Abstract
Metastatic bone tumors to the hand are extremely rare. We present a case of metastatic prostate cancer to the right middle finger distal phalanx. To our knowledge, there is one other case of metastatic prostate cancer to the hand in the literature. In our case, a 59-year-old man with a history of widely metastatic prostate cancer presented to urgent care and was diagnosed with a nail plate avulsion injury. He was referred to hand surgery and treated with amputation of the right middle finger distal phalanx. The pathology reported high-grade poorly differentiated adenocarcinoma with primary lesion from the prostate.
Keywords: prostate cancer, acral metastasis, metastatic cancer, distal phalanx tumor, finger tumor
Introduction
Malignant bone tumors of the hand are uncommon. Bone tumors of the hand, both malignant and benign, account for 6% of all bone tumors of the skeleton. 1 Metastatic tumors of the hand are exceedingly rare and portend a worse prognosis, constituting 0.1% of all osseous metastases. 2 The most common primary source of acral metastatic disease is by far the lung, accounting for 44% of malignant bone tumors of the hand. 3 Other common sources of acral metastatic disease are breast and gastrointestinal malignancies.
It is not uncommon for fingertip metastatic disease to mimic infection, and this often leads to delayed care for the patient. 4 The presumed reason for this is the rarity with which medical providers see and evaluate patients with malignant hand lesions. The following is a case report of metastatic prostate cancer to the hand initially misdiagnosed as a nail plate avulsion injury, including a detailed discussion of metastatic prostate cancer.
Case Report
The patient is a 59-year-old man who was diagnosed with metastatic prostate cancer 7 months prior to hand surgery consultation. His symptoms first began with back and pelvic pain. Work-up revealed mixed lytic and blastic lesions of the appendicular skeleton, with a large pelvic soft tissue mass. His prostate-specific antigen (PSA) was 49, and transrectal ultrasound-guided biopsy revealed prostatic adenocarcinoma, ductal type, solid variant, with a Gleason score of 10. This is compatible with high-grade poorly differentiated adenocarcinoma. 5
Treatment consisted of palliative radiotherapy and chemotherapy with docetaxel. Despite this, he was noted to have progressive spread of metastatic disease to his brain and lungs. He also developed a mass in the right middle finger distal phalanx. He injured the hand when placing it in his pant pocket. He was seen in an urgent care and was diagnosed with a nail plate avulsion injury with presumed infection. Per referral paperwork, an attempt at irrigation and debridement was done. No radiographs were obtained at the urgent care. He was started on oral antibiotics and referred for hand surgery consultation.
A few days after that, he was seen in the hand surgery office. The right middle finger distal phalanx was grossly swollen with purple discoloration, a fungating subungual mass, and bloody drainage (Figures 1-3). Radiographs were obtained demonstrating near-total erosion of the distal phalanx with soft tissue calcifications (Figures 4 and 5). The finger was very painful for the patient and made self-care challenging. Given the degree of deformity, he was indicated for palliative amputation.
Figure 1.
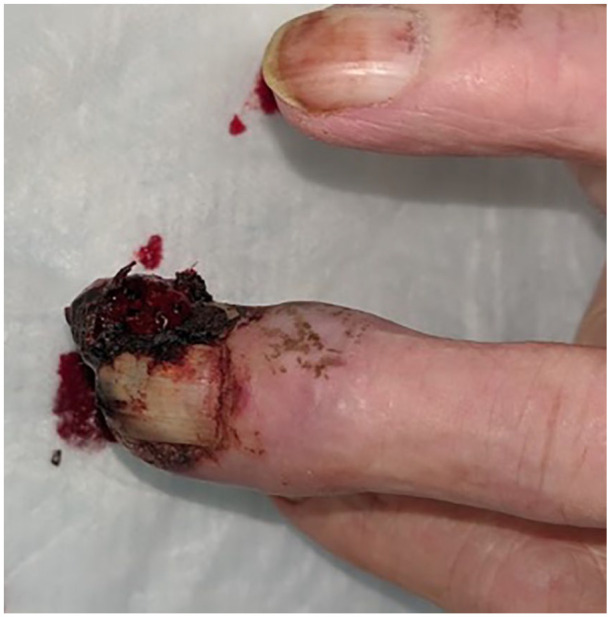
Dorsal view of the right middle finger demonstrating fungating subungual mass.
Figure 3.
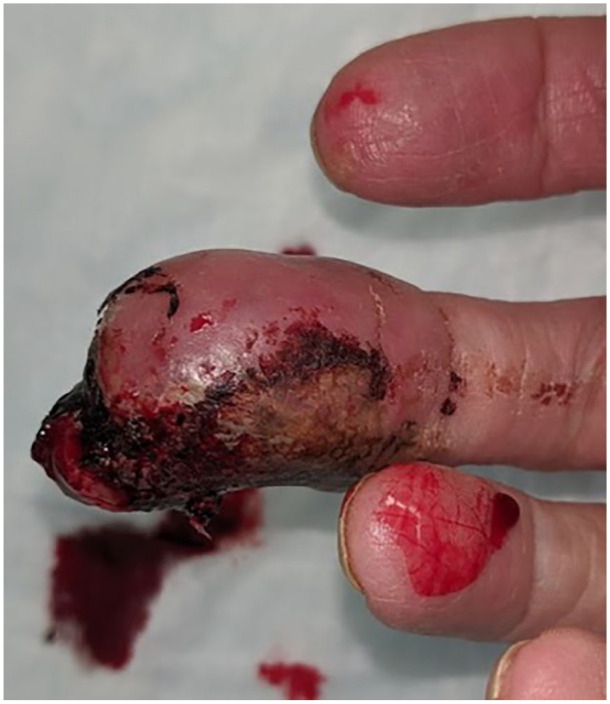
Palmer view of the right middle finger distal phalanx.
Figure 4.
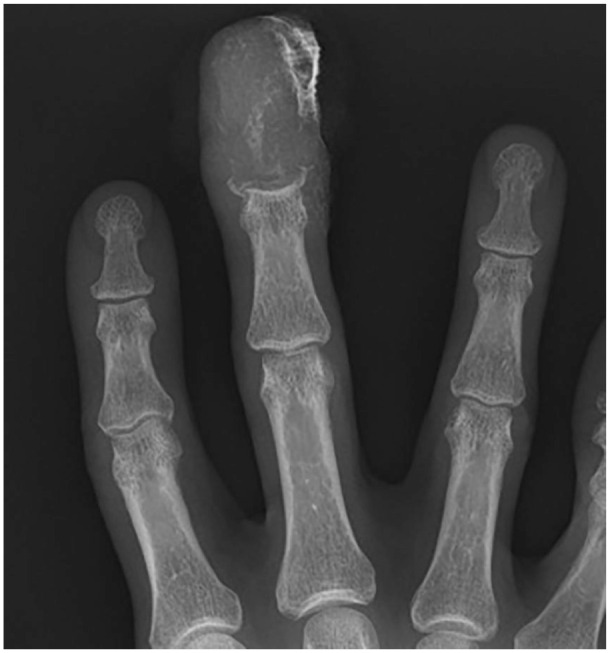
Posteroanterior radiograph of the right hand demonstrating erosive changes to the middle finger distal phalanx.
Figure 5.
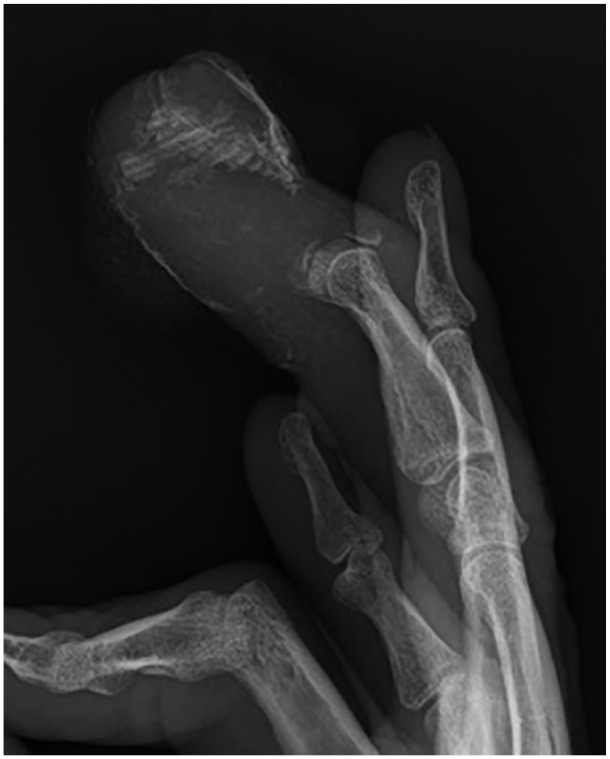
Lateral radiograph of the right hand demonstrating erosive changes to the middle finger distal phalanx with associated soft tissue calcifications.
Figure 2.
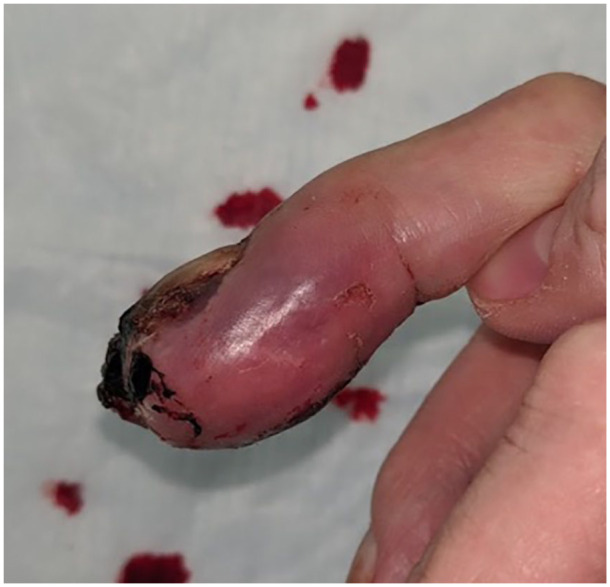
Lateral view of the right middle finger demonstrating deformity and purple discoloration of the distal phalanx.
He underwent an uncomplicated amputation of the finger through the middle phalanx with local-only anesthesia. Pathology returned with metastatic carcinoma consistent with prostate origin. He was seen once postoperatively for suture removal and opted for self-guided therapy for motion and desensitization modalities. He reported improvement in his pain and hand functionality. He had worsening symptoms from his diffusely metastatic prostate cancer and soon thereafter opted for palliative care. He died 2 months after the finger amputation.
Discussion
Metastatic prostate cancer to the hand is extremely rare and has been documented only once previously in the literature. In 2016, Nagano et al 6 was the first to report metastatic prostate cancer to the bone and soft tissue of the hand. In this case report, the patient’s prostate cancer first metastasized to the S1 vertebra before developing a painless mass in the first web space of the right hand. Treatment consisted of thumb disarticulation, disarticulation of the second metacarpal, and pollicization of the remaining index finger. The patient did not have local recurrence, but succumbed to metastatic prostate cancer 4 years thereafter.
In addition, within the literature, Sui et al 7 was the first to report the finding of prostate cancer metastasis to the distal phalanx of the left hallux in a case report in 2016. Their patient developed lung and vertebral bone metastases 1 year after initial prostate cancer diagnosis. Eight months after that, the patient had worsening chronic pain and swelling of the left hallux distal phalanx. The patient was initially treated with incision and drainage for presumed infection. However, the patient’s symptoms persisted, and soon thereafter amputation of the phalanx was performed, with immunohistochemical analysis confirming metastatic prostate cancer. The patient succumbed to cardiac and respiratory failure 28 months after the initial prostate cancer diagnosis.
As previously stated, metastatic tumors to the hand account for only 0.1% of all skeletal metastases. The most common primary tumors that metastasize to the hand are lung, kidney, and breast. 8 The most common location of metastasis to the hand is the distal phalanx. Prostate cancer commonly metastasizes to the pelvic lymph nodes and axial skeleton. However, prostate cancer rarely metastasizes to the bone distal to both the elbow and the knee. 9 In this case, prostate cancer widely metastasized throughout the body, and one of those locations was the right middle finger distal phalanx.
Well-differentiated prostatic adenocarcinoma has typical pathological features that make it easy to diagnose. In contrast, poorly differentiated prostatic adenocarcinoma often lacks the typical pathologic features, making it challenging to diagnose. 10 Prostate-specific antigen immunohistochemical staining is effective in associating metastatic disease with the origin, prostate adenocarcinoma. However, high-grade poorly differentiated adenocarcinoma may lack PSA staining, such as in this case. Our patient’s primary tumor was positive for p504, CAM5.2, and pancytokeratin and negative for PSA, synaptophysin, chromogranin, TTF-1, and NKX3.1. The pathologist felt that the cytology, architecture, and clinical location of the primary tumor strongly suggested a prostatic origin. The immunostain profile, negative for PSA and NKX3.1, is unusual but can happen with high-grade poorly differentiated prostatic adenocarcinoma. The metastasis to the right middle finger distal phalanx showed tumor cells that were positive for pancytokeratin, whereas negative for p40, NKX3.1, TTF-1, and Napsin A. Given this immunoprofile and the morphologic compatibility with the patient’s primary tumor biopsy, this lesion was determined to be consistent with poorly differentiated adenocarcinoma of prostate origin.
Informing patients of the grade and stage of cancer, as well as risk stratification, is an important step that guides treatment. All treatment options including the risk and benefits should be explained to the patient. As with our patient, when there are bone metastases present, the median survival is 3 to 3.5 years. 11 Shared decision-making is critical to achieve patient compliance and optimize outcomes. This patient was informed of the prognosis of his disease along with expectations and risks of the surgery. The patient elected to proceed with right middle finger amputation as a palliative measure, and there were no complications with the surgery that was done under local-only anesthesia. The ability to perform the procedure under local-only anesthesia was very beneficial as it mitigated the risk of general anesthesia and allowed the patient to position himself in a position of comfort for the procedure given his diffuse pelvic girdle metastatic disease.
There are several key learning points from this case report. It is common to misdiagnose finger tumors as infections. There needs to be a high index of suspicion for the possibility of a tumor mimicking infection, especially in a patient with a known history of metastatic disease. Performing a thorough history and physical examination is a key component to avoid making this common misdiagnosis. Radiographs are essential to obtain to evaluate for bony and soft tissue changes secondary to either tumor or infection. Depending on the clinical situation, a biopsy should be obtained. As is often taught, tumors mimic infections and infections mimic tumors, which require analysis with cultures and pathology specimens to avoid misdiagnoses.
Footnotes
Ethical Approval: Not required.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There is no highly identifiable personal information within this case report.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Richard D. Lander  https://orcid.org/0000-0002-5621-4951
https://orcid.org/0000-0002-5621-4951
Marc J. O’Donnell  https://orcid.org/0000-0003-4814-3206
https://orcid.org/0000-0003-4814-3206
References
- 1. Hsu CS, Hentz VR, Yao J. Tumours of the hand. Lancet Oncol. 2007;8:157-166. [DOI] [PubMed] [Google Scholar]
- 2. Kerin R. The hand in metastatic disease. J Hand Surg Am. 1987;12:77-83. [DOI] [PubMed] [Google Scholar]
- 3. Kumar PP, Kovi J. Metastases to bones of the hands and feet. J Natl Med Assoc. 1978;70(11):837-840. [PMC free article] [PubMed] [Google Scholar]
- 4. Soylemez S, Demiroglu M, Yayla MA, et al. Lung metastasis mimicking fingertip infection. Case Rep Oncol Med. 2015;2015:708789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donohue JF, Bianco FJ, Jr, Kuroiwa K, et al. Poorly differentiated prostate cancer treated with radical prostatectomy: long-term outcome and incidence of pathological downgrading. J Urol. 2006;176:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagano A, Ohno T, Oshima K, et al. Metastatic prostate cancer of hand. Case Rep Orthop. 2016;2016:1472932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sui X, Hu Y, Zhang C, et al. Prostate cancer metastasis to the distal phalanx of the left hallux: the first confirmed case and literature review. Oncol Lett. 2016;12(2):1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henderson M, Neumeister MW, Bueno RA., Jr. Hand tumors: II. Benign and malignant bone tumors of the hand. Plast Reconstr Surg. 2014;133(6):814e-821e. [DOI] [PubMed] [Google Scholar]
- 9. Ho PSS, Yip LY, Nguyen M, et al. A painful finger: an unusual presentation of Von Hippel-Lindau-associated advanced renal cell carcinoma. Case Rep Oncol. 2020;13(1):245-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parwani AV, Ali SZ. Prostatic adenocarcinoma metastases mimicking small cell carcinoma on fine-needle aspiration. Diagn Cytopathol. 2002;27(2):75-79. [DOI] [PubMed] [Google Scholar]
- 11. Kirby R. Case study: management of advanced prostate cancer with soft tissue metastases. Prostate Cancer Prostatic Dis. 2005;8(3):290-292. [DOI] [PubMed] [Google Scholar]


