as demand). ICU capacity strain not only has adverse consequences for patient outcomes but may also adversely affect the well-being of members of the healthcare delivery team (6, 7).
The severe acute respiratory syndrome coronavirus 2 (COVID-19) pandemic created a huge demand in the number of patients requiring critical care worldwide. Meeting this demand placed an unprecedented capacity strain on healthcare systems, and particularly on ICUs (8–10) with hospitals having to rapidly expand their critical care capacity. This translated into hospitals increasing the number of, and staffing for, beds within ICUs and creating new “surge” critical care beds in areas outside of recognized ICUs. Meeting the staffing and resources challenges for these additional beds resulted in reduced critical care staffing ratios within ICUs and redeployment of healthcare personnel with little to no experience in providing critical care. On top of this, resources such as equipment (e.g., mechanical ventilators) and medications (e.g., sedatives) may have been, at times, in short supply (11, 12).
The COVID-19 pandemic offered the opportunity to explore the relationship between patient outcome and unprecedented ICU capacity strain. Using the national clinical audit of the Intensive Care National Audit & Research Centre (ICNARC) Case Mix Programme, this study compared acute hospital mortality in patients admitted during periods of typical ICU capacity strain with patients admitted during periods with pandemic levels of ICU capacity strain outside of the typical range. We then compared trends between the first two “waves” of the COVID-19 pandemic in the United Kingdom (13).
METHODS
We conducted a large, multicenter, observational cohort study using data for all admissions to adult general ICUs participating in the ICNARC Case Mix Programme in England, Wales, and Northern Ireland. Two cohorts were identified: a pre-pandemic reference cohort of patients admitted March 1, 2019, to February 29, 2020, and the analysis cohort of patients admitted March 1, 2020, to February 28, 2021 (covering the first two “waves” of the COVID-19 pandemic in the United Kingdom). Only ICUs contributing data to both the reference and analysis cohorts were included in the analyses. In the analysis cohort, non-COVID-19 patients were distinguished from COVID-19 patients. In both cohorts, patients with missing data required to calculate ICU length of stay were excluded. Patients admitted prior to March 1 but remaining in ICU on March 1 and patients with missing outcome contributed to the calculation of ICU capacity strain but were excluded from the analysis of the impact of strain on outcome.
The ICNARC Case Mix Programme is the national clinical audit for adult critical care collecting, validating, and pooling case mix and outcome data for individual patient admissions and covering 100% of adult general ICUs (including both standalone and combined intensive/high-dependency care units) in England, Wales, and Northern Ireland. During the pandemic, the Case Mix Programme expanded to include patients admitted to temporary/expanded critical care areas. If patients were cared for in a temporary/expanded area by an ICU team that contributed data to both the reference and analysis cohorts, and if data for those patients was included with the ICUs own data submission (rather than as a new ICU), then those patients were included in the analysis. The geographical distribution of cases within the United Kingdom varied over time and is beyond the scope of this analysis. Detailed information on geographical distribution can be found in the ICNARC report on COVID-19 in critical care (14) and previously published analyses (13).
Approval for the collection and use of patient identifiable data from the Case Mix Programme was obtained under Section 251 of the National Health Service Act 2006 (PIAG 2-10(f)/2005). The Confidentiality Advisory Group advisory function was previously carried out by the National Information Governance Board’s Ethics and Confidentiality Committee (2009–2013) and prior to that, the Patient Information Advisory Group (2001–2008). Approval by a research ethics committee was not required, as the analysis was performed as a service evaluation.
Exposure of Interest: ICU Capacity Strain
ICU bed census on the date of ICU admission was used as the measure of ICU capacity strain, as previously described by Wilcox et al (5). Typical ICU capacity strain, for each ICU, was defined with respect to the range observed in the reference cohort. Aligning with Wilcox et al (5), “typical high” and “typical low” strain were defined as more than 10% above or below the median bed census observed for each ICU from the reference cohort. We initially defined “pandemic high” ICU capacity strain as values exceeding the maximum ICU bed census for each ICU during the pre-pandemic reference period, but in response to reviews, further subdivided this into “pandemic high” (up to 50% greater than the pre-pandemic maximum) and “pandemic extreme” (more than 50% greater than the pre-pandemic maximum). We also defined, a priori, a category of “pandemic low” (less than the pre-pandemic minimum). ICU capacity strain was assessed on the day of admission to ICU.
Outcomes
The primary outcome was acute hospital mortality. All patients were followed up until ultimate discharge from acute hospital, after any transfers occurring between acute hospitals.
Statistical Analysis
Baseline characteristics and unadjusted outcomes were tabulated using standard summary statistics. We fitted multilevel, mixed effects, logistic regression models for each outcome, including random effects for each ICU, and with the following covariates: ICU capacity strain (categories, as defined above); age (linear); sex; ethnic group (White/Black/Asian/mixed/other/not stated); index of multiple deprivation (quintile for area of residence) (15); body mass index (< 18.5/18.5 to < 25/25 to < 30/30 to < 40/40+); severe comorbidities (absence/presence of: cardiovascular—symptoms at rest; respiratory—symptoms with light activity or home ventilation; renal—renal replacement therapy for end-stage kidney disease; liver—biopsy-proven cirrhosis, portal hypertension, or hepatic encephalopathy; hematological malignancy; metastatic disease; immunocompromise due to disease or treatment); dependency prior to hospital admission (none/some/total); location prior to ICU admission (emergency department or not in hospital/theater/ward/other critical care unit/other acute hospital); urgency of surgery (elective/emergency); cardiopulmonary resuscitation prior to ICU admission (out-of-/in-hospital/none); ICNARC Physiology Score (linear) (16); and primary reason for ICU admission (categorized according to the ICNARC risk prediction model [17], with additional category for COVID-19).
Nine models were fitted. First, three separate models were fitted for: 1) all patients combined; 2) COVID-19 patients only; and 3) non-COVID-19 patients. Second, these three separate models were then fitted for: 1) patients admitted during the first “wave” (admissions up to August 31, 2020) and 2) patients admitted during the second “wave” (admissions from September 1, 2020). Joint significance was assessed using chi-square statistics for all ICU capacity strain categories simultaneously within each model.
RESULTS
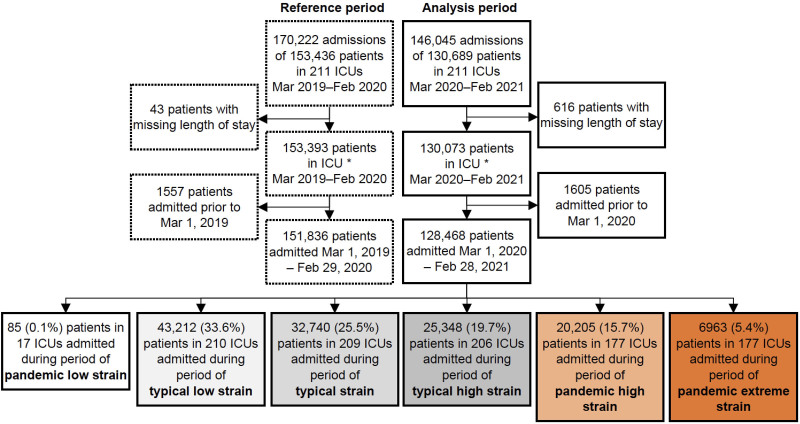
The pre-pandemic reference cohort included 170,222 admissions (for 153,436 patients) admitted between March 1, 2019, and February 29, 2020, and the analysis cohort included 146,045 admissions (for 130,689 patients) admitted between March 1, 2020, and February 28, 2021 (Fig. 1). Both cohorts included 211 ICUs in 207 hospitals. In the analysis cohort, one-fifth of patients were admitted during periods ICU occupancy strain outside of the range observed during the pre-pandemic reference period (pandemic high or pandemic extreme).
Figure 1.
Flow diagram of admissions during pre-pandemic reference and analysis periods.
For the analysis cohort (Supplementary Table 1, http://links.lww.com/CCM/G988), baseline characteristics, unadjusted outcomes, and ICU length of stay are presented overall and across categories of ICU capacity strain (note: pandemic low/low categories were combined due to small numbers in pandemic low [n = 85]). Because the proportion of patients with COVID-19 varies substantially between categories of ICU occupancy strain, patterns in other baseline characteristics are best interpreted among patients with COVID-19 (Supplementary Table 2, http://links.lww.com/CCM/G988) and non-COVID-19 (Supplementary Table 3, http://links.lww.com/CCM/G988), separately. One example that illustrates this, is the patterns for discharge at night, a known indicator of pressure on ICU beds. Little difference is seen across ICU capacity strain categories for COVID-19 patients, however, for non-COVID-19 patients, the proportion discharged at night rises from 6.7% to 12.2% as ICU capacity strain increases.
Comparing non-COVID-19 patients admitted during the pandemic (analysis cohort) with patients admitted pre-pandemic (reference cohort), baseline characteristics, unadjusted outcomes, and ICU lengths of stay were remarkably similar (Supplementary Table 4, http://links.lww.com/CCM/G988). Other than the proportion of COVID-19 patients increasing, there were only modest differences in the characteristics of patients admitted in “wave 1” versus “wave 2” (Supplementary Table 5, http://links.lww.com/CCM/G988). However, indicators of strain such as out-of-hours discharge increased.
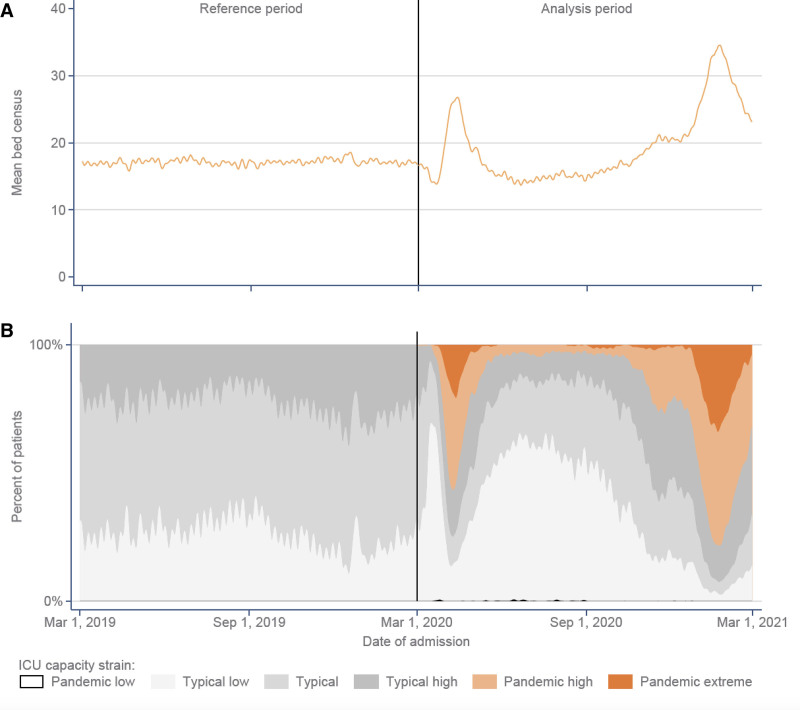
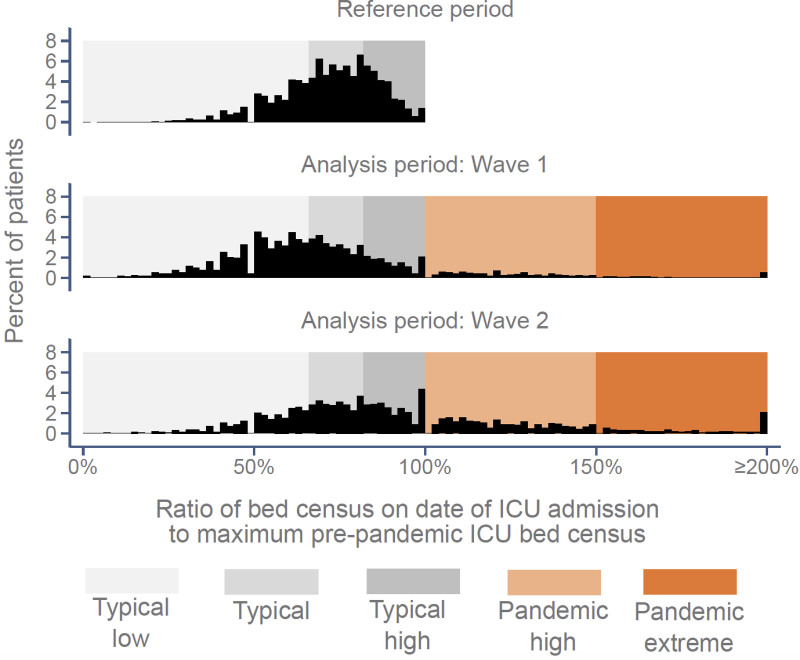
During the pre-pandemic reference period, the median (interquartile range) bed census (the number of other patients in ICU on the index patient’s day of admission, across all ICUs) was 17.3 (sd: 11.2 and ranged from 14.4 to 19.6 over the year) (Fig. 2A). During the analysis period, immediately prior to the first “wave,” as elective surgery was canceled, the mean bed census decreased to 12.8 (sd: 8.4, March 21, 2020) and then rose to a maximum of 27.8 (sd: 24.5, April 15, 2020) in the first “wave.” Between the first and second “waves,” the mean bed census dropped to a minimum of 12.3 (sd: 8.2, June 21, 2021) and then rose again to a maximum of 35.1 (sd: 31.9, January 25, 2021) in the second “wave.” During the peaks of both pandemic waves, most patients were exposed to ICU capacity strain exceeding the range previously experienced by the admitting ICU (i.e., pandemic high or pandemic extreme), while, immediately prior to the first “wave” and in the period between the “waves,” most patients were exposed to typical low ICU capacity strain (Fig. 2B). A total of 177 out of 211 ICUs (84%) experienced periods of pandemic high or pandemic extreme strain, during which time 21.1% of patients were admitted (Fig. 3). Only 85 patients were admitted during periods of ICU capacity strain below the pre-pandemic reference minimum, so “pandemic low” was combined with “typical low” for analysis purposes.
Figure 2.
ICU capacity strain between March 1, 2019, and February 28, 2021. A, Mean bed census (the number of other patients in ICU on the index patient’s day of admission, averaged across all ICUs). B, ICU capacity strain: pandemic low, less than observed range of values for the ICU during the pre-pandemic period; typical low, more than 10% below the pre-pandemic median bed census for each ICU but within the observed range; typical, within ± 10% of the pre-pandemic median; typical high, more than 10% above the pre-pandemic median up to the pre-pandemic maximum; pandemic high, up to 50% greater than the pre-pandemic maximum; and pandemic extreme, more than 50% greater than the pre-pandemic maximum bed census for the ICU.
Figure 3.
Distributions of patient-level ICU bed census on date of admission compared with the maximums observed for the admitting ICU during the pre-pandemic reference period.
There was some evidence of triaging access to ICU, with both COVID-19 and non-COVID-19 patients admitted during periods of higher ICU capacity strain having slightly higher (worse) ICNARC Physiology Scores and non-COVID-19 patients admitted during periods of higher ICU occupancy also having slightly lower levels of dependency prior to ICU admission. Unadjusted acute hospital mortality for COVID-19 patients was 44% for those admitted during periods of typical high, pandemic high, or pandemic extreme ICU occupancy strain, compared with 41% for those admitted during periods of typical low or typical ICU occupancy (Supplementary Table 2, http://links.lww.com/CCM/G988). For non-COVID-19 patients, unadjusted mortality increased linearly from 21.6% (typical low) to 36.5% (pandemic extreme).
In models using patients exposed to typical ICU capacity strain as the reference category and after accounting for differences in baseline characteristics, a significant, monotonic (dose-response) relationship was observed between exposure to higher ICU capacity strain and higher acute hospital mortality, overall and for patients with COVID-19 and non-COVID-19 (Table 1). However, patterns differed between COVID-19 and non-COVID-19 patients and between patients admitted during the first and second waves of the pandemic. During the “wave 1,” the adjusted association between ICU capacity strain and acute hospital mortality was only apparent and significant for non-COVID-19 patients, who were at 37% higher odds of acute hospital mortality if admitted during periods of pandemic extreme ICU occupancy strain (odds ratio [OR], 1.37; 95% CI, 1.05–1.81). During “wave 2,” COVID-19 patients admitted during periods of pandemic high or pandemic extreme ICU occupancy were also at increased risk of acute hospital mortality (OR, 1.17; 95% CI, 1.05–1.30 and OR, 1.15; 95% CI, 1.00–1.31, respectively), while the pattern for non-COVID-19 patients (OR, 1.16; 95% CI, 1.06–1.28 and OR, 1.28; 95% CI, 1.10–1.50, respectively) remained comparable to “wave 1.”
TABLE 1.
Adjusted Hospital Mortality Across Categories of ICU Capacity Strain
| Adjusted OR for Hospital Mortality | ICU Capacity Strain Within Reference Range | ICU Capacity Strain Outside Reference Range | p > χ2 | |||
|---|---|---|---|---|---|---|
| Typical Low | Typical | Typical High | Pandemic High | Pandemic Extreme | ||
| Both waves | ||||||
| All patients | 0.93 (0.89–0.97) | Reference | 1.07 (1.02–1.12) | 1.12 (1.06–1.18) | 1.11 (1.03–1.20) | p = 8 × 10–12 |
| COVID-19 | 0.92 (0.83–1.01) | Reference | 1.02 (0.93–1.11) | 1.12 (1.03–1.22) | 1.10 (0.99–1.23) | p = 0.0002 |
| Non- COVID-19 | 0.95 (0.90–1.00) | Reference | 1.10 (1.04–1.17) | 1.16 (1.08–1.25) | 1.30 (1.14–1.48) | p = 3 × 10–11 |
| Wave 1 only | ||||||
| All patients | 0.94 (0.88–1.00) | Reference | 1.05 (0.96–1.13) | 1.05 (0.96–1.16) | 1.08 (0.93–1.25) | p = 0.02 |
| COVID-19 | 0.88 (0.76–1.02) | Reference | 0.90 (0.76–1.05) | 1.00 (0.85–1.17) | 1.01 (0.82–1.25) | p = 0.25 |
| Non- COVID-19 | 0.96 (0.89–1.03) | Reference | 1.11 (1.01–1.23) | 1.10 (0.96–1.27) | 1.37 (1.05–1.81) | p = 0.001 |
| Wave 2 only | ||||||
| All patients | 0.93 (0.87–1.00) | Reference | 1.08 (1.01–1.15) | 1.14 (1.06–1.22) | 1.13 (1.02–1.24) | p = 2 × 10–6 |
| COVID-19 | 0.93 (0.81–1.07) | Reference | 1.07 (0.96–1.20) | 1.17 (1.05–1.30) | 1.15 (1.00–1.31) | p = 0.003 |
| Non- COVID-19 | 0.97 (0.89–1.05) | Reference | 1.10 (1.02–1.20) | 1.16 (1.06–1.28) | 1.28 (1.10–1.50) | p = 0.00005 |
OR = odds ratio.
DISCUSSION
For patients admitted to ICU during the pandemic, higher than typical levels of high ICU capacity strain were significantly associated with higher acute hospital mortality, after accounting for differences in baseline characteristics. In the first “wave,” with fewer periods of pandemic high strain, the association of higher strain with higher acute hospital mortality was observed solely for non-COVID-19 patients. In the second “wave,” with more periods of pandemic high strain, the association was observed for both non-COVID-19 and COVID-19 patients.
Interestingly, the United States has more ICU beds per capita than almost any other country in the world, and by comparison, with the United Kingdom by almost an order of magnitude (18). Although this has been a cause for criticism as a potential source of inefficiency and high-cost/low-cost care (19), the COVID-19 pandemic tested this thesis, as countries across the world were forced to triage ICU resources. In a nationally representative survey of 169 U.S. hospitals, Kerlin et al (20) report highly variable responses to the need to scale up ICU capacity including canceling or postponing procedures to preserve ICU bed capacity and increase or preserve access to equipment such as mechanical ventilators. These efforts were focused primarily on reducing the overall demand for ICU care by non-COVID-19 patients. Similar efforts were made in the United Kingdom, likely meeting some success in “wave 1,” however in “wave 2,” where the greatest influx of patients was seen, and sustained for a longer period of time, such efforts likely fell short and ICU capacity strain was at its greatest which may be why a greater impact is seen on patient outcome. In an international survey of critical care practitioners caring for patients with COVID-19, at different stages of the pandemic up to one third of responders reported that limits were placed on administering mechanical ventilation, and more than half reported changes to policies and practices for cardiopulmonary resuscitation (21). The need to place limitations on mechanical ventilation is indicative of levels of ICU capacity strain reached in those countries. Although regional differences may exist with regards to pandemic ICU capacity strain within our dataset, this was more likely to have happened in “wave 2” when the greatest number of patients were being care for—numbers at which care for both COVID-19 and non-COVID-19 patients was affected.
Our findings of the association of strain with mortality are in keeping with those recently published by Bravata et al (8), who, despite using a different measure of strain, found a similar association between strain and acute hospital mortality for COVID-19 patients, a finding that was consistent across two pandemic waves at 88 Veterans Affairs hospitals in the United States. However, the relationship between strain for non-COVID-19 patients was not explored (8). More generally in the nonpandemic literature, despite variation in how ICU capacity strain is measured, studies using the most commonly described indicators of strain consistently report an association with adverse outcomes, deviations in care processes, and changes in resource use (22–28). Furthermore, in a large retrospective cohort of 558 U.S. hospitals large surges in COVID-19 case load were found to significantly impact patient survival, eroding potential benefits of emerging therapies (29). The reported surge index (a severity weighted measure of COVID-19 caseload relative to pre-COVID-19 bed capacity) was associated with increased risk of death both on the ward and in the ICU; a finding that was also stronger in the second “wave” of cases experience (29).
The strengths of this study include the nationally representative cohort of ICUs across England, Wales, and Northern Ireland and the inclusion of both COVID-19 and non-COVID-19 patients in our investigation. Another strength was the availability of a reference cohort to determine ICU capacity strain under nonpandemic conditions to understand the additional strain for each ICU during pandemic conditions. One limitation of our study was a greater level of missing data in the analysis cohort compared with the reference cohort, likely due to the pressures of the pandemic (0.5% vs 0.03% for ICU length of stay; 1.1% vs 0.6% for acute hospital mortality)—but with both cohorts having very low absolute levels of missing data. We used only one measure of ICU capacity strain, bed census, which does not account for other human factors that likely weighed in during the pandemic. Other than bed occupancy levels, we do not have other data on key organizational factors, such as staffing levels, to contextualize our results. Previous work has identified some differences in prognostic factors for patients with COVID-19, compared with standard critical care risk prediction models (30), and risk-adjusted mortality changed across the “waves” of the pandemic (14). While we addressed these potential limitations by including additional prognostic factors in adjusted models and by fitting separate models for patients with and without COVID-19 and for the two “waves” of the pandemic, there remains scope for residual confounding, especially in light of apparent triaging of patients during periods of extreme occupancy strain. Last, a limitation of our findings is the underlying assumption that ICUs are adequately staffed for their typical strain, and there were no units in a state of persistent capacity strain. However, we attempted to minimize this by using a minimum of 1 years’ worth of data, as it would be unlikely that an ICU would be able to sustain such a level of function prior to the pandemic. Although it is possible that timely goals of care discussion may have been prioritized during different periods of pandemic levels of ICU capacity strain, it is unlikely to explain the significant numbers of deaths seen during these periods. Additional data items on treatment limitations have been recently added to the Case Mix Programme data collection but were not available during the study period.
The range in ICU capacity strain, as measured by bed census, was highly variable. These fluctuations in ICU bed census would make it very challenging to predict the number of healthcare providers (doctors, nurses, other allied professionals) that would be required from day to day or week to week to staff units appropriately. As such, this poses a major challenge for future pandemics trying to organize appropriate staffing for ICUs in such high degrees of variation.
CONCLUSIONS
For both COVID-19 and non-COVID-19 patients admitted to ICU during periods of unprecedented ICU capacity strain, higher strain was significantly associated with higher acute hospital mortality, after accounting for differences in baseline characteristics.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Wilcox ME, Chong CA, Niven DJ, et al. : Do intensivist staffing patterns influence hospital mortality following ICU admission? A systematic review and meta-analyses. Crit Care Med 2013; 41:2253–2274 [DOI] [PubMed] [Google Scholar]
- 2.Shahin J, Harrison DA, Rowan KM: Relation between volume and outcome for patients with severe sepsis in United Kingdom: Retrospective cohort study. BMJ 2012; 344:e3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn JM, Goss CH, Heagerty PJ, et al. : Hospital volume and the outcomes of mechanical ventilation. N Engl J Med 2006; 355:41–50 [DOI] [PubMed] [Google Scholar]
- 4.Gershengorn HB, Harrison DA, Garland A, et al. : Association of intensive care unit patient-to-intensivist ratios with hospital mortality. JAMA Intern Med 2017; 177:388–396 [DOI] [PubMed] [Google Scholar]
- 5.Wilcox ME, Harrison DA, Patel A, et al. : Higher ICU capacity strain is associated with increased acute mortality in closed ICUs. Crit Care Med 2020; 48:709–716 [DOI] [PubMed] [Google Scholar]
- 6.Bagshaw SM, Opgenorth D, Potestio M, et al. : Healthcare provider perceptions of causes and consequences of ICU capacity strain in a large publicly funded integrated health region: A qualitative study. Crit Care Med 2017; 45:e347–e356 [DOI] [PubMed] [Google Scholar]
- 7.Opgenorth D, Stelfox HT, Gilfoyle E, et al. : Perspectives on strained intensive care unit capacity: A survey of critical care professionals. PLoS One 2018; 13:e0201524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bravata DM, Perkins AJ, Myers LJ, et al. : Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open 2021; 4:e2034266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M, Ning J, Du Y, et al. : Modelling the evolution trajectory of COVID-19 in Wuhan, China: Experience and suggestions. Public Health 2020; 183:76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards-Belle A, Orzechowska I, Gould DW, et al. ; ICNARC COVID-19 Team: COVID-19 in critical care: Epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med 2020; 46:2035–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beitler JR, Mittel AM, Kallet R, et al. : Ventilator sharing during an acute shortage caused by the COVID-19 pandemic. Am J Respir Crit Care Med 2020; 202:600–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montmeat D, Gard C, Raux M, et al. : Shortage of sedatives and neuromuscular blockers during COVID-19 pandemic: The result of an overstocking procedure in French hospitals? Anaesth Crit Care Pain Med 2020; 39:585–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doidge JC, Gould DW, Ferrando-Vivas P, et al. : Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med 2021; 203:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intensive Care National Audit & Research Centre: ICNARC Report on COVID-19 in Critical Care: England, Wales and Northern Ireland. 2021. Available at: https://www.icnarc.org/ouraudit/audits/cmp/reports. Accessed June 3, 2021
- 15.Government Ministry of Housing, Communities & Local Government: The English Indices of Deprivation 2019 (IoD2019). 2019. Available at: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019. Accessed July 1, 2021
- 16.Harrison DA, Parry GJ, Carpenter JR, et al. : A new risk prediction model for critical care: The Intensive Care National Audit & Research Centre (ICNARC) model. Crit Care Med 2007; 35:1091–1098 [DOI] [PubMed] [Google Scholar]
- 17.Ferrando-Vivas P, Jones A, Rowan KM, et al. : Development and validation of the new ICNARC model for prediction of acute hospital mortality in adult critical care. J Crit Care 2017; 38:335–339 [DOI] [PubMed] [Google Scholar]
- 18.Wunsch H, Angus DC, Harrison DA, et al. : Comparison of medical admissions to intensive care units in the United States and United Kingdom. Am J Respir Crit Care Med 2011; 183:1666–1673 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO): WHO Director-General’s Statement on IHR Emergency Committee on Novel Coronavirus (2019-nCoV). 2019. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov). Accessed September 28, 2021
- 20.Kerlin MP, Costa DK, Davis BS, et al. : Actions taken by US hospitals to prepare for increased demand for intensive care during the first wave of COVID-19: A national survey. Chest 2021; 160:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahlster S, Sharma M, Lewis AK, et al. : The coronavirus disease 2019 pandemic’s effect on critical care resources and health-care providers: A global survey. Chest 2021; 159:619–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagshaw SM, Wang X, Zygun DA, et al. : Association between strained capacity and mortality among patients admitted to intensive care: A path-analysis modeling strategy. J Crit Care 2018; 43:81–87 [DOI] [PubMed] [Google Scholar]
- 23.Kohn R, Harhay MO, Weissman GE, et al. : Ward capacity strain: A novel predictor of delays in intensive care unit survivor throughput. Ann Am Thorac Soc 2019; 16:387–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weissman GE, Gabler NB, Brown SE, et al. : Intensive care unit capacity strain and adherence to prophylaxis guidelines. J Crit Care 2015; 30:1303–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua M, Halpern SD, Gabler NB, et al. : Effect of ICU strain on timing of limitations in life-sustaining therapy and on death. Intensive Care Med 2016; 42:987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner J, Gabler NB, Ratcliffe SJ, et al. : Outcomes among patients discharged from busy intensive care units. Ann Intern Med 2013; 159:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Town JA, Churpek MM, Yuen TC, et al. : Relationship between ICU bed availability, ICU readmission, and cardiac arrest in the general wards. Crit Care Med 2014; 42:2037–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpern SD: ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care 2011; 17:648–657 [DOI] [PubMed] [Google Scholar]
- 29.Kadri SS, Sun J, Lawandi A, et al. : Association between caseload surge and COVID-19 survival in 558 U.S. hospitals, March to August 2020. Ann Intern Med 2021; 174:1240–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrando-Vivas P, Doidge J, Thomas K, et al. ; ICNARC COVID-19 Team: Prognostic factors for 30-day mortality in critically ill patients with coronavirus disease 2019: An observational cohort study. Crit Care Med 2021; 49:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]