Abstract
Background: Therapeutic electrical stimulation (ES) applied to repaired nerve is a promising treatment option to improve regeneration. However, few studies address the impact of ES following nerve graft reconstruction. The purpose of this study was to determine if ES applied to a nerve repair using nerve isograft in a rodent model could improve nerve regeneration and functional recovery. Methods: Adult rats were randomized to 2 groups: “ES” and “Control.” Rats received a tibial nerve transection that was repaired using a tibial nerve isograft (1.0 cm length), where ES was applied immediately after repair in the applicable group. Nerve was harvested 2 weeks postrepair for immunohistochemical analysis of axon growth and macrophage accumulation. Independently, rats were assessed using walking track and grid-walk analysis for up to 21 weeks. Results: At 2 weeks, more robust axon regeneration and greater macrophage accumulation was observed within the isografts for the ES compared to Control groups. Both walking track and grid-walk analysis revealed that return of functional recovery was accelerated by ES. The ES group demonstrated improved functional recovery over time, as well as improved recovery compared to the Control group at 21 weeks. Conclusions: ES improved early axon regeneration into a nerve isograft and was associated with increased macrophage and beneficial M2 macrophage accumulation within the isograft. ES ultimately improved functional recovery compared to isograft repair alone. This study supports the clinical potential of ES to improve the management of nerve injuries requiring a nerve graft repair.
Keywords: nerve, basic science, autograft, muscle, nerve regeneration, diagnosis, microsurgery, specialty
Introduction
Despite their regenerative ability, repaired peripheral nerve injuries typically result in modest functional recovery. 1 A major factor affecting functional recovery in repaired nerve injuries is the inability of axons to regenerate long distances to reach their appropriate end-organ target (ie, muscle and skin),2,3 as well as the potential misdirection of axon growth to inappropriate targets. 4 Additionally, axon growth is further challenged by cellular and structural changes to the nerve microenvironment that accompany injury and repair. Taken together, these cumulatively limit functional recovery potential.
Therapeutic electrical stimulation (ES) applied to repaired nerve is a promising treatment option to overcome these issues and improve upon reconstructive limitations.5-7 ES therapy applied to nerve injury and repair scenarios improves axon regeneration and functional recovery, as demonstrated in animal models. 8 In rodent models of nerve transection and direct repair, ES applied after the nerve repair improves the speed at which axons cross the repair site, as well as the accuracy at which motor and sensory axons regenerate to their appropriate end-organ targets.9-12
However, as promising as ES appears in its neuro-regenerative abilities, the majority of animal studies have only evaluated ES in end-to-end nerve repairs. End-to-end neurorrhaphies are generally considered to have superior outcomes compared to nerve grafting. However, in practice an end-to-end repair is frequently not feasible. 13 Nerve grafting presents additional regenerative challenges, as the axons must cross multiple suture lines, grow through the less permissive microenvironment of a nerve graft, and regenerate longer distances. Given this increased regenerative challenge, it remains unclear if ES provides similar benefits in nerve graft repair as direct repair.
The capabilities of ES to improve nerve repair using grafting still remains relatively understudied. Recently, it was demonstrated that a clinically relevant ES protocol in a rodent animal model of nerve isograft repair (considered an animal equivalent of autograft repair) improved axon regeneration across the nerve grafts. Therefore, conceptionally, this work demonstrated that ES improves axon regeneration, but it is unclear if functional recovery can also be achieved. 14 Our studies sought to address these shortcomings. Our studies provide additional evidence regarding ES’s efficacy in the context of nerve grafting reconstruction. Furthermore, our studies assess whether a clinically relevant ES protocol applied to a nerve repair using a clinically relevant, nerve isograft repair in a rodent model could improve early axon regeneration into the isograft, as well as functional recovery.
Materials and Methods
Animals
Adult male Lewis rats weighing 226 to 250 g (Charles River Laboratories; Wilmington, MA) were used for both experimental and donor animals to generate sciatic nerve isografts. Animals were housed in a central housing facility and received food (PicoLab rodent diet 20, Purina Animal Nutrition LLC; St. Louis, MO) and water ad libitum. All procedures were carried out in strict accordance with the guidelines set forth by the National Institutes of Health and were approved by the Division of Comparative Medicine at the institution. Specifically, surgical procedures and perioperative care measures were conducted in compliance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited Washington University Institutional Animal Care and Use Committee under animal protocol #20170111.
Experimental Design
The study was executed using 2 arms. Eight (Arm 1) and 18 (Arm 2) Lewis rats were randomized equally to 1 of 2 groups: 0 min ES (Control group) or 60 min ES (ES group). These groups sought to determine the effect to nerve regeneration following tibial nerve transection and nerve isograft repair. In both arms, animals received a tibial nerve transection followed by immediate nerve isograft repair, described in the Surgical Procedures section. Arm 1 assessed whether ES had an impact on early axon outgrowth and macrophage recruitment and accumulation within the nerve grafts, measured using immunohistochemistry (IHC) on nerve harvested 2 weeks after procedures. Two weeks was chosen as previous studies demonstrated ES resulted in near maximal numbers of axons crossing a suture line by 2 weeks postoperatively compared to control.9,15 Arm 2 assessed the long-term outcome and recovery, where functional recovery was evaluated over 21 weeks, and the quality of nerve regeneration was evaluated using histology from this nerve harvested at 21 weeks. A 21-week endpoint was chosen based on previous studies demonstrating that a tibial nerve transection-repair model took at least 16 weeks to recover functionally to its maximum using walking track analysis, 16 and at least 16 weeks to recover its capable muscle contractile force.17,18
Surgical Procedures
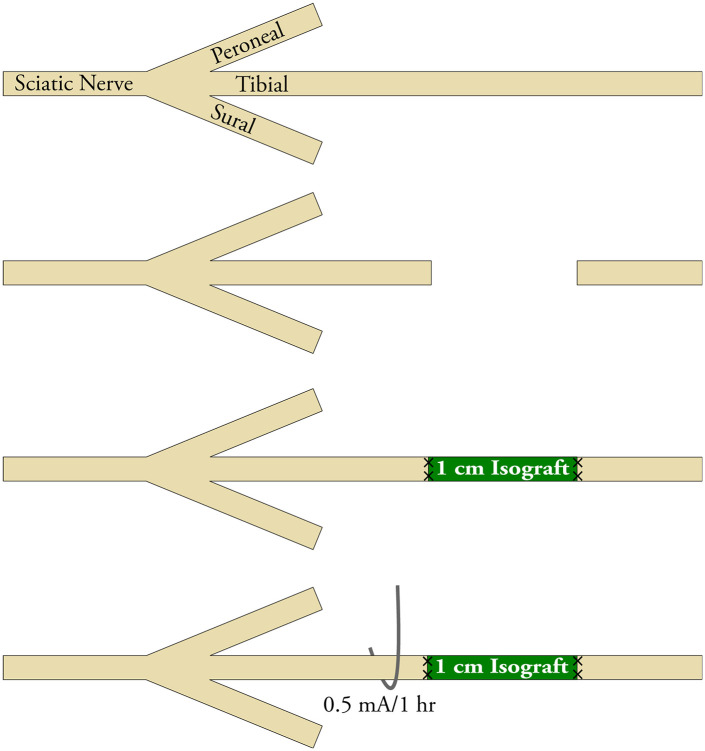
All procedures were carried out in strict accordance with the guidelines set forth by the National Institutes of Health and were approved by the Division of Comparative Medicine at the institution. Surgical procedures and peri-operative care measures were conducted in compliance with the AAALAC accredited Washington University Institutional Animal Care. Surgeries were performed using aseptic technique and with the aid of an operating microscope. Anesthesia was delivered using a cocktail of ketamine (75 mg/kg; Zoetis Inc, Kalamazoo, MI) and dexmedetomidine (0.5 mg/kg; Zoetis Inc). During surgery, animals were placed on a warming pad for body temperature maintenance and given 1 mL of normal saline subcutaneously for hydration. The tibial nerve was exposed using a gluteal muscle-splitting approach. To obtain donor tibial nerve for isograft, the tibial nerve was measured and dissected to achieve the needed length bilaterally in donor animals. In the animals randomized to the experimental groups, the right sciatic nerve was exposed, and the tibial nerve transected 3 mm distal to the trifurcation. The defect was reconstructed using 1 cm reversed isografts with 9-0 nylon micro-suture proximally and distally. Then, in applicable groups, a stainless steel 304 wire electrode (Component Supply Company, Sparta, TN) was hooked around the tibial nerve 2 mm proximal to the cut and repair site (Figure 1). The nerve was hooked by fashioning the wire into a half circle and applying gentle upward tension to the wire to secure it to the nerve without compressing the nerve. A return current electrode was placed securely in musculocutaneous facia in the dorsum of the animal just proximal to the injured nerve. In the ES group, the ES device (Checkpoint® Stimulator/Locator, Checkpoint Surgical, Inc, Cleveland, OH) delivered 60 minutes of intraoperative current at 0.5 mA, at a pulse width of 100 µs and frequency of 16 Hz, setting based on previous studies. 19 To confirm ES, the device used provided feedback via a LED light indicating whether current was provided and circuit complete. Evidence of excitation of nerve was also verified based on the contractions of the foot for the period of stimulation. After the completion, the electrodes were gently removed from the nerve and fascia. Wounds were closed in a layered fashion, using 4-0 vicryl suture for muscle and 4-0 nylon suture for skin (Ethicon, Somerville, NJ). Animals were recovered with a subcutaneous injection of atipamezole HCl (1 mg/kg, Antisedan®, Orion Corporation, Finland) and placed on a warming pad postoperatively. Postoperative pain and hypersensitivity were managed using a single dose of Buprenorphine SR (1 mg/kg, ZooPharm, Windsor, CO) given intraoperatively. Animals were returned to the central housing facility and closely monitored for infection, distress, and other morbidities. For all nonsurvival procedures described later, animals were killed by i.p. administered pentobarbital (150mg/kg).
Figure 1.
Schematic of electrical stimulation (ES) applied after nerve grafting. Experimental groups receiving ES therapy had a mono-polar electrode hooked around the tibial nerve proximal to the cut and repair site. A return electrode was placed securely in musculocutaneous facia.
Note. ES was delivered immediately after nerve graft repair for 60 minutes using intraoperative constant current at 0.5 mA, a pulse width of 100 µs, and a frequency of 16 Hz.
Immunohistochemistry IHC
In Arm 1, to assess axon regeneration and macrophage populations within nerve grafts, animals were sacrificed at 2 weeks postoperatively and nerve samples were explanted and immediately placed in 4% paraformaldehyde in phosphate buffered saline (PBS) overnight followed by immersion in 30% sucrose in PBS solution for 24 to 48 hours. Samples were frozen in OCT Compound (VWR, Radnor, PA) and sectioned at 15 µm onto pretreated charged glass slides. Sections were rehydrated and blocked using 5% normal goat serum diluted in PBS before primary antibody staining. Primary antibodies (Abcam, Boston, MA) were applied overnight and included axons (β-III-Tubulin [1:500]) and macrophages (CD68 [1:500], CD206 (1:250)). Sections were then washed in PBS and stained for appropriate secondary antibodies (1:500) for 1 hour at room temperature (Thermofisher Scientific, Waltham, MA). All sections were mounted with Fluoroshield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Abcam) and then imaged using a Fluoview FV1000 confocal microscope system (Olympus, Waltham, MA). A minimum of 3 sections were analyzed and averaged for each nerve using ImageJ (NIH) to obtain a value for each single animal (n = 1). For axon density, an ImageJ macro was used to both threshold a minimum expression to qualify as positive expression, and then quantify the percentage of the area positive for expression from standardized distances. For cell counts, a 600x standardized standard field was used and colocalization of the marker(s) with DAPI was considered a positive cell.
Walking Track Functional Assessment
In Arm 2, behavioral recovery was monitored weekly for up to 15 weeks, and then biweekly until the endpoint at 21 weeks. Animal hind feet were glazed with water-soluble, nontoxic paint before the animal was placed on a plexiglass track lined with construction paper. Each animal walked down the track until at least 3 pairs of footprints with clear markings were obtained. On each footprint, parameters were measured to calculate the tibial functional index (TFI) developed by Bain 20
Grid-Walk Assessment
Animals in Arm 2 also underwent a grid-walk behavioral assessment for functional recovery at the 21-week endpoint following walking track assessment. Animals were placed on an elevated grid with a grid size measuring 3.5 cm by 3.5 cm. After animals had acclimated to the grid for at least 5 mins, they were recorded with a video camera for at least 5 minutes moving upon the grid. From the video considering the injured limb, the total number of steps with that foot and steps that resulted in a foot placement missing the mesh and going through the grid (slipped steps) was measured. Foot fault was calculated as the proportion of slipped steps to total steps.
Nerve Histomorphometry
At 21 weeks postoperatively after behavioral testing was completed, en bloc specimens of the tibial nerve mid-graft and tibial nerve 5 mm distal to the repair site underwent histomorphometric analysis as previously described.21,22 Sectioned slides were analyzed to quantify nerve fiber counts, percent neural tissue, fiber sizes, and myelin thickness. All analyses were done by an observer blinded to the experimental groups.
Statistical Analysis
Prism 8 (GraphPad Software Inc, LaJolla, CA) was used for all statistical analyses. Data are presented as mean ± standard deviation (SD). Data for walking track analyses were analyzed using 2-way analysis of variance (ANOVA) with repeated measures, followed by Tukey’s multiple comparisons test for posthoc analysis between groups at each time point and within each group over time. All other data were analyzed using unpaired t-tests. The study was adequately powered (β = 0.80), and significance (α) was set at 0.05 (P < .05).
Results
ES Improved Early Axon Regeneration and Increased Macrophage Accumulation Within Isografts
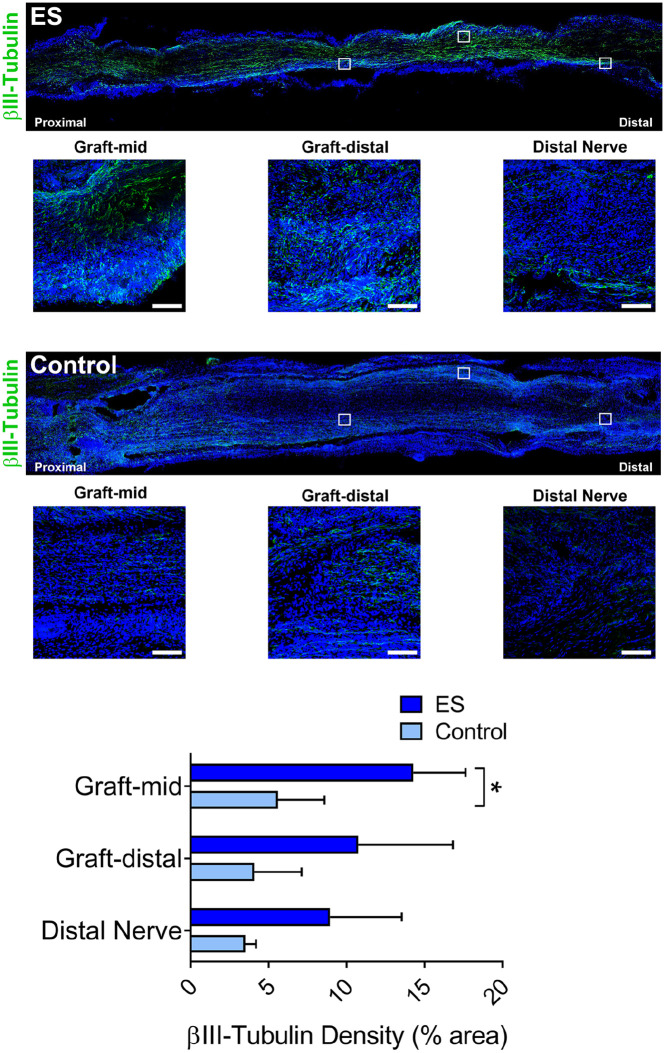
Both experimental groups demonstrated axon regeneration, as measured by βIII-tubulin staining for axon density. Both groups had positive βIII-tubulin within the mid-graft and distal graft, as well as distal nerve indicating that some quantity of axons traversed the entire distance by 2 weeks after surgery (Figure 2). The ES group demonstrated improved axon regeneration into the nerve isograft, based on increased axon density (% βIII-tubulin area) in the mid-graft vs the Control group (P < .01). No significant differences in axonal density were observed within the distal graft or distal nerve between groups.
Figure 2.
ES improved early axon regeneration within isografts. Representative immunofluorescence images and quantification of axons (βIII-tubulin) within nerve isografts 2 weeks postrepair. Inset boxes represent magnified fields presented within the graft and distal nerve.
Note. Scale bars represent 20 µm. Mean ± SD, n = 4/group; * indicates P < .05. ES = electrical stimulation.
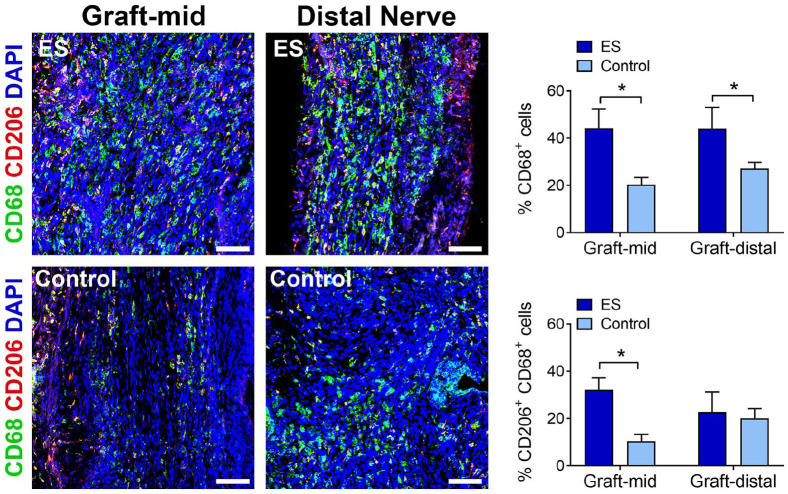
At this 2-week endpoint, macrophage accumulation within the isograft was also observed for both experimental groups (Figure 3). The ES group contained an increased proportion of macrophages (CD68+ cells) among all cells within both the mid-graft (P < .01) and distal graft (P < .05) compared to the Control group. Furthermore, the ES group also contained an increased proportion of M2 macrophages (CD206+ and CD68+ ) among all cells within the mid-graft (P < .05), but not distal graft (P > .05), compared to the Control group.
Figure 3.
ES increased macrophage accumulation within isografts. Representative immunofluorescence images and quantification of macrophages (pan: CD68; M2: CD206) within nerve isografts 2 weeks postrepair. Scale bars represent 20 µm. Mean ± SD, n = 4/group; * indicates P < .05. ES = electrical stimulation.
ES Improved Functional Recovery Following Nerve Repair Using Isografts
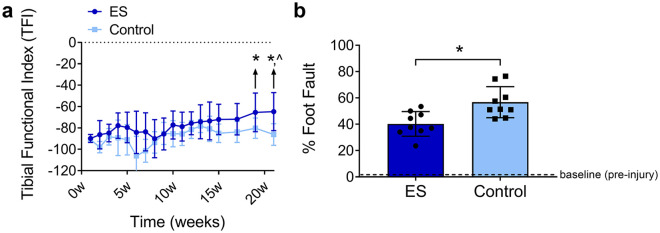
All animals in the study demonstrated functional impairment after the nerve injury and repair (Figure 4). Walking track analysis was performed weekly until Week 15, and then biweekly until Week 21, where both groups demonstrated a trend toward improved recovery over time. However, only the ES group demonstrated improved recovery at Week 21 compared to Week 1 based on TFI values (P < .05; Figure 4a). As well, the ES group demonstrated increased functional recovery based on TFI values compared to the Control group starting at Week 19 (P < .05), which was sustained until the end of measurements at Week 21 (P < .01). Additionally, at Week 21, grid-walk analysis was performed, which revealed that the ES group had improved recovery, as measured by fewer foot faults compared to the Control group (P < .01; Figure 4b). Neither group recovered to their preinjury measurements.
Figure 4.
ES improved functional recovery across isografts. Quantification of walking track using tibial functional index (a) and grid-walk analysis (b) at 21 weeks postrepair. Mean ± SD, n = 9/group; * indicates P < .05 vs Control and ^ indicates P < .05 vs postinjury (1 week).
Note. ES = electrical stimulation.
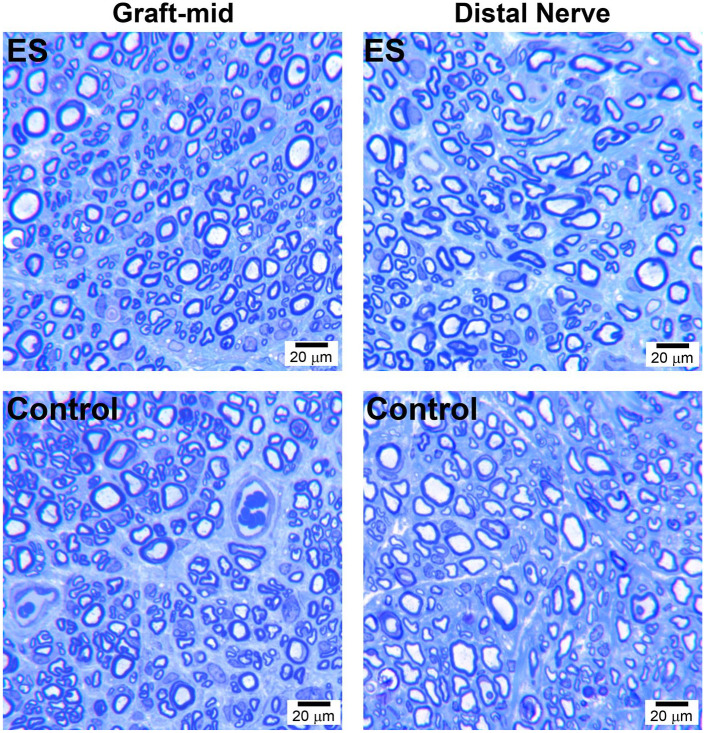
After behavioral assessments, the quality of final nerve regeneration was assessed within the mid-graft and distal nerve at Week 21, which demonstrated robust axon regeneration for both experimental groups (Figure 5 and Table 1). Nerve isograft and distal nerve demonstrated no histomorphometric differences at the end of the experiment, regardless of experimental treatment, across all histomorphometric parameters.
Figure 5.
Final nerve regeneration quality within and across isografts. Representative histological images of repaired nerve within the isograft (graft-mid) and distal nerve at 21 weeks postrepair. Scale bars represent 20 µm. ES = electrical stimulation.
Table 1.
Histomorphometric Data 21 Weeks Posttibial Nerve Injury and Isograft Repair.
| Group | Myelinated axon number |
Nerve density (#/µm2) |
Percent nerve (%) |
Fiber width (µm) |
Myelin width (µm) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Graft-mid | Distal nerve | Graft-mid | Distal nerve | Graft-mid | Distal nerve | Graft-mid | Distal nerve | Graft-mid | Distal nerve | |
| ES (60 minutes) | 20 138 ± 4356 | 6783 ± 1846 | 35 556 ± 6502 | 23 844 ± 8001 | 47 ± 5 | 38 ± 6 | 3.32 ± 0.25 | 3.72 ± 0.35 | 0.77 ± 0.06 | 0.82 ± 0.06 |
| Control (no ES) | 18 797 ± 4200 | 6189 ± 2031 | 35 235 ± 5542 | 23 721 ± 4956 | 44 ± 5 | 40 ± 9 | 3.20 ± 0.18 | 3.72 ± 0.32 | 0.77 ± 0.05 | 0.82 ± 0.05 |
Note. ES = electrical stimulation.
Discussion
In order to understand the potential of therapeutic ES to improve nerve grafting outcomes for the clinic, we explored the effect of ES on nerve regeneration across 1 cm isografts in a rat tibial nerve gap injury model. We determined that ES not only improved early axon regeneration across the isograft, but was associated with increased macrophage and beneficial M2 macrophage accumulation within the isograft, and ultimately improved functional recovery compared to no ES.
Previous studies that employed an end-to-end nerve repair model demonstrated that axonal growth across the repair site was accelerated due to ES, where ES resulted in near maximal numbers of axons crossing the suture line by 2 weeks instead of 4 weeks postoperatively. 9 As well, recently it was also demonstrated that ES in a nerve isograft repair improved axon regeneration across nerve isografts. 14 Our results similarly demonstrated that ES improved the speed of axon regeneration across a repair site, as axon density within the isografts was increased compared to the control. Therefore, our results corroborate that ES in the context of nerve grafting still accelerates axon regeneration across a repair site.
In addition to assessing early axon regeneration, ES increased the proportion of macrophages and M2 macrophages within isografts. This general correlation of increased macrophages due to ES therapy has been noted in other studies of ES applied to nerve,23,24 but not previously to nerve grafts. This novel finding holds importance as macrophages play a key beneficial role in regeneration. Following nerve injury, macrophages accumulate to promote nerve regeneration, as well as angiogenesis.25,26 Furthermore, additional studies delineate a beneficial role for so-called “M2” macrophages in nerve regeneration, where these macrophages promote Schwann cell functions including myelination during regeneration. 27 While we observed an increase in macrophages and M2 macrophages, it is unclear if this increase is directly or indirectly attributable to ES. As axon regeneration improved with ES, the resulting increase in macrophage accumulation might be due to the greater number of axons, and thus only indirectly associated with the ES therapy. Alternatively, the increased macrophage accumulation might be directly due to ES, potentially implicating macrophage recruitment in the mechanism of improved nerve regeneration with ES. This mechanism is not yet clear, and further exploration is required to uncover the mechanism of action for macrophage accumulation due to ES.
Long-term recovery was evaluated through both histological and functional analyses, and showed that ES significantly improved functional recovery compared to Control. Both walking track and grid-walk analysis revealed that return of functional recovery was facilitated by ES, as the ES group demonstrated superior functional recovery compared to Control. These results corroborate other animal studies employing ES therapy during nerve repair.8,28 But, these findings are novel for the context of nerve grafting reconstruction. Despite this evidence, final nerve regeneration based on histology, revealed no differences at 21 weeks. While these data appear to conflict with the early axon regeneration outcomes measured at 2 weeks and the functional outcomes, the rodent model is known to have histological assessment limitations at late endpoints. 29 Rodent models are known to have superior neural regeneration compared to humans, 30 and as well, later endpoints often find no histological differences between experimental groups in rodent models of nerve injury and regeneration.26,29,31,32 Early histological assessments, such as the IHC assessed at 2 weeks in our studies, provide valuable information indicating more rapid axon regeneration that can be measured more sensitively than at later endpoints. Therefore, the 21-week endpoint serves not to demonstrate any superior axon regeneration between the groups, but to validate that no issues prevented regeneration in this nerve isograft repair model. Specifically, the control group was capable of regenerating similar numbers of axons across the isograft, but it proceeded at a slower rate than the ES group and did not result in similar levels of functional improvement. Interpreting the overall results, it appears that improved early axon regrowth is key to improved function when delivering ES in an isograft model.
Conclusion
In conclusion, ES applied following nerve injury and graft repair improves early axon regeneration across a nerve graft. And, this early axon outgrowth stimulated by ES may in turn promote and improve functional recovery compared to graft repair alone. Based on these preclinical model findings, the use of ES to promote clinical outcomes following reconstruction of nerve gap injuries is supported.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All institutional and national guidelines for the care and use of laboratory animals were followed.
Statement of Informed Consent: This study does not contain any studies with human subjects.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.D.W has been the recipient of sponsored research agreements from Checkpoint Surgical, Inc. and has consulted for Foundry Therapeutics, LLC and The Foundry, LLC. No personal compensation was provided. Checkpoint Surgical, Inc. did not influence or affect the experimental design or outcome of the studies. None of the other authors have any conflicts of interest to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded in part by a research grant from the American Association for Hand Surgery (M.D.W.), a medical student research grant from the American Society for Reconstructive Microsurgery (G.C.K.), by an industry sponsored research agreement from Checkpoint Surgical, Inc. to Washington University (M.D.W.), and by in part by the National Institutes of Neurological Disorders and Stroke of the National Institutes of Health (NIH) under award number K08 NS096232 (A.S.W.) to Washington University.
ORCID iDs: Joseph Roh  https://orcid.org/0000-0002-2764-707X
https://orcid.org/0000-0002-2764-707X
Amy M. Moore  https://orcid.org/0000-0002-4951-1325
https://orcid.org/0000-0002-4951-1325
Susan E. Mackinnon  https://orcid.org/0000-0002-5561-6027
https://orcid.org/0000-0002-5561-6027
Matthew D. Wood  https://orcid.org/0000-0001-8132-6827
https://orcid.org/0000-0001-8132-6827
References
- 1. Taylor CA, Braza D, Rice JB, et al. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87(5):381-385. [DOI] [PubMed] [Google Scholar]
- 2. Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15(5 Pt 2):3886-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995;15(5 Pt 2):3876-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wood MD, Mackinnon SE. Pathways regulating modality-specific axonal regeneration in peripheral nerve. Exp Neurol. 2015;265:171-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan KM, Curran MW, Gordon T. The use of brief post-surgical low frequency electrical stimulation to enhance nerve regeneration in clinical practice. J Physiol. 2016;594(13):3553-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon T, Amirjani N, Edwards DC, et al. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol. 2010;223(1):192-202. [DOI] [PubMed] [Google Scholar]
- 7. Wong JN, Olson JL, Morhart MJ, et al. Electrical stimulation enhances sensory recovery: a randomized controlled trial. Ann Neurol. 2015;77(6):996-1006. [DOI] [PubMed] [Google Scholar]
- 8. Gordon T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics. 2016;13(2):295-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al-Majed AA, Neumann CM, Brushart TM, et al. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20(7):2602-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brushart TM, Hoffman PN, Royall RM, et al. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22(15):6631-6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. English AW, Schwartz G, Meador W, et al. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol. 2007;67(2):158-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordon T, Udina E, Verge VM, et al. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control. 2009;13(4):412-441. [DOI] [PubMed] [Google Scholar]
- 13. Mackinnon SE. Surgical management of the peripheral nerve gap. Clin Plast Surg. 1989;16(3):587-603. [PubMed] [Google Scholar]
- 14. Zuo KJ, Shafa G, Antonyshyn K, et al. A single session of brief electrical stimulation enhances axon regeneration through nerve autografts. Exp Neurol. 2020;323:113074. [DOI] [PubMed] [Google Scholar]
- 15. Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12(12):4381-4390. [PubMed] [Google Scholar]
- 16. Hare GM, Evans PJ, Mackinnon SE, et al. Walking track analysis: a long-term assessment of peripheral nerve recovery. Plast Reconstr Surg. 1992;89(2):251-258. [PubMed] [Google Scholar]
- 17. Ladak A, Schembri P, Olson J, et al. Side-to-side nerve grafts sustain chronically denervated peripheral nerve pathways during axon regeneration and result in improved functional reinnervation. Neurosurgery. 2011;68(6):1654-1665; discussion 1665-1666. [DOI] [PubMed] [Google Scholar]
- 18. Hendry JM, Alvarez-Veronesi MC, Snyder-Warwick A, et al. Side-to-side nerve bridges support donor axon regeneration into chronically denervated nerves and are associated with characteristic changes in Schwann cell phenotype. Neurosurgery. 2015;77(5):803-813. [DOI] [PubMed] [Google Scholar]
- 19. Jo S, Pan D, Halevi AE, et al. Comparing electrical stimulation and tacrolimus (FK506) to enhance treating nerve injuries. Muscle Nerve. 2019;60(5):629-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83(1):129-138. [DOI] [PubMed] [Google Scholar]
- 21. Hunter DA, Moradzadeh A, Whitlock EL, et al. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166(1):116-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunter DA, Pan D, Wood MD, et al. Design-based stereology and binary image histomorphometry in nerve assessment. J Neurosci Methods. 2020;336:108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLean NA, Verge VM. Dynamic impact of brief electrical nerve stimulation on the neural immune axis-polarization of macrophages toward a pro-repair phenotype in demyelinated peripheral nerve. Glia. 2016;64(9):1546-1561. [DOI] [PubMed] [Google Scholar]
- 24. Oliveira KMC, Barker JH, Berezikov E, et al. Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model. Sci Rep. 2019;9(1):11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cattin AL, Burden JJ, Van Emmenis L, et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162(5):1127-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan D, Mackinnon SE, Wood MD. Advances in the repair of segmental nerve injuries and trends in reconstruction. Muscle Nerve. 2019;61:726-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stratton JA, Holmes A, Rosin NL, et al. Macrophages regulate Schwann cell maturation after nerve injury. Cell Rep. 2018;24(10):2561-2572.e6. [DOI] [PubMed] [Google Scholar]
- 28. Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci. 2016;43(3):336-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brenner MJ, Moradzadeh A, Myckatyn TM, et al. Role of timing in assessment of nerve regeneration. Microsurgery. 2008;28(4):265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mackinnon SE, Hudson AR, Hunter DA. Histologic assessment of nerve regeneration in the rat. Plast Reconstr Surg. 1985;75(3):384-388. [DOI] [PubMed] [Google Scholar]
- 31. Vannucci B, Santosa KB, Keane AM, et al. What is normal? Neuromuscular junction reinnervation after nerve injury. Muscle Nerve. 2019;60(5):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santosa KB, Keane AM, Jablonka-Shariff A, et al. Clinical relevance of terminal Schwann cells: an overlooked component of the neuromuscular junction. J Neurosci Res. 2018;96(7):1125-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]