Abstract
Aims:
Diabetic bladder dysfunction (DBD) is a prevalent diabetic complication thought to progress from overactive (OAB) to underactive (UAB) bladder. Previously we found OAB at 15 weeks in the Akita mouse, a genetic model of Type 1 diabetes. The first aim of this study assesses bladder function at 30 weeks to assess progression. In addition, inflammation triggered by the NLRP3 inflammasome is implicated in DBD. In a second aim we assessed a role for NLRP3 by crossing Akita mice with NLRP3−/− mice.
Main methods:
Akita mice were bred with NLRP3−/− mice. The effect of diabetes was assessed by comparing nondiabetic to diabetic mice (all NLRP3+/+). The effect of diabetes in the absence of the NLRP3 inflammasome was assessed by comparing nondiabetic/NLRP3−/− to diabetic/NLRP3−/− mice. Mice were assessed at 30 weeks for blood glucose (glucometer), inflammation (Evans blue), bladder morphology (histology) and bladder function (urodynamics).
Key Findings:
At 30 weeks blood glucose of nondiabetics and diabetics was not affected by the presence of absence of NLRP3. Diabetic/NLRP3+/+ mice showed bladder inflammation and detrusor hypertrophy which was blocked in the diabetic/NLRP3−/− mice, clearly showing a role for NLRP3. When bladder function was examined, diabetic/NLRP3+/+ showed an increase in voiding volume and a decrease in frequency, two signs of underactive bladder. However, in the NLRP3−/− mice, diabetes was unable to effectuate these changes, demonstrating that NLRP3-induced inflammation is responsible for UAB symptoms in these mice
Significance:
Akita diabetic mice progress from OAB to UAB. NLRP3 is a possible target to treat DBD.
Keywords: diabetes, bladder, immunity, cystitis, urodynamics, complications
1.0. Introduction
Diabetes is a developing epidemic [1, 2] with numerous complications that erode quality of life and basic functioning. One of the most common is diabetic bladder dysfunction (DBD), a term for the constellation of urinary symptoms associated with diabetes. This complication is particularly perplexing as strict glucose control does not prevent its development [3–5].
Contemporary thinking postulates that DBD progresses from an early overactive bladder (OAB) towards an underactive (UAB) or decompensated state [6–8], although in reality patients may present with a plethora of symptoms typical of one or more of these states, such as urinary frequency and urgency, detrusor underactivity and bladder decompensation [9]. Even if there is a symptomatic progression in humans, the wide range of presenting symptoms may mask its recognition. Definitive longitudinal studies are sorely needed in humans, as are animal models that reliably undergo this progression. Such models are essential to developing therapeutics, as there are currently no targeted therapies for DBD. Previously we documented that female Akita mice, a genetic model of type 1 diabetes, develop OAB at 15 weeks of age [10]. In the present study we have examined these mice at a later time point (30 weeks) to determine if their OAB progresses towards an UAB phenotype as the animals progress to middle age.
Diabetes is characterized by an underlying inflammation that is responsible for the detrimental consequences of its complications [11–14]. The mechanisms underlying inflammation is just beginning to be understood, but it is important to note that diabetes is not just a disease of high blood glucose but, in fact, is characterized by metabolic derangement and the production of numerous diabetes metabolites [15–17], many of which activate the NACHT, LRR and PYD Domains-Containing Protein 3 (NLRP3) inflammasome. NLRP3 is a member of the Nod-like receptor family that responds to numerous molecules that trigger sterile inflammation, known as Damage (or Danger) Associated Molecular Patterns or DAMPS. NLRP3 senses DAMPS, oligomerizes and activates a protease known as caspase-1. Caspase-1 in turn cleaves pro-IL-1β and pro-IL18 to their mature forms, which are released to act as pro-inflammatory cytokines, triggering a wider inflammatory response. In chronic cases such as diabetes, which typically occur without a sudden and large release of DAMPS, the inflammatory response is muted and results in a chronic state of low-level inflammation known as meta-inflammation. [18–20].
In the bladder, NLRP3 is localized to the urothelia [21, 22] and we have shown that many diabetic metabolites (e.g. monosodium urate, HMGB1, C6-ceramide) directly activate this sensor and trigger inflammation [10]. Moreover, this inflammation is significantly activated in urothelia from the Akita diabetic mice at the time point where we detected overactivity (15 weeks). Finally, by cross-breeding a NLRP3 knock out mouse with the Akita strain, we showed bladder inflammation and overactivity was a direct result of NLRP3 activation. In the present study, we have extended the work to determine if NLRP3 inflammation is responsible for bladder dysfunction detected at the later time point of 30 weeks which correlates to middle adulthood in mice.
2.0. Materials and Methods
2.1. Animals
All protocols performed by the investigators were approved by the Institutional Animal Care and Use Committee at Duke University Medical Center and strictly adhere to the NIH Guide for the Care and Use of Laboratory Animals. Mice were bred by the Breeding Core Facility at Duke University through an independently approved protocol and genotypes assessed by Transnetyx, Inc. (Cordova, TN) around 3 weeks of age. Immediately thereafter they were transferred to the investigators IACUC protocol (≈5 weeks of age) where they remained until they reached the appropriate age for the study (30 weeks). Only female mice were used.
All founder mice were purchased (and replaced when necessary) by the breeding core from Jackson Laboratory (Bar Harbor, MA). These consisted of Akita mice (C57BL/6J-Ins2Akita/J mice; stock number: 003548) [23] and NLRP3−/− mice (B6.129S6-Nlrp3tm1Bhk/J (stock number: 021302) [24]. It should be noted that the NLRP3−/− origin strain was 129S6/SvEvTac, which is different from the C57BL/6J background the Akita mice were created on. However, the NLRP3−/− strain has been backcrossed to C57BL/6J for >11 generations (https://www.jax.org/). Also, Akita mice have a mutation in the ins2 gene which creates the type 1 diabetic condition. Because the homozygote Ins2 mutation is often lethal, the heterozygote Akita mice were used. Blood glucose becomes elevated in the heterozygote Akita mice (200–300 mg/dL) around 4 weeks of age and remains high thereafter [25, 26].
The breeding core used these founder mice to create 4 distinct experimental groups for this study.
Nondiabetic, NLRP3+/+ - homozygote wild type Ins2 genes; homozygote wild type NLRP3 genes - i.e. control mice
Diabetic, NLRP3+/+ - heterozygote for Akita mutation at the Ins2 gene; homozygote wildtype NLRP3 genes - i.e. Akita diabetic.
Nondiabetic, NLRP3−/−, nondiabetic - homozygote wild type Ins2 genes, both NLRP3 genes deleted - i.e. NLRP3 knockout control.
Diabetic, NLRP3−/− - heterozygote for Akita mutation at the Ins2 gene, both NLRP3 genes knocked out - i.e. Akita diabetic mice with NLRP3 knocked out
2.2. Blood Glucose
Glucose levels in the blood were assess using the AimStrip Plus blood glucose testing system and blood harvested from the submandibular vein using a lance. Mice were not calorically restricted and collections were taken at approximately the same time each day (between 1 and 2 PM). For mice slated for urodynamics glucose levels were assessed prior to implantation of suprapubic tubes.
2.4. Evans Blue Dye Extravasation
Bladder inflammation was assessed by the extravasation of Evans blue dye, as previously described [21, 27–29]. Briefly, mice are restrained and then injected (i.v., tail vein) with 10 mg/kg dye. 1 h later they were sacrificed by isoflurane exposure and the bladder removed and weighed. The bladder was then submersed in 1 ml formamide and incubated at 50°C overnight. The next day the absorbance of the formamide at 620 nm was assessed and the concentration of dye calculated from a standard curve. The amount of extravasated dye was then normalized to bladder weight.
2.5. Histology
Isolated bladders were fixed (10% neutral buffered formalin) at 4°C overnight. They were then transferred to 70% ethanol and stored at 4°C until transferred to the Duke Histology Core (< 1 week). The core facility embedded them in paraffin blocks en places 5 um thick sections on slides. The slides were then stained with hematoxylin and eosin with standard techniques. Micrographs were obtained on an Axio Imager 2 microscope (Zeiss, Oberkochen, Germany) running Zen software (Zeiss). The entire cross section was obtained using the tiling and stitching feature of the software. Calibration bars were inserted and the final files were exported as TIFF files and cropped as indicated.
2.6. Surgery
For mice undergoing urodynamic analysis, suprapubic tubes were implanted in the bladder one week prior to analysis. Briefly, animals were anesthetized with an i.p. injection of ketamine hydrochloride (50 mg/kg) and xylazine (5 mg/kg). Mice were given injections of amikacin (10 mg/kg, s.c.) for antibiotic prophylaxis and carprofen (5 mg/kg, s.c.) for pain relief. A low, vertical, midline abdominal incision was made and the bladder externalized. Using a 6–0 silk suture with a tapered needle a purse string suture was placed in the bladder dome. A hole was then cut in the middle of the purse string, PE-10 tubing inserted and the purse string was tightened. The PE-10 tubing had a flared intravesicular end to insure it remained securely in the bladder. The tube was then tunneled subcutaneously and externalized at the interscapular region where it was secured using a 6–0 silk suture. The end of the tube was then sealed by heat. A 6–0 PGA suture was then used to stich closed first the abdominal wall and then the abdominal skin. Finally, the skin at the catheter exit site was closed around the catheter.
2.7. Urodynamics
One week following catheter placement the animals were placed in a Ballman-type restrainer (Natsume Seisakusho Co., Tokyo, Japan), following appropriate training. The sealed end of the catheter was cut and slid inside a length of P50 tubing. The junction between the two was then adhered and sealed with cyanoacrylate adhesive. The restrainer was then placed inside a Small Animal Cystometry Lab Station (Med Associates, St. Albans, VT) where it was situated above a digital analytical balance to measure voided volume. The P50 tubing was attached to a syringe pump with an in-line pressure transducer and sterile saline infused at a rate of 15 μl/min for 60 – 180 min. Near continuous reading (4 per sec) were recorded from both the pressure transducer and the scale using Med-CMG software (Med Associates, St. Albans, VT). Micturition cycles were allowed to stabilize (typically 30–45 min) and at least 3–8 cycles recorded before halting the infusion pump, which always took place immediately after a void. After halting the pump the tubing was detached from the pressure transducer and attached to a 3 ml syringe. The plunger was then withdrawn and any fluid recovered (the post void residual volume (PVR)) was measured by expelling the fluid onto the scale and recording its weight. Analysis of the micturition cycles (typically 5–8 per sample) took place using CMG Analysis software (version 1.06; Med Associates, St. Albans, VT). One cycle was defined as the time intravesicular pressure returned to baseline after a previous void until it returned to baseline following the next void. Voiding pressure is defined as the peak intravesical pressure occurring at the time of a void. The void volume was the amount of change on the scale associated with the voiding pressure peak and the intercontraction interval (ICI) the time between successive voiding pressure peaks. Frequency in voids per hour was determined by first measuring the intercontraction interval (ICI) or the seconds between successive peaks in intravesical pressure that occurred at the time of a void and then normalizing to time using the formula 3600/ICI. Bladder capacity was calculated by adding the average voiding volume to the recovered PVR. Voiding efficiency was calculated as the voiding volume divided by the bladder capacity, times 100.
2.8. Statistical Analysis
All parameters were assessed by a two-tailed Student’s t-test using GraphPad InStat software (La Jolla, CA). Statistical significance was defined as p<0.05 and these results are indicated on the graphs. In addition, we also performed a one-way ANOVA followed by a Student-Newman-Keuls post hoc test to compare all groups. The results of that comparison, which did not change any conclusions, can be found in the supplementary material.
3.0. Results
3.1. NLRP3 expression does not affect blood glucose levels
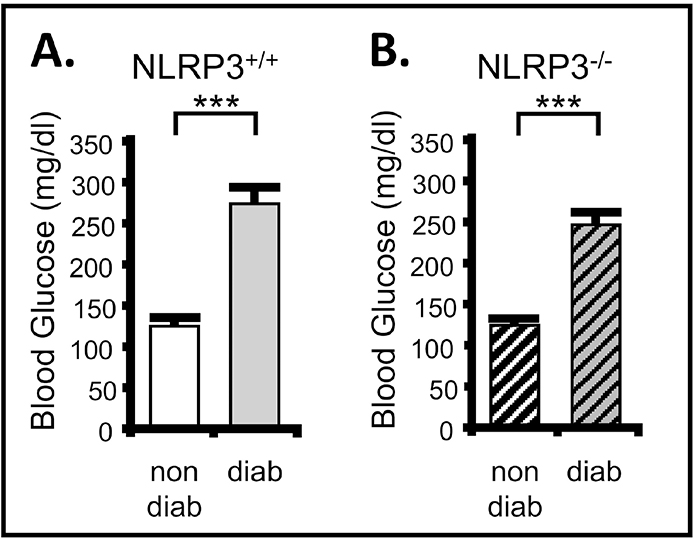
In order to assess the importance of NLRP3 on development and progression of DBD we have bred the Akita type 1 mouse with a NLRP3 knock out to create diabetic mice that lack NLRP3, as described in the Methods section. Because we sought to explore a role for NLRP3-induced inflammation, is was critical to demonstrate that knocking out NLRP3 had no effect on blood glucose levels (i.e. diabetes). As shown in Figure 1A, at 30 weeks of age diabetic Akita mice had a greatly elevated blood glucose level over nondiabetic control. Importantly, genetic deletion of NLRP3 (Figure 1B) had no effect on serum glucose levels for either group, demonstrating that NLRP3 has no direct effect in the regulation of blood glucose. These results are similar to that reported earlier at 15 weeks [10].
Figure 1. Blood glucose is significantly increased in the Akita diabetic mouse relative to nondiabetic controls and this is not effected by genetic deletion of NLRP3.
Blood glucose levels were assessed at week 30 of life using the AimStrip Plus blood glucose testing system and blood from the submandibular vein. Measurements were taken from all non-fasted animals at approximately the same time of day (between 1 and 2 PM). A. Blood glucose is significantly increased in the diabetic mouse with a NLRP3+/+ genotype. n=32 (nondiabetic), 25 (diabetic). B. Blood glucose is elevated in the diabetic mouse with a NLRP3−/− genotype. n=33 (nondiabetic), 30 (diabetic). For all graphs bars represent the mean ± SEM. ***p<0.0001 by Student’s two tailed t-test. non-diab = nondiabetic. diab = diabetic. ANOVA followed by Student-Newman-Keuls post hoc test was also used to compare all groups. The only additional significant differences found were between nondiabetic/NLRP3+/+ and diabetic/NLRP3−/− as well as diabetic/NLRP3+/+ and nondiabetic/NLRP3−/−. These comparisons can be found in the supplemental material.
3.2. Inflammation is present in the bladder at 30 weeks but blocked in the absence of NLRP3
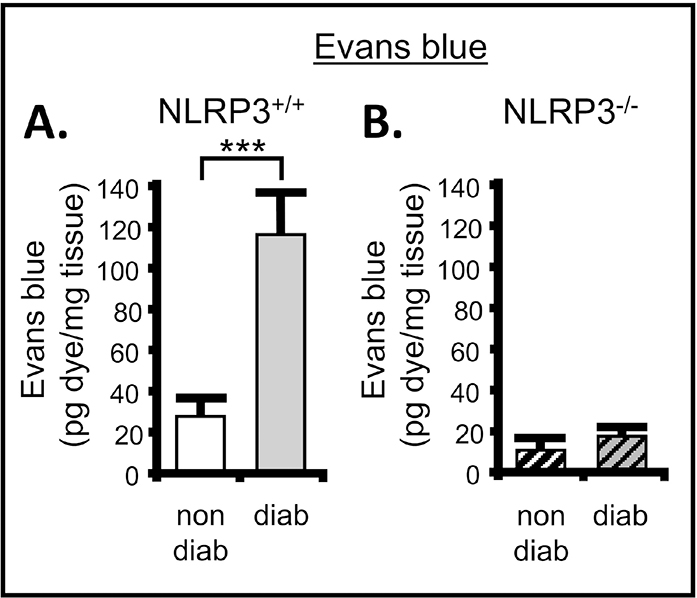
To determine if NLRP3-mediated inflammation is driving progression of DBD it was essential to establish that inflammation remained blocked after 30 weeks, as it was at 15 weeks [10]. Using the Evans blue dye extravasation assay of inflammation we show, in Figure 2A, a significant increase of inflammation in the bladder at 30 weeks of age in the diabetics compared to nondiabetic controls. However, inflammation remained near control levels in the diabetic mice that lacked NLRP3 expression (Figure 2B). Again, these results are similar to levels previously reported at 15 weeks [10].
Figure 2. There is significant inflammation in the bladder of diabetic mice which is not present when NLRP3 is deleted.
The Evans blue dye extravasation assays was used to assess inflammation in the bladder of the Akita mouse in the presence of NLRP3 or with NLRP3 genetically deleted. A. Diabetes significantly increased the amount of Evans blue dye in the bladder compared to control at 30 weeks when NLRP3 was intact (NLRP+/+). n = 10 (nondiabetic) and 3 (diabetic). B. Diabetes did not affect the movement of Evans blue into the bladder in the absence of NLRP3. n = 5 (nondiabetic) and 12 (diabetic). For all graphs bars represent the mean ± SEM. ***P < 0.0001 by paired Student two-tailed t test. non-diab = nondiabetic. diab = diabetic. ANOVA followed by Student-Newman-Keuls post hoc test was also used to compare all groups. The only additional significant differences found were between diabetic/NLRP3+/+ and nondiabetic/NLRP3−/− as well as diabetic/NLRP3−/−. These comparisons can be found in the supplemental material.
3.3. There is an increase in the size of the detrusor in the bladder at 30 weeks which is blocked in the absence of NLRP3.
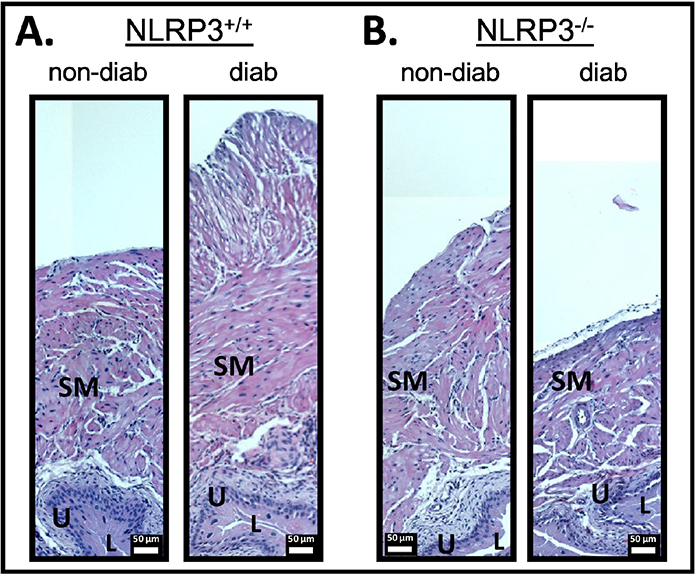
Detrusor hypertrophy is a well-known consequence of inflammation in the bladder and has been associated with DBD models [30]. Moreover, in cyclophosphamide-induced cystitis it has been shown to be mediated by IL-1β [31], the cytokine output of the NLRP3 inflammasome. Figure 3 shows slides from each of the 4 groups stained with hematoxylin and eosin and cropped to illustrate the difference in the bladder wall. Sections for the diabetic NLRP3+/+ mouse showed clear increase in the size of the detrusor layer that remained near normal size in the NLRP3−/− mouse.
Figure 3. There is significant hypertrophy of the smooth muscle layer in the bladder of diabetic mice which is not present when NLRP3 is deleted.
Whole bladders from each group with harvested, fixed, embedded, sectioned and sections (5 μm) stained for Hematoxylin and Eosin and visualized using standard methods, as described in the methods section. SM = bladder smooth muscle; U = urothelia; L = lumen. The lumen of the bladder is necessarily collapsed (i.e. not inflated) and is only represented by small while areas. Error bars represent 50 μm.
3.4. After 30 weeks Akita mice develop bladder underactivity in an NLRP3-dependent manner
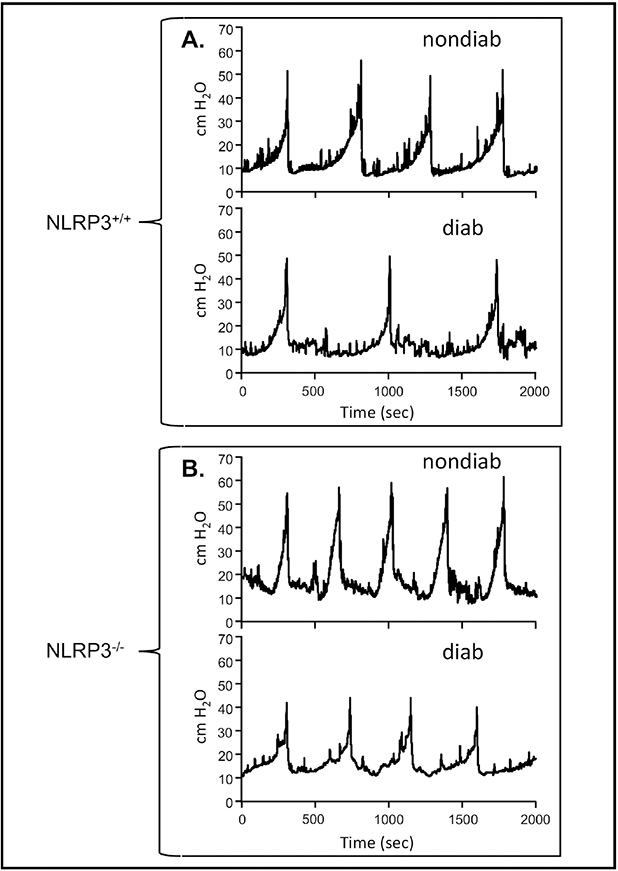
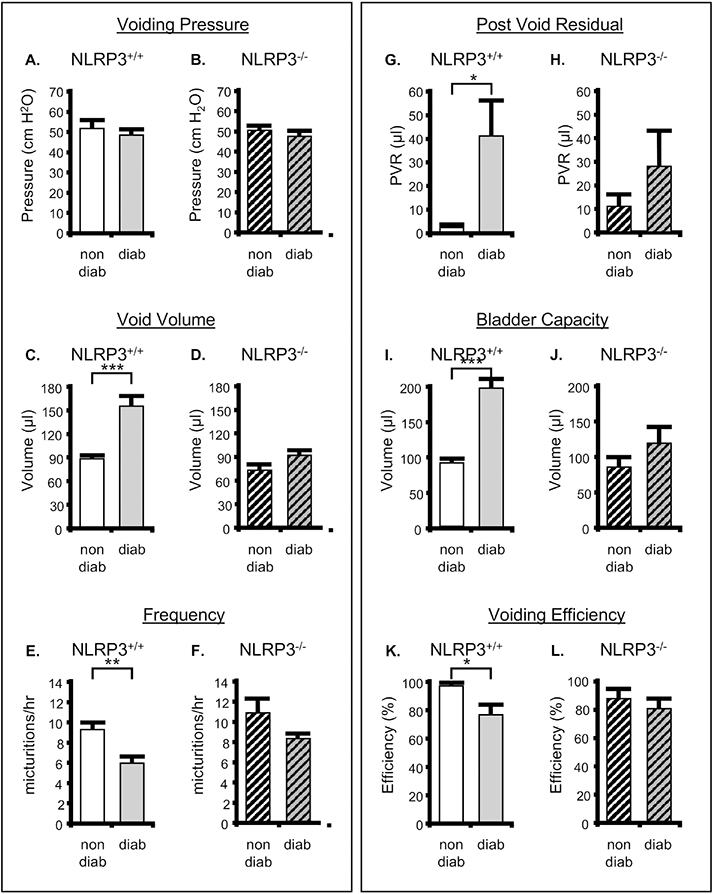
To assess bladder function at 30 weeks, mice were subjected to urodynamics. Figure 4A shows representative pressure tracings from nondiabetic and diabetic mice that possessed wild type NLRP3 while Figure 4B depicts the same pathological states in mice with NLRP3 deleted. Quantitative information gleaned from these graphs, along with urine scale measurements, post-void residual assessments, and bladder capacity and efficiency calculations, are depicted in Figure 4. Not surprisingly, there was no effect of diabetes or NLRP3 expression on voiding pressure (Figure 5A and B). However, at 30 weeks diabetes significantly increased the voided volume of urine (Figure 5C) and this change was blocked when NLRP3 was deleted (Figure 5D). Associated with this increase in void volume was a concomitant decrease in frequency (Figure 5E) which was also absent in the knock out diabetic animals (Figure 5F). Even with the increased void volume seen in the diabetic mice we also detected an increased post void residual volume remaining in the bladder after a void (Figure 5G), a finding that leads to a significantly increased bladder capacity (Figure 5I) and a reduced voiding efficiency (Figure 5K). Tellingly, all these parameters were reduced back to values not significantly different from non-diabetic controls when NLRP3 is knocked out (Figures 5H, J and L).
Figure 4. NLRP3 is responsible for bladder dysfunction associated with DBD: Representative tracings of intravesicular pressures over time.
Each panel is a single representative tracing (or part of a tracing) of the luminal pressure (cm H2O) in the bladder and how it changes over several micturition cycles. These tracings were obtained during cystometry for each of the four experimental groups reported in the Methods section and recorded using an in-line pressure transducer. The tracings were chosen to represent only several micturition cycles (more may have occurred) and aligned so the peak of the first cycle aligns for each tracing to allow easy visual determination of the time between voids which is used to calculate urinary frequency. Typically, three to eight micturition cycles were quantitated per animal. Tracings from the scale used to measure voiding volume is not shown but voids align with the peaks in pressure A. Representative tracing from the NLRP3+/+ strains. B. Representative tracings from the NLRP3−/− strains. non-diab = nondiabetic. diab = diabetic.
Figure 5. NLRP3 is responsible for bladder dysfunction associated with DBD: results of various parameters measured through cystometry.
The results are shown for nondiabetic and diabetic mice that either express NLRP3 (NLRP3+/+) or have that gene deleted (NLRP3−/−). All studies were performed at 30 weeks of age, and animals were implanted with a suprapubic catheter 1 week prior to analysis. A. Peak voiding pressure in nondiabetic and diabetic mice (both NLRP3+/+). B. Peak voiding volume in nondiabetic and diabetic mice with NLRP3 deleted (NLRP3−/−). C. Voiding volume in nondiabetic and diabetic mice (both NLRP3+/+). D. Voiding volume in nondiabetic and diabetic mice with NLRP3 deleted (NLRP3−/−). E and F. Frequency of voiding in the indicated animals calculated as 3600/intercontraction interval. G and H. The post void residual volume, or volume of urine remaining in the bladder immediately after the last void, in the indicated animals. I and J. The bladder capacity of the indicated animals calculated as the voided volume + PVR. K and L. The voiding efficiency of the indicated animals was calculated as 100 (voided volume)/(voided volume + PVR). For all graphs, bars represent the mean ± SEM. For Void Pressure, Void Volume and Frequency: n = 11 and 13 for nondiabetic and diabetic mice, respectively, that are NLRP3+/+. n = 9 and 10 for nondiabetic and diabetic mice, respectively, that are NLRP3−/−. For PVR, Voiding Capacity and Voiding frequency: : n = 8 and 9 for nondiabetic and diabetic mice, respectively, that are NLRP3+/+. n = 9 and 7 for nondiabetic and diabetic mice, respectively, that are NLRP3−/−. *P < 0.05, **P < 0.01; ***P < 0.001 by a Student two-tailed t test. non-diab = nondiabetic. diab = diabetic. ANOVA followed by Student-Newman-Keuls post hoc test was also used to compare all groups. Voiding pressure – no significant differences were found. Voiding volume – the only additional significant differences found were between diabetic/NLRP3+/+ and nondiabetic/NLRP3−/− as well as diabetic/NLRP3−/−. Frequency – the only additional significant differences found were between diabetic/NLRP3+/+ and nondiabetic/NLRP3−/− as well as diabetic/NLRP3−/−. These comparisons can be found in the supplemental material. PVR – no additional differences were found. Capacity - the only additional significant differences found were between diabetic/NLRP3+/+ and nondiabetic/NLRP3−/− as well as diabetic/NLRP3−/−. Efficiency – no significant differences were detected.
4.0. Discussion
Exploring the progression of DBD in the female Akita mouse, we previously found bladder overactivity at 15 weeks of age [32] whereas in this study at 30 weeks there was a profound change towards an underactive/decompensated state. Together these results clearly demonstrate progression of DBD and define specific time points where the specific states can reliably be assessed. In addition, we cross bred the Akita diabetic mice with NLRP3−/− mice to assess the importance of NLRP3-driven inflammation to the development of DBD. After confirming that the bladder inflammation and detrusor hypertrophy was indeed blocked by knocking out NLRP3, the urodynamic results indicated that every single parameter remained indistinguishable from control values. This was somewhat astounding after 30 weeks of pronounced and uncontrolled high glucose/diabetes. Thus, the female Akita model shows a distinct and reproducible progression of DBD driven by NLRP3-induced inflammation.
Progression of DBD in humans is in need of better documentation and animal models are sorely lacking. One of the most commonly used models is the streptozotocin model (type 1) model. Daneshgari et al. have used this model effectively to study DBD [6, 7] and have shown, with the correct dosing, that two progressive phases are apparent; an early phase OAB phase and a later UAB stage. He expressed this progression as the “Temporal Hypothesis for Diabetic Bladder Dysfunction” which defines the contemporary idea of DBD progression. Additional labs have also noted this progression with streptozotocin [33, 34] although some have not [35], a result likely due to dose of streptozotocin, analysis time point, strain of mice etc. In addition, progressive DBD has only been found with a very limited number of models, perhaps most notably with what is considered a model of Type 2 diabetes by Klee et al. [30]. That model uses animals fed a high-fat diet to trigger diabetes but also requires 2 low doses of streptozotocin which may indicate a contribution of Type 1 diabetes. Perhaps the most undesirable effect of any streptozotocin model is that we have found that streptozotocin itself functions as a DAMP and directly activates NLRP3 in urothelial cells in culture [10]. Such complications have led many, including us, to seek out genetic models.
Arguably the three most well-known genetic models of diabetes are the Non obese Diabetic (NOD) mouse, the diabetes prone biobreeding rat (BB-DP) and the Akita mouse [36]. Unfortunately, the first two models involve severe autoimmune responses and show a highly variable time of onset for diabetes if it develops at all. For example, diabetes only develops in 60% of NOD mice with the time of onset typically ranging from 12–30 weeks [37]. The female Akita mice appears to avoid these complications. There has been one report of UAB in male Akita at 20 weeks of age [25], but it is unknown if these male mice display OAB at an earlier time progression. It is known that male Akita display a more profound diabetic phenotype than females and so it is conceivable their temporal progression is compressed in response. One additional study examined DBD in male and female Akita mice [38] but did so only with mice with insulin implants designed to reflect poorly controlled diabetes. Unfortunately, their urodynamic studies were reported as being vastly “underpowered” and “not statistically robust” and so cannot be reliably compared to the present study. Overall, this study, in conjunction with our previous one [10], has defined very specific and reliable time points in which female Akita mice display OAB symptoms (15 weeks) and a later UAB phenotype. Consequently, itshould be extraordinarily useful in understanding the progression of DBD.
The mechanism by which diabetes triggers DBD is worth considering in light of our studies. We propose that in the early stages hyperglycemia causes oxidative stress which leads to tissue damage, in agreement with others (23). Tissue damage results in the release of DAMPs that trigger inflammation through activation of NLRP3 in urothelia [21, 22, 27]. In addition, diabetic metabolites found stored in the urine further activate NLRP3 and drive inflammation forward [10]. This inflammation then triggers OAB in the acute phase which may be mediated by NLRP3-dependent increases in c-fibers [10]. Persistent inflammation then brings about a number of seemingly permanent changes such as denervation [32] and possibly fibrosis, although the presence of fibrosis during DBD is controversial at best [39–41]. Preventing inflammation from the beginning, as with NLRP3−/− mice, prevents both the initiation and progression of bladder dysfunction. Such a conclusion begs the question if inflammation is really necessary for progression of DBD or only it’s initiation, and the current study does not answer that although it does suggest either 1) the chronic stage of DBD is dependent on the acute stage or 2) both the acute and chronic stages are driven by inflammation, or 3) both 1 and 2 are correct. An intriguing study that would address these question would require conditional (and preferably urothelia-specific) NLRP3 knock out mice where the protein could be inactivated after the early overactive state (15 weeks) and see if underactivity develops at 30 weeks. Conversely, NLRP3 inhibitors such as glyburide or MCC950 could be employed after 15 weeks to pharmacologically block NLRP3.
Finally, the present results are not exclusively relevant to patients with diabetes. It is now recognized that obese but non-diabetic humans also suffer from a chronic low level of inflammation that is mechanistically similar to, but less severe, than those with full-blown diabetes. In fact, even non-obese, otherwise normal people experience some increased level of baseline inflammation as they get older which could be an essential part of aging or a response to a modern environment, diet, etc. This phenomenon of chronic low-grade inflammation, as a contributing factor in most age-related diseases has been referred to as “inflammaging” [42, 43]. Therefore, the results of this current investigation, and others [44] could provide insight into how aging humans develop bladder dysfunction even in the absence of a known pro-inflammatory disease such as diabetes.
5.0. Conclusion
In summary, this study has shown the appearance of an underactive bladder phenotype in female Akita mice at 30 weeks which represents the progression from an underactive phenotype at 15 weeks [10]. Moreover, NLRP3-induced inflammation is essential to the development of DBD in these mice.
Supplementary Material
Highlights.
Female Akita mice (type 1), develop underactive bladder at 30 weeks
This is the first genetic model to show progression of diabetic bladder dysfunction
Knocking out NLRP3 prevents bladder inflammation in diabetic mice at 30 weeks
Knocking out NLRP3 prevents bladder underactivity in diabetic mice at 30 weeks
NLRP3-induced inflammation is responsible for diabetic bladder dysfunction
6.0. Acknowledgments
The authors would like to thank the Breeding Core at Duke University Medical Center for their invaluable help with the breeding program for these mice. They would also like to thank Yasheng Gao and the Light Microscopy Core Facility at Duke University for their help obtaining images.
7.0. Funding Sources
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases to J. Todd Purves (R01DK117890) and Michael R. Odom (K12 DK100024). Additional funds were provided by the Duke University School of Medicine.
Footnotes
8.0 Conflict of Interest Policy
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9.0 References
- 1.Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, and Marks JS, Diabetes trends in the U.S.: 1990–1998, Diabetes Care, 23 (2000) 1278–1283. 10.2337/diacare.23.9.1278 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report Atlanta, GA: U.S. Dept of Health and Human Services, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html, 2020 (accessed 25 March, 2022) [Google Scholar]
- 3.Genuth S, Insights from the diabetes control and complications trial/epidemiology of diabetes interventions and complications study on the use of intensive glycemic treatment to reduce the risk of complications of type 1 diabetes, Endocr Pract, 12 Suppl 1 (2006) 34–41. 10.4158/EP.12.S1.34 [DOI] [PubMed] [Google Scholar]
- 4.Sarma AV, Kanaya A, Nyberg LM, Kusek JW, Vittinghoff E, Rutledge B, Cleary PA, Gatcomb P, Brown JS, Diabetes C, I. Complications Trial/Epidemiology of Diabetes, and G. Complications Research, Risk factors for urinary incontinence among women with type 1 diabetes: findings from the epidemiology of diabetes interventions and complications study, Urology, 73 (2009) 1203–1209. 10.1016/j.urology.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Den Eeden SK, Sarma AV, Rutledge BN, Cleary PA, Kusek JW, Nyberg LM, McVary KT, Wessells H, Diabetes C, and Complications Trial G /Epidemiology of Diabetes Research, Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study, Diabetes Care, 32 (2009) 664–670. 10.2337/dc07-2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daneshgari F, Huang X, Liu G, Bena J, Saffore L, and Powell CT, Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice, Am J Physiol Regul Integr Comp Physiol, 290 (2006) R1728–1735. 10.1152/ajpregu.00654.2005 [DOI] [PubMed] [Google Scholar]
- 7.Daneshgari F, Liu G, and Imrey PB, Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function, J Urol, 176 (2006) 380–386. 10.1016/S0022-5347(06)00582-9 [DOI] [PubMed] [Google Scholar]
- 8.Daneshgari F, Liu G, Birder L, Hanna-Mitchell AT, and Chacko S, Diabetic bladder dysfunction: current translational knowledge, J Urol, 182 (2009) S18–26. 10.1016/j.juro.2009.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez CS, Kanagarajah P, and Gousse AE, Bladder dysfunction in patients with diabetes, Curr Urol Rep, 12 (2011) 419–426. 10.1007/s11934-011-0214-0 [DOI] [PubMed] [Google Scholar]
- 10.Hughes FM Jr., Hirshman NA, Inouye BM, Jin H, Stanton EW, Yun CE, Davis LG, Routh JC, and Purves JT, NLRP3 Promotes Diabetic Bladder Dysfunction and Changes in Symptom-Specific Bladder Innervation, Diabetes, 68 (2019) 430–440. 10.2337/db18-0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lontchi-Yimagou E, Sobngwi E, Matsha TE, and Kengne AP, Diabetes mellitus and inflammation, Curr Diab Rep, 13 (2013) 435–444. 10.1007/s11892-013-0375-y [DOI] [PubMed] [Google Scholar]
- 12.Calle MC, and Fernandez ML, Inflammation and type 2 diabetes, Diabetes Metab, 38 (2012) 183–191. 10.1016/j.diabet.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 13.Halim M, and Halim A, The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes), Diabetes Metab Syndr, 13 (2019) 1165–1172. 10.1016/j.dsx.2019.01.040 [DOI] [PubMed] [Google Scholar]
- 14.Luc K, Schramm-Luc A, Guzik TJ, and Mikolajczyk TP, Oxidative stress and inflammatory markers in prediabetes and diabetes, J Physiol Pharmacol, 70 (2019) 10.26402/jpp.2019.6.01 [DOI] [PubMed] [Google Scholar]
- 15.Shin JJ, Lee EK, Park TJ, and Kim W, Damage-associated molecular patterns and their pathological relevance in diabetes mellitus, Ageing Res Rev, 24 (2015) 66–76. 10.1016/j.arr.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 16.Ighodaro OM, Molecular pathways associated with oxidative stress in diabetes mellitus, Biomed Pharmacother, 108 (2018) 656–662. 10.1016/j.biopha.2018.09.058 [DOI] [PubMed] [Google Scholar]
- 17.Fleming T, and Nawroth PP, Reactive metabolites as a cause of late diabetic complications, Biochem Soc Trans, 42 (2014) 439–442. 10.1042/BST20130265 [DOI] [PubMed] [Google Scholar]
- 18.Hotamisligil GS, Inflammation and metabolic disorders, Nature, 444 (2006) 860–867. 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- 19.Russo S, Kwiatkowski M, Govorukhina N, Bischoff R, and Melgert BN, Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites, Front Immunol, 12 (2021) 746151. 10.3389/fimmu.2021.746151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boni-Schnetzler M, and Meier DT, Islet inflammation in type 2 diabetes, Semin Immunopathol, 41 (2019) 501–513. 10.1007/s00281-019-00745-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes FM Jr., Vivar NP, Kennis JG, Pratt-Thomas JD, Lowe DW, Shaner BE, Nietert PJ, Spruill LS, and Purves JT, Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation, Am J Physiol Renal Physiol, 306 (2014) F299–308. 10.1152/ajprenal.00297.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes FM Jr., Turner DP, and Purves JT, The Potential Repertoire of the Innate Immune System in the Bladder: Expression of Pattern Recognition Receptors in the Rat Bladder and a Rat Urothelial Cell Line (MYP3 cells), Int Urol Nephrol, 47 (2015) 1953–1964. 10.1007/s11255-015-1126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, and Izumi T, A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse, J Clin Invest, 103 (1999) 27–37. 10.1172/JCI4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovarova M, Hesker PR, Jania L, Nguyen M, Snouwaert JN, Xiang Z, Lommatzsch SE, Huang MT, Ting JP, and Koller BH, NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice, J Immunol, 189 (2012) 2006–2016. 10.4049/jimmunol.1201065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolber PC, Jin H, Nassar R, Coffman TM, Gurley SB, and Fraser MO, The effects of Ins2(Akita) diabetes and chronic angiotensin II infusion on cystometric properties in mice, Neurourol Urodyn, (2013) 10.1002/nau.22511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshioka M, Kayo T, Ikeda T, and Koizumi A, A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice, Diabetes, 46 (1997) 887–894. [DOI] [PubMed] [Google Scholar]
- 27.Hughes FM Jr., Hill HM, Wood CM, Edmondson AT, Dumas A, Foo WC, Oelsen JM, Rac G, and Purves JT, The NLRP3 Inflammasome Mediates Inflammation Produced by Bladder Outlet Obstruction, J Urol, 195 (2016) 1598–1605. 10.1016/j.juro.2015.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes FM Jr., Kennis JG, Youssef MN, Lowe DW, Shaner BE, and Purves JT, The NACHT, LRR and PYD Domains-Containing Protein 3 (NLRP3) Inflammasome Mediates Inflammation and Voiding Dysfunction in a Lipopolysaccharide-Induced Rat Model of Cystitis, J Clin Cell Immunol, 7 (2016) 396. 10.4172/2155-9899.1000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inouye BM, Hughes FM Jr., Jin H, Lütolf R, Potnis K, Routh JC, Rouse C, Foo W-C, and Purves JT, Diabetic Bladder Dysfunction is Associated with Bladder Inflammation Triggered Through Hyperglycemia not Polyuria, Res Rep Urol, 10 (2018) 219–225. 10.2147/RRU.S177633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klee NS, Moreland RS, and Kendig DM, Detrusor contractility to parasympathetic mediators is differentially altered in the compensated and decompensated states of diabetic bladder dysfunction, Am J Physiol Renal Physiol, 317 (2019) F388–F398. 10.1152/ajprenal.00178.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haldar S, Dru C, Choudhury D, Mishra R, Fernandez A, Biondi S, Liu Z, Shimada K, Arditi M, and Bhowmick NA, Inflammation and pyroptosis mediate muscle expansion in an interleukin-1beta (IL-1beta)-dependent manner, J Biol Chem, 290 (2015) 6574–6583. 10.1074/jbc.M114.617886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes FM Jr., Sexton SJ, Ledig PD, Yun CE, Jin H, and Purves JT, Bladder decompensation and reduction in nerve density in a rat model of chronic bladder outlet obstruction are attenuated with the NLRP3 inhibitor glyburide, Am J Physiol-Renal, 316 (2019) F113–F120. 10.1152/ajprenal.00400.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshizawa T, Hayashi Y, Yoshida A, Yoshida S, Ito Y, Yamaguchi K, Yamada S, and Takahashi S, Concomitant alteration in number and affinity of P2X and muscarinic receptors are associated with bladder dysfunction in early stage of diabetic rats, Int Urol Nephrol, 50 (2018) 451–458. 10.1007/s11255-018-1800-6 [DOI] [PubMed] [Google Scholar]
- 34.Yang XF, Wang J, Rui W, Xu YF, Chen FJ, Tang LY, Ren WK, Fu LJ, Tan B, Huang P, and Cao HY, Time-dependent functional, morphological, and molecular changes in diabetic bladder dysfunction in streptozotocin-induced diabetic mice, Neurourol Urodyn, 38 (2019) 1266–1277. 10.1002/nau.24008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eika B, Levin RM, Monson FC, Murphy M, and Longhurst PA, 3H-thymidine uptake by the rat urinary bladder after induction of diabetes mellitus, J Urol, 150 (1993) 1316–1320. 10.1016/s0022-5347(17)35768-3 [DOI] [PubMed] [Google Scholar]
- 36.Daneshgari F, Leiter EH, Liu G, and Reeder J, Animal models of diabetic uropathy, J Urol, 182 (2009) S8–13. 10.1016/j.juro.2009.07.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkinson MA, and Leiter EH, The NOD mouse model of type 1 diabetes: as good as it gets?, Nat Med, 5 (1999) 601–604. 10.1038/9442 [DOI] [PubMed] [Google Scholar]
- 38.Kim AK, Hamadani C, Zeidel ML, and Hill WG, Urological complications of obesity and diabetes in males and females of three mouse models: temporal manifestations, Am J Physiol Renal Physiol, 318 (2020) F160–F174. 10.1152/ajprenal.00207.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Liu S, Yuan ZW, Liu JH, Li JM, Chen T, and Fang KW, MicroRNA-27a Suppresses Detrusor Fibrosis in Streptozotocin-Induced Diabetic Rats by Targeting PRKAA2 Through the TGF-beta1/Smad3 Signaling Pathway, Cell Physiol Biochem, 45 (2018) 1333–1349. 10.1159/000487560 [DOI] [PubMed] [Google Scholar]
- 40.Yankelevitch-Yahav R, Franko M, Huly A, and Doron R, The forced swim test as a model of depressive-like behavior, J Vis Exp, (2015) 10.3791/52587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues AA Jr., Suaid HJ, Tucci S Jr., Fazan VP, Foss MC, Cologna AJ, and Martins AC, Long term evaluation of functional and morphological bladder alterations on alloxan-induced diabetes and aging: experimental study in rats, Acta Cir Bras, 23 Suppl 1 (2008) 53–58; discussion 58. 10.1590/s0102-86502008000700010 [DOI] [PubMed] [Google Scholar]
- 42.Fulop T, Larbi A, Pawelec G, Khalil A, Cohen AA, Hirokawa K, Witkowski JM, and Franceschi C, Immunology of Aging: the Birth of Inflammaging, Clin Rev Allergy Immunol, (2021) 10.1007/s12016-021-08899-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santoro A, Bientinesi E, and Monti D, Immunosenescence and inflammaging in the aging process: age-related diseases or longevity?, Ageing Res Rev, 71 (2021) 101422. 10.1016/j.arr.2021.101422 [DOI] [PubMed] [Google Scholar]
- 44.Chen L, He PL, Yang J, Yang YF, Wang K, Amend B, Stenzl A, Zhang YM, Wang ZL, Xing SS, and Luo X, NLRP3/IL1beta inflammasome associated with the aging bladder triggers bladder dysfunction in female rats, Mol Med Rep, 19 (2019) 2960–2968. 10.3892/mmr.2019.9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.