Abstract
Objective
We assessed levels of anxiety and depression in patients with Crohn disease (CD) to identify predictors of health-related quality of life (HRQOL).
Methods
In this case-control study, we enrolled 50 adult patients with CD and 50 matched, healthy controls. All participants completed self-administered questionnaires including the Self-rating Anxiety Scale (SAS), Self-rating Depression Scale (SDS), Short Form-36 Health Survey (SF-36), and Short Inflammatory Bowel Disease Questionnaire (IBDQ, patients only). We analyzed the relationship between HRQOL and influencing factors.
Results
Mean total scores on the SAS, SDS, and SF-36 were significantly different between patients and controls. IBDQ scores among patients in the active phase of CD were significantly lower than those in remission phase. SF-36 scores were significantly lower in patients with CD compared with healthy controls. SF-36 scores among patients with active CD were significantly lower than scores among those in remission, and SF-36 scores in patients without complications were significantly higher than in those with complications. SF-36 scores in patients with good nutritional status were also significantly higher than scores in malnourished patients with CD.
Conclusions
Depression, anxiety, disease activity, complications, and nutritional status were predictive factors of decreased HRQOL in patients with CD.
Keywords: Crohn disease, health-related quality of life, short form-36 health survey, active disease, complication, nutritional status
Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn disease (CD), has a nonspecific etiology and the incidence and prevalence of IBD are increasing. 1 To date, there is no definite cure for CD, and treatment is concentrated on managing the inflammatory response and maintaining remission with a focus on maintenance therapy. 2
Psychological effects from CD and medications are experienced by many patients. Anxiety disorders and depression can have a profound effect on quality of life (QOL), including the ability to work and maintain a family life. There is no doubt that stress is a triggering and exacerbating factor connected with the course and symptoms of CD. 3 Although an annual increase in the number of patients with CD had been documented in China, very few studies have focused on both health-related quality of life (HRQOL) and psychological symptoms in these patients.
CD involves a chronic progressive process that is characterized by intermittent recurrence. Disease activity influences a number of mechanisms, including metabolic disturbances associated with chronic inflammation, protein-losing enteropathy, chronic blood loss, and malabsorption, as well as side effects of medication. 4 Higher CD disease activity increases the likelihood that patients will have poorer QOL and advancing malnutrition. 5 Many of these patients have severe forms of gastrointestinal (GI) and systemic illness, and some patients experience a series of complications. These outcomes can temporarily or permanently lower patients’ HRQOL. 6
The Self-rating Anxiety Scale (SAS) and Self-rating Depression Scale (SDS) are self-report questionnaires used to assess disease symptoms, severity, and clinical status.7,8 HRQOL is defined as a multidimensional concept that is used to assess health-related physical, emotional, and social functions. 9 Patients with CD have higher rates of depression and may have higher rates of other anxiety-related disorders. 10
In this study, we investigated depression and anxiety levels as well as HRQOL among patients with CD to identify predictive risk factors of decreased HRQOL in these patients.
Methods
Patients
In this case-control study, we recruited patients with CD from the First Hospital of Soochow University between January 2019 and December 2020. The diagnostic criteria for all patients were those set out in the 2007 guidelines of the Chinese Medical Association “The Specification Consensus for Right Diagnosis and Treatment of Inflammatory Bowel Disease in China.” 11 In our patients, CD was classified as active disease or remission according to the CD Activity Index (CDAI). Healthy volunteers without a family history of IBD or related diseases were also recruited from the First People’s Hospital of Soochow University during the same period and included as normal controls. An anonymous, self-administered questionnaire was sent to all participants via an Internet platform.
The Ethics Committee of the First Hospital of Soochow University approved the study protocol. The patients participating in the study provided written formed consent. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 12
Clinical features of patients
A demographic survey was used to collect information on sex, age, marital status (married, unmarried or divorced), education level (middle school or below, college degree or above), medical insurance (none or residents’ basic health insurance), use of biologic therapy (yes or no), surgery (yes or no), and disease status (active or remission). The criteria used for clinical disease activity were those stipulated in the CDAI. 13 The Patient-Generated Subjective Global Assessment (PG-SGA) incorporates a numerical score as well as a global rating of nutritional status (well-nourished, malnourished or severely malnourished). Patients were scored and categorized as follows: 0–1, no nutritional intervention required at present; 2–3, educational intervention by a dietitian or clinician for symptom management; 4–8, intervention by a dietitian indicated with physician consultation; and ≥9, critical need for dietary intervention and symptom management. 14 For each component of the PG-SGA, 0–4 points are awarded according to the impact of symptoms on nutritional status. The higher the score, the greater the risk of malnutrition.
Emotional status
Patients’ anxiety levels were judged using the Self-rating Anxiety Scale (SAS) and depression was estimated using the Self-rating Depression Scale (SDS). In both scales, scores for each item range from 1 to 4. The scores for each scale were multiplied by 1.25. A score of 50 or more on the SAS indicates anxiety, and a score of more than 53 on the SDS indicates sustained depression. Higher scores on these scales are related to a greater likelihood of psychological morbidity.
Health-related quality of life (HRQOL)
The 10-item Inflammatory Bowel Disease Questionnaire (IBDQ) has been applied effectively in patients with IBD. 15 The IBDQ includes physical, social, emotional, and systemic domains. The questionnaire is graded on a scale from 1 to 7 (severe to no problem at all, respectively). Total scores range from 10 (poor) to 70 (best).
The Medical Outcomes Study Questionnaire Short Form 36, or Short Form-36 Health Survey (SF-36), address general health parameters and is designed for the evaluation of general populations and for health policies. 16 The SF-36 is also applied in clinical practice and research related to certain diseases. The SF-36 includes 36 items in two dimensions, the physical component summary (PCS) and mental component summary (MCS). The PCS comprises four subscales: physical function (PF), role limitations owing to physical health (RP), bodily pain (BP), and general health perceptions (GH). The MCS also comprises four subscales: vitality (VT), social functioning (SF), role limitations owing to emotional problems (RE), and mental health (MH). Scores range from 0 to 100 for each subscale in both dimensions, with higher scores indicating better health.
Statistical analysis
All demographic, clinical, physical, and psychological variables were included in descriptive analyses. Categorical variables are reported as frequency (percentage), and continuous variables are reported as mean ± standard deviation or standard error. The Student t test was used to compare differences in the SAS, SDS, IBDQ, and SF-36 scores between two groups. For demographic and clinical variables in patients with CD, we used multiple regression analyses to identify the determinants of HRQOL. We used IBM SPSS version 19.0 to analyze all data (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Fifty adult patients with CD, and 50 matched healthy controls were enrolled in this study. Fifty adult patients with CD (14 male and 36 female patients, age 14–60 years), and 50 matched healthy controls (19 male and 31 female patients, age 15–57 years), were enrolled in this study. General demographic information of the included patients with CD are summarized in Table 1, according to the Montreal revision (2005) of the Vienna classification, which is considered the international standard of subtyping in CD. Overall, 26 (52%) patients were less than age 16 years, 20 (40%) were between age 17 and 40 years, and 4 (8%) were more 40 years old. The region of involvement was the terminal ileum in 28 (56%) patients, colon in 19 (38%) patients, and ileocolon in 3 (6%) patients. As for the behavior of CD, 8 (16%) patients were classified as having non-stricturing non-penetrating disease (designated as B1), 39 (78%) as having stricturing disease (B2), and 3 (6%) patients as having penetrating disease (B3). The occurrence of perianal fistulae and abscesses is considered a “modifier,” denoted with a “P” (for perianal) added to B1, B2 or B3. A total of 4 (8%) patients were classified as B1P, 2 (4%) as B2P, and 2 (4%) as B3P.
Table 1.
Montreal phenotype classification in patients with CD.
| Montreal phenotype classification | n (%) |
|---|---|
| Age (A), y | |
| A1 (≤16) | 26 (52%) |
| A2 (17–40) | 20 (40%) |
| A3 (>40) | 4 (8%) |
| Location (L) | |
| L1: terminal ileum | 28 (56%) |
| L2: colon | 19 (38%) |
| L3: ileocolon | 3 (6%) |
| L4: upper GI | 0 (0%) |
| L1 + L4: terminal ileum + upper GI | 0 (0%) |
| L2 + L4: colon + upper GI | 0 (0%) |
| L3 + L4: ileocolon + upper GI | 0 (0%) |
| L4: upper GI only | 0 (0%) |
| Behavior (B) | |
| B1: non-stricturing, non-penetrating | 8 (16%) |
| B2: stricturing | 39 (78%) |
| B3: penetrating | 3 (6%) |
| Occurrence of perianal fistulae and abscesses (P) | |
| B1P: non-stricturing, non-penetrating with perianal fistulae and abscesses | 4 (8%) |
| B2P: stricturing with perianal fistulae and abscesses | 2 (4%) |
| B3P: penetrating with perianal fistulae and abscesses | 2 (4%) |
CD, Crohn disease; GI, gastrointestinal.
SAS and SDS in patients with CD
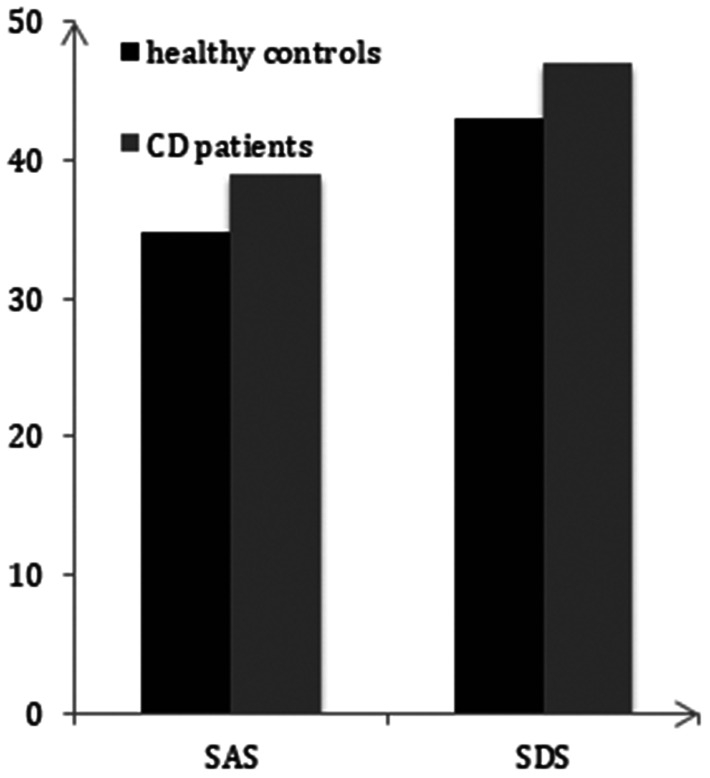
In our study, both the mean SAS score (38.78 ± 6.4) and mean SDS score (47.08 ± 9.5) among patients with CD was found to be significantly higher than scores (SAS = 34.57 ± 6.7, SDS = 43.23 ± 8.7) among control participants (p < 0.05) (Figure 1).
Figure 1.
SAS and SDS total scores between patients with CD and healthy controls (mean ± standard error).
*p < 0.05 indicates a significant difference between patients with CD and healthy controls.
SAS, Self-rating Anxiety Scale; SDS, Self-rating Depression Scale.
HRQOL in patients with CD
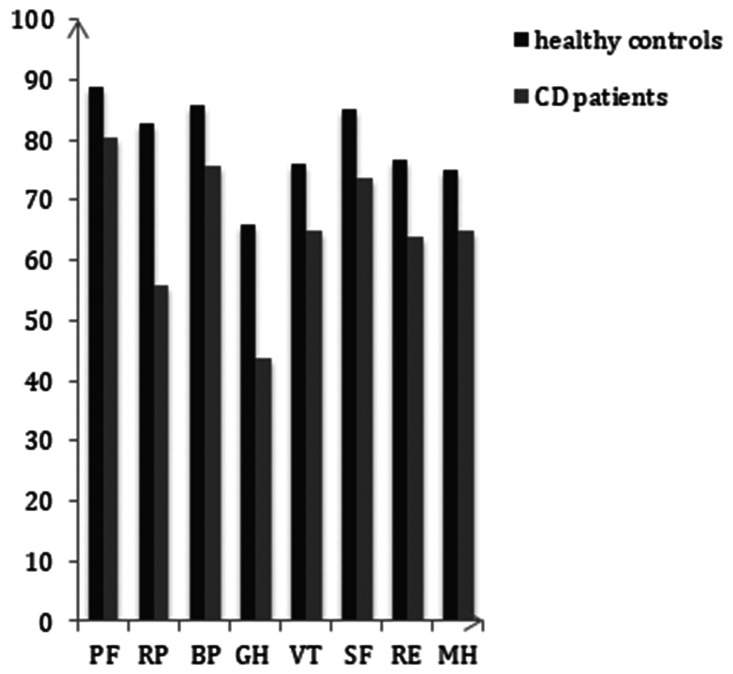
In patients with CD, mean IBDQ scores in the active phase of disease were lower than those in the remission phase (Table 2). Mean physical symptoms scores were 49.50 ± 7.62 and 60.12 ± 4.01 among patients in active phase and those in remission phase, respectively. Mean systemic symptom scores in these groups were 23.92 ± 5.07 and 26.24 ± 3.97, and mean emotional function scores were 57.13 ± 15.62 and 67.34 ± 15.17, respectively. Mean respective social function scores were 22.15 ± 9.08 and 25.44 ± 2.03. These scores demonstrated significantly lower HRQOL among patients with active disease than among patients in remission (p < 0.05). The total SF-36 score was significantly different between patients with CD and healthy controls (p < 0.05). Comparing each subscale, scores for PF, RP, and MH differed between patients with active disease and those in remission (p < 0.05). PF, RF, BP, RE and MH scores in patients with complications were significantly lower than those in patients with no complications. Subscale scores of GH and VT were relatively lower for malnourished patients than for well-nourished ones (p < 0.05) (Table 3 and Figure 2).
Table 2.
IBDQ scores according to disease activity indices.
| IBDQ domain | Crohn disease (N = 50) |
||
|---|---|---|---|
| Active * (n = 33) | Remission (n = 17) | P‡ | |
| Physical symptoms | 49.50 ± 7.62 | 60.12 ± 4.01 | p < 0.05 |
| Systemic symptoms | 23.92 ± 5.07 | 26.24 ± 3.97 | p < 0.05 |
| Emotional function | 57.13 ± 15.62 | 67.34 ± 15.17 | p < 0.05 |
| Social function | 22.15 ± 9.08 | 25.44 ± 2.03 | p < 0.05 |
*Disease activity assessed using the Crohn Disease Activity Index.
‡Student t test.
Values in the table are mean ± standard error.
IBDQ, Inflammatory Bowel Disease Questionnaire.
Table 3.
SF-36 domain scores in patients according to clinical factors.
| Clinical stage |
Clinical features |
Nutritional status |
||||
|---|---|---|---|---|---|---|
| Domain | Active | Remission | Complications | No complications | Well-nourished | Malnourished |
| PF | 60.02 ± 20.12 | 73.81 ± 20.12* | 61.27 ± 23.08 | 70.79 ± 23.08# | 80.23 ± 30.11 | 76.32 ± 21.07 |
| RP | 31.59 ± 40.72 | 56.37 ± 42.34* | 30.26 ± 33.15 | 48.34 ± 46.89# | 48.337 ± 21.07 | 39.45 ± 37.30 |
| BP | 67.69 ± 24.21 | 74.21 ± 22.14 | 66.12 ± 21.09 | 81.14 ± 23.09# | 72.43 ± 27.318 | 71.10 ± 26.00 |
| GH | 40.02 ± 21.34 | 46.62 ± 21.49 | 43.69 ± 21.91 | 50.47 ± 18.28 | 50.63 ± 23.21@ | 38.01 ± 18.53 |
| VT | 52.83 ± 17.83 | 60.51 ± 17.79 | 59.83 ± 16.43 | 63.01 ± 18.66 | 70.23 ± 18.80@ | 50.26 ± 17.28 |
| SF | 75.49 ± 24.07 | 80.62 ± 24.97 | 82.75 ± 23.43 | 86.17 ± 25.27 | 90.39 ± 20.02 | 87.06 ± 20.33 |
| RE | 38.56 ± 41.13 | 42.02 ± 42.65 | 24.43 ± 44.37 | 58.52 ± 39.09# | 60.65 ± 45.00 | 55.04 ± 44.21 |
| MH | 63.32 ± 11.71 | 80.03 ± 14.64* | 57.27 ± 12.25 | 75.24 ± 17.01# | 76.30 ± 21.13 | 72.15 ± 17.57 |
Notes: Versus active, p ≤ 0.05; versus complications, p ≤ 0.05; versus well-nourished, p ≤ 0.05.
Values in the table are mean ± standard error.
SF-36, Short Form-36 Health Survey; PF, physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health.
Figure 2.
SF-36 summary scores and subscale scores between patients with CD and healthy controls (mean ± standard error).
*p < 0.05 indicates a significant difference between patients with CD and healthy controls.
CD, Crohn disease; SF-36, Short Form-36 Health Survey; PF, physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health.
Mean SF-36 domain scores of patients with CD and different clinical factors
As shown in Table 4, we conducted multivariate regression analyses to assess the relationship between IBDQ domains and general characteristics of patients with CD. We found a significant correlation between IBDQ domains and disease activity status, as well as nutritional risk (p < 0.05). No significant correlations were found between IBDQ domains and sex, age, marital status, educational background, medical insurance, use of biologics, and surgery (p > 0.05).
Table 4.
Multivariate regression analyses of the relationship between HRQOL and predictor variables.
| IBDQ scores (mean ± SE) | t | P | |
|---|---|---|---|
| Sex | 1.045 | >0.05 | |
| Male | 161.67 ± 33.1 | ||
| Female | 157.67 ± 34.2 | ||
| Age, y | 0.837 | >0.05 | |
| <35 | 158.07 ± 36.4 | ||
| ≥35 | 162.53 ± 30.6 | ||
| Marital status | 0.840 | >0.05 | |
| Married | 157.71 ± 34.9 | ||
| Unmarried or divorced | 161.03 ± 32.4 | ||
| Educational level | 0.127 | >0.05 | |
| Middle school or below | 155.71 ± 33.1 | ||
| College degree or above | 160.53 ± 31.7 | ||
| Medical insurance | 0.713 | >0.05 | |
| No insurance | 157.03 ± 31.9 | ||
| Residents’ basic health insurance | 163.81 ± 30.5 | ||
| Use of biologic therapy | 0.306 | >0.05 | |
| Yes | 156.09 ± 31.2 | ||
| No | 161.23 ± 34.7 | ||
| Surgery | 0.997 | >0.05 | |
| Yes | 153.09 ± 32.5 | ||
| No | 160.19 ± 31.4 | ||
| Disease status | 2.357 | <0.05 | |
| Active | 154.83 ± 27.5 | ||
| Remission | 187.14 ± 30.1 | ||
| Nutritional risk | 2.171 | <0.05 | |
| Yes | 153.26 ± 31.1 | ||
| No | 185.01 ± 29.7 |
IBDQ, Inflammatory Bowel Disease Questionnaire; HRQOL, health-related quality of life.
Discussion
The relationship between psychiatric symptoms and the GI environment are well studied in various GI disorders. In comparison with healthy controls, more severe symptoms of depression and anxiety are reported among individuals with non-erosive reflux disease, irritable bowel syndrome, and functional dyspepsia. 17 Patients with IBD may have a higher lifetime rate of depression and anxiety disorders. 10
In the current study, we investigated the status of patients with CD in terms of psychological characteristics and HRQOL levels. Our evaluation of patients’ SAS and SDS scores showed that patients with CD exhibited more severe symptoms of anxiety and depression. Our findings are generally in accordance with those of other studies. Kim et al. revealed that the prevalence rates of depression and anxiety decreased during periods of IBD remission to 33% and 27%, respectively. 18 The evidence suggests a possible cyclical relationship in CD whereby GI symptoms increase an individual’s risk for worsening mood symptoms, which subsequently increase their risk for worsening physical symptoms.
The IBDQ is a commonly used tool for the assessment of QOL in patients with CD. 19 An association between clinical symptoms and QOL is reasonable and has been previously confirmed in web-based surveys by Kappelman et al. and Long et al. using the IBDQ.20,21 We found that all IBDQ subscale scores were significantly lower in the active-phase group than those among patients in remission. We found statistically significant differences between active phase and remission phase in scores related to GI symptoms, systemic symptoms, emotional function, and social function. The main intestinal complications of CD include acute inflammation of the intestines, ulcers, obstruction, and perforation. Many studies have revealed that mood dysfunction and psychological stress can trigger relapses in IBD.22–24 Among patients with CD, anxiety is associated with mood, stress, abdominal pain, and lower socioeconomic status. 25 Patients with poor coping skills or little social support, as well as those with certain personality traits such as neuroticism, may be predisposed to feeling frustrated or sad and to avoiding social interactions.
Clinical observations have indicated that stressful experiences can adversely influence the course of CD. The notion of perceived stress involves an individual’s subjective perception of and emotional response to stress. Stress is a triggering and exacerbating factor connected with the course and symptoms of IBD. 26 A negative relationship between psychological stress and the development of IBD has been found. 27 Together with patients’ awareness that they have an incurable disease that has an uncertain course and prognosis as well concerns about the need for surgery or increased risk of developing carcinoma, these factors are likely to lead to anxiety and depression in patients with IBD. 28
CD can negatively affect QOL owing to depression and anxiety as well as social isolation and altered self-image. 29 Our findings support the strong discriminating capability of the IBDQ in assessing clinical symptoms, with a strong correlation between symptom scores and each score on the SF-36. In our study, patients with CD had lower SF-36 scores than scores in healthy controls. A bidirectional relationship exists between psychological comorbidity and CD, with each producing an effect on the course of the other. 24 Hence, social and psychological support and interventions should be provided earlier in patients diagnosed with CD and should be included in further investigations.
Our findings are in line with those of other studies showing that active disease is a typical risk factor for poor QOL in patients with IBD and that QOL among patients in remission is better than that among individuals with active disease. 30 There are few reports in China evaluating the correlation of HRQOL with the clinical status of CD. Our data showed that disease activity, having complications, and malnourishment were correlated with lower HRQOL. Our research results showed that disease activity was an independent predictive factor for impaired HRQOL, with patients who had active CD showing significantly lower SF-36 mean scores (PE, RP, MH) than those in remission. Patients with more complications, such as perianal abscess and fistula formation, had lower HRQOL (PF, RP, BP, RE, and MH scores) than those without complication.
In one study, malnourished patients were more likely than well-nourished patients to be underweight and to have more active flare-ups and more IBD-related hospital admissions over the preceding 12 months. 31 Malnourished patients have greater unintentional weight loss, a pronounced reduction in food intake, and more GI-related symptoms, including diarrhea, which could be the result of disease activity, surgery, or medications. In all SF-36 domains, clinically malnourished patients with CD had lower HRQOL (GH and VT scores), which is likely to have an impact on the progression of clinical symptoms, such as unintentional weight loss, abdominal pain, and diarrhea. Hence, nutritional support could be an appropriate therapy for malnourished patients with CD.
Multivariate regression analyses also showed that disease activity and nutritional status were correlated with HRQOL. There was no correlation with sex, age, marital status, educational level, or biologic therapy, in line with the findings of other studies. 32
There are some limitations in our research. First, the number of patients with CD enrolled in our study was small. Second, very few of our patients were using biologic therapy; therefore, we could not effectively assess whether this was an independent risk factor of poor HRQOL. Hence, studies including larger samples from multiple centers and follow-up visits are needed in future investigations.
Conclusion
Depression and anxiety significantly influence HRQOL in patients with CD. We identified depression, anxiety, disease activity, complications, and malnourished status as predictive factors of decreased HRQOL in these patients.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received financial support for the research, authorship, and/or publication of this article from the Clinical Immunology Key Laboratory Open Research Project of Jiangsu Province (KJS2110) and Suzhou Science & Technology plan project (SYS201747).
ORCID iDs: Nan Gao https://orcid.org/0000-0003-4904-3186
Zhenguo Qiao https://orcid.org/0000-0002-9079-956X
References
- 1.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54. [DOI] [PubMed] [Google Scholar]
- 2.Nahon S, Lahmek P, Saas C, et al. Socioeconomic and psychological factors associated with nonadherence to treatment in inflammatory bowel disease patients: results of the ISSEO survey. Inflamm Bowel Dis 2011; 17: 1270–1276. [DOI] [PubMed] [Google Scholar]
- 3.Hisamatsu T, Inoue N, Yajima T, et al. Psychological aspects of inflammatory bowel disease. J Gastroenterol 2007; 42: 34–40. [DOI] [PubMed] [Google Scholar]
- 4.Hebuterne X, Filippi J, Schneider SM. Nutrition in adult patients with inflammatory bowel disease. Curr Drug Targets 2014; 15: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 5.Gibson PR, Weston AR, Shann A, et al. Relationship between disease severity, quality of life and health-care resource use in a cross-section of Australian patients with Crohn’s disease. J Gastroenterol Hepatol 2007; 22: 1306–1312. [DOI] [PubMed] [Google Scholar]
- 6.Graff LA, Walker JR, Lix L, et al. The relationship of inflammatory bowel disease type and activity to psychological functioning and quality of life. Clin Gastroenterol Hepatol 2006; 4: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 7.Zung WW. A rating instrument for anxiety disorders. Psychosomatics 1971; 12: 371–379. [DOI] [PubMed] [Google Scholar]
- 8.Zung WW. A self-rating depression scale. Arch Gen Psychiatry 1965; 12: 63–70. [DOI] [PubMed] [Google Scholar]
- 9.De Boer M, Grootenhuis M, Derkx B, et al. Health-related quality of life and psychosocial functioning of adolescents with inflammatory bowel disease. Inflamm Bowel Dis 2005; 11: 400–406. [DOI] [PubMed] [Google Scholar]
- 10.Walker JR, Ediger JP, Graff LA, et al. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol 2008; 103: 1989–1997. [DOI] [PubMed] [Google Scholar]
- 11.Ren WH, Lai M, Chen Y, et al . Validation of the mainland Chinese version of the inflammatory bowel disease questionnaire (IBDQ) for ulcerative colitis and Crohn’s disease. Inflam Bowel Dis 2007; 13: 903–910. [DOI] [PubMed] [Google Scholar]
- 12.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 13.Harvey RF, Bradshaw JM. A simple index of Crohn’s disease activity. Lancet 1980; 1: 514. [DOI] [PubMed] [Google Scholar]
- 14.Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002; 56: 779–785. [DOI] [PubMed] [Google Scholar]
- 15.Jowett SL, Seal CJ, Barton JR, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol 2001; 96: 2921–2928. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE JrandSherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 17.Hartono JL, Mahadeva S, Goh KL. Anxiety and depression in various functional gastrointestinal disorders: do differences exist? J Dig Dis 2012; 13: 252–257. [DOI] [PubMed] [Google Scholar]
- 18.Kim ES, Cho KB, Park KS, et al. Daegukyungbook Gastrointestinal Study Group (DGSG). Predictive factors of impaired quality of life in Korean patients with inactive inflammatory bowel disease: association with functional gastrointestinal disorders and mood disorders. J Clin Gastroenterol 2013; 47: 38–44. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989; 96: 804–810. [PubMed] [Google Scholar]
- 20.Kappelman MD, Long MD, Martin C, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014; 12: 1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long MD, Kappelman MD, Martin CF, et al . Development of an Internet-based cohort of patients with inflammatory bowel diseases [CCFA Partners]: methodology and initial results. Inflamm Bowel Dis 2012; 18: 2099–2106. [DOI] [PubMed] [Google Scholar]
- 22.Levenstein S, Prantera C, Varvo V, et al. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol 2000; 95: 1213–1220. [DOI] [PubMed] [Google Scholar]
- 23.Bitton A, Dobkin PL, Edwardes MD, et al. Predicting relapse in Crohn’s disease: a biopsychosocial model. Gut 2008; 57: 1386–1392. [DOI] [PubMed] [Google Scholar]
- 24.Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut 2005; 54: 1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahon S, Lahmek P, Durance C, et al. Risk factors of anxiety and depression in inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 2086–2091. [DOI] [PubMed] [Google Scholar]
- 26.Maunder RG, Levenstein S. The role of stress in the development and clinical course of inflammatory bowel disease: epidemiological evidence. Curr Mol Med 2008; 8: 247–252. [DOI] [PubMed] [Google Scholar]
- 27.Eugenicos MP, Ferreira NB. Psychological factors associated with inflammatory bowel disease. Br Med Bull 2021; 6: 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis 2009; 15: 1105–1118. [DOI] [PubMed] [Google Scholar]
- 29.Karwowski CA, Keljo D, Szigethy E. Strategies to improve quality of life in adolescents with inflammatory bowel disease. Inflamm Bowel Dis 2009; 15: 1755–1764. [DOI] [PubMed] [Google Scholar]
- 30.Luo XP, Mao R, Chen BL, et al. Over-reaching beyond disease activity: the influence of anxiety and medical economic burden on health-related quality of life in patients with inflammatory bowel disease. Patient Prefer Adherence 2016; 11: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulley J, Todd A, Flatley C, et al. Malnutrition and quality of life among adult inflammatory bowel disease patients. JGH Open 2019; 4: 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper JM, Collier J, James V, et al. Beliefs about personal control and self-management in 30-40 year olds living with inflammatory bowel disease: a qualitative study. Int J Nurs Stud 2010; 47: 1500–1509. [DOI] [PubMed] [Google Scholar]