ABSTRACT
The cycloserine concentrations in plasma and bone that were collected during operations on 28 osteoarticular tuberculosis (TB) patients treated daily with a 500-mg cycloserine-containing regimen were determined. The median concentrations in plasma and bone were 16.29 μg/mL (interquartile range [IQR], 6.47 μg/mL) and 24.33 μg/g (IQR, 14.68 μg/g), respectively. The median bone/plasma penetration ratio was 0.76 (range, 0.33 to 1.98). Cycloserine could effectively penetrate bone and acquire concentrations comparable to those in plasma, which favors its usage in osteoarticular TB treatment.
KEYWORDS: cycloserine, osteoarticular tuberculosis, plasma, bone, drug penetration
TEXT
Osteoarticular tuberculosis (TB) is one of the most common forms of extrapulmonary tuberculosis (1, 2). Multidrug-resistant (MDR) (defined as resistance to both isoniazid and rifampicin) osteoarticular TB has been reported worldwide (3–6). Cycloserine (CS) is a group B second-line drug categorized by the World Health Organization (WHO) and plays an important role in the treatment of MDR-TB (7–9). The efficacy and safety of using cycloserine are highly dependent on its plasma concentration, and the recommended peak serum concentration range is ∼20 to 35 μg/mL (10, 11). However, concentrations below the desired range were common in pulmonary TB patients in clinical practice (12, 13). Although cycloserine has also been administered in osteoarticular TB patients with confirmed or suspected drug resistance, the pharmacokinetic (PK)/pharmacodynamic (PD) data for such patients are very limited (14–16).
A total of 28 osteoarticular TB patients treated daily with a 500-mg cycloserine-containing regimen for at least 5 days prior to orthopedic surgery were enrolled between April 2017 and September 2019 in Beijing Chest Hospital (Table 1). The steady state of cycloserine in plasma was considered reached. One dose of cycloserine was administered before surgery. Blood and bone were collected simultaneously during the operation. Cycloserine concentrations were determined by high-performance liquid chromatography–tandem mass spectrometry for both specimen types.
TABLE 1.
Demographic and clinical characteristics of the participants in the current studya
| Characteristic | Value for group |
P value | ||
|---|---|---|---|---|
| Total | 120–240 min postdose | 245–420 min postdose | ||
| No. of patients | 28 | 16 | 12 | |
| Mean age (yrs) ± SD (range) | 47.82 ± 17.06 (17–78) | 53.31 ± 17.00 (17–78) | 43.17 ± 16.00 (24–60) | 0.2236 |
| No. of male/no. of female patients | 16/12 | 9/7 | 7/5 | 1.0000 |
| Mean BMI ± SD (range) | 21.46 ± 3.12 (15.04–26.81) | 21.77 ± 3.07 (15.94–26.81) | 21.06 ± 3.14 (15.04–26.12) | 0.5744 |
| % (no.) of patients with site of osteoarticular TB | 0.2850 | |||
| Spinal | 85.71 (24/28) | 93.75 (15/16) | 75.00 (9/12) | |
| Site other than spine | 14.29 (4/28) | 6.25 (1/16) | 25.00 (3/12) | |
| No. of patients with prior TB infection | 3 | 2 | 1 | |
| Duration (mo) of receiving TB treatment before operation (range) | 1 (0–48) | 1 (0–48) | 0.5 (0–19) | 1.0000 |
| Mean sampling time after preoperative dose (min) ± SD (range) | 219.12 ± 73.69 (120–420) | 165.31 ± 34.35 (120–220) | 290.83 ± 45.77 (245–420) | |
| Mean dose/wt (mg · kg−1) ± SD (range) | 8.50 ± 1.39 (5.95–11.11) | 8.35 ± 1.24 (5.95–11.11) | 8.65 ± 1.57 (6.25–11.11) | 0.5963 |
| % (no.) of patients with CT finding | ||||
| Bone destruction | 100 (28/28) | 100 (16/16) | 100 (12/12) | |
| Soft-tissue swelling | 96.43 (27/28) | 100 (16/16) | 91.67 (11/12) | 0.4286 |
| Paravertebral/psoas abscess | 46.43 (13/28) | 37.50 (6/16) | 58.33 (7/12) | 0.4454 |
| Pleural thickening | 14.29 (4/30) | 12.50 (2/16) | 16.67 (2/12) | 1.0000 |
| % (no.) of patients with bacteriological examination outcome | ||||
| Culture positive | 42.86 (12/28) | 37.50 (6/16) | 50.00 (6/12) | 0.7022 |
| Xpert positive | 71.43 (20/28) | 75.00 (12/16) | 66.67 (8/12) | 0.6908 |
| RR-TB rate | 10.00 (2/20) | 8.33 (1/12) | 12.50 (1/8) | 0.5604 |
| % (no.) of patients with pathological examination outcome | ||||
| Granulomatous inflammation | 82.14 (23/28) | 81.25 (13/16) | 83.33 (10/12) | 1.0000 |
| Necrosis | 89.29 (25/28) | 93.75 (15/16) | 83.33 (10/12) | 0.5604 |
| TB molecular test positiveb | 80.77 (21/26) | 80.00 (12/15) | 81.82 (9/11) | 1.0000 |
BMI, body mass index; CT, computed tomography; RR-TB, rifampicin resistant-tuberculosis.
Molecular testing was not performed on two patients.
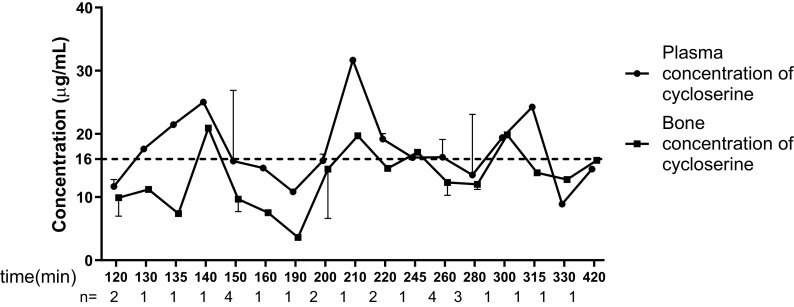
The median concentrations in plasma and bone of the enrolled patients were 16.29 μg/mL (interquartile range [IQR], 6.47 μg/mL) and 24.33 μg/g (IQR, 14.68 μg/g), respectively. The cycloserine concentrations in samples collected over time are presented in Fig. 1. The median bone/plasma penetration ratio was calculated to be 0.76 (range, 0.33 to 1.98), which demonstrated a tendency toward a gradual increase over time after dosing (Fig. 2). The median time from preoperative dosing to intraoperative sampling was 215 min (range, 120 to 420 min). Patients were grouped according to the sampling time. None of the analyzed demographic characteristics showed any significant differences between the 120- to 240-min group and the 241- to 420-min group (Table 2). The plasma concentrations in these two groups were accordant, whereas the bone concentration in the 120- to 240-min group was lower than that in the 241- to 420-min group, even though the difference was not significant (median, 20.71 μg/g versus 26.62 μg/g [P = 0.09]). Notably, the 120- to 240-min group had a significantly lower bone/plasma penetration ratio than the 241- to 420-min group (median, 0.63 versus 1.04 [P < 0.05]). A plausible explanation for this increased efficiency in penetration might be the incomplete bone diffusion at an earlier time period after dosing; therefore, the penetration ratio increased accordingly over time after dosing. We assume that with the extension of the time after dosing, the bone concentration will surpass the blood concentration because of the delayed diffusion of cycloserine into the circulation system for renal excretion.
FIG 1.
Median plasma and bone concentrations of cycloserine over the sampling time in 28 osteoarticular tuberculosis patients. The median concentration is presented if more than one patient was enrolled at the same time point. The dotted line presents the MICs of 12 isolates recovered from the enrolled patients.
FIG 2.
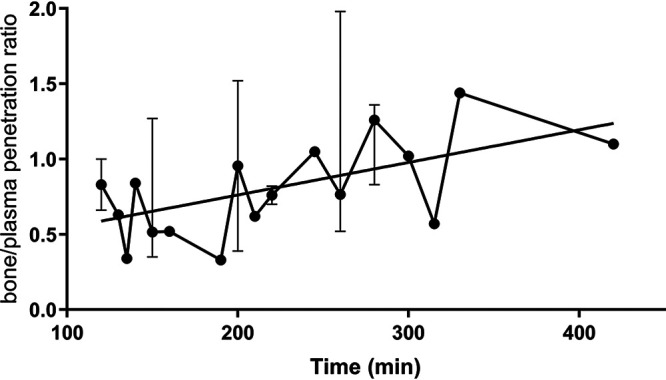
Median bone/plasma penetration ratios of cycloserine over the sampling time in the 28 osteoarticular tuberculosis patients. The median ratio is presented if more than one patient was enrolled at the same time point.
TABLE 2.
Cycloserine concentrations in the participants in the current study
| Cycloserine parameter | Median value (IQR) for group |
P value | ||
|---|---|---|---|---|
| Total (n = 28) | 120–240 min postdose (n = 16) | 241–420 min postdose (n = 12) | ||
| Plasma concn (μg/mL) | 16.29 (6.47) | 16.57 (7.86) | 15.87 (6.17) | 0.4118 |
| Bone concn (μg/g) | 24.33 (14.68) | 20.71 (16.03) | 26.62 (14.72) | 0.0904 |
| Bone/plasma penetration ratio | 0.76 (0.56) | 0.63 (0.42) | 1.04 (0.67) | 0.0172 |
Detailed information of the patients is provided in Table 3 (17). Only 8 (25.0%; 7/28) of our enrolled patients had a plasma concentration within the recommended range of 20 to 35 μg/mL, while none had concentrations above 35 μg/mL. van der Galiën et al. developed a limited sampling strategy to obtain reliable PK data with minimal sampling and found that 4 h after dosing of cycloserine had the best coefficient with the area under the concentration-time curve (AUC) compared with the full AUC (R2 = 0.9956) (16). They also found that the median time to the maximum concentration of drug in serum (Cmax) was 3.84 h, and the AUC from 0 to 24 h (AUC0–24) was also significantly correlated with a threshold cycle (CT) of 4, with a beta coefficient of 0.957. Our sampling time centered on 3 to 4 h postdosing, which enforces the main findings of our study. However, the outcomes for 15 patients whose sampling time fell into 3 to 5 h after dosing did not present a significant difference compared with the total enrolled patients. For these 15 patients, the median plasma and bone concentrations were 16.29 μg/mL (IQR, 6.47 μg/mL) and 24.33 μg/g (IQR, 14.68 μg/g), with a median bone/plasma penetration ratio of 0.76 (IQR, 0.56). Thus, inadequate cycloserine plasma concentrations seemed common in these osteoarticular TB patients. Even among the 4 patients with low body weight (<50 kg), only one achieved a favorable concentration, which highlighted the importance of therapeutic drug monitoring (TDM). The WHO recommends a dose for cycloserine at 10 to 15 mg/kg of body weight; based on our study, the highest dosage in this range needs to be considered. Alghamdi et al. suggested an increase in the dose of cycloserine (from 500 to 1,000 mg) to acquire a favorable plasma concentration (18).
TABLE 3.
Cycloserine concentrations in samples collected during operations
| Patient ID | Sex | BMI/wt (kg) | Dose/wt | Sampling time after morning dosing (min) | Plasma concn (μg/mL) | Bone concna |
Bone/plasma ratio | MIC of cycloserine (μg/mL) | |
|---|---|---|---|---|---|---|---|---|---|
| μg/mL | μg/g | ||||||||
| 1 | Female | 24.22/62 | 8.06 | 120 | 12.776 | 12.839 | 24.3941 | 1.00 | NA |
| 2 | Male | 25.50/79 | 6.33 | 120 | 10.555 | 6.955 | 13.2145 | 0.66 | NA |
| 3 | Male | 20.37/66 | 7.58 | 130 | 17.615 | 11.185 | 21.2515 | 0.63 | NA |
| 4 | Male | 19.59/60 | 8.33 | 135 | 21.455 | 7.375 | 14.0125 | 0.34 | NA |
| 5 | Female | 21.48/55 | 9.09 | 140 | 25.030 | 20.924 | 39.7556 | 0.84 | 16 |
| 6 | Male | 16.44/47.5 | 10.53 | 150 | 12.651 | 16.067 | 30.5273 | 1.27 | NA |
| 7 | Male | 21.89/64 | 7.81 | 150 | 16.322 | 8.744 | 16.6136 | 0.54 | NA |
| 8 | Female | 23.88/65 | 7.69 | 150 | 15.048 | 7.363 | 13.9897 | 0.49 | NA |
| 9 | Male | 24.97/60 | 8.33 | 150 | 30.415 | 10.613 | 20.1647 | 0.35 | 16 |
| 10 | Male | 26.81/84 | 5.95 | 160 | 14.603 | 7.522 | 14.2918 | 0.52 | NA |
| 11 | Female | 24.03/60 | 8.33 | 190 | 10.815 | 3.603 | 6.8457 | 0.33 | 16 |
| 12 | Female | 23.23/58 | 8.62 | 200 | 14.671 | 22.241 | 42.2579 | 1.52 | 16 |
| 13 | Female | 20.76/60 | 8.33 | 200 | 16.814 | 6.611 | 12.5609 | 0.39 | 16 |
| 14 | Male | 15.94/45 | 11.11 | 210 | 31.675 | 19.710 | 37.449 | 0.62 | NA |
| 15 | Male | 18.19/57 | 8.77 | 220 | 18.329 | 15.009 | 28.5171 | 0.82 | 16 |
| 16 | Female | 20.94/57 | 8.77 | 220 | 20.029 | 14.046 | 26.6874 | 0.70 | NA |
| 17 | Male | 22.31/66 | 7.58 | 245 | 16.256 | 17.094 | 32.4786 | 1.05 | 16 |
| 18 | Female | 22.83/57 | 8.77 | 260 | 13.495 | 26.778 | 50.8782 | 1.98 | 16 |
| 19 | Male | 20.76/60 | 8.33 | 260 | 19.762 | 10.225 | 19.4275 | 0.52 | NA |
| 20 | Female | 24.84/62 | 8.06 | 260 | 17.149 | 10.433 | 19.8227 | 0.61 | 16 |
| 21 | Male | 24.82/76 | 6.58 | 260 | 15.471 | 14.187 | 26.9553 | 0.92 | NA |
| 22 | Female | 26.12/80 | 6.25 | 280 | 23.092 | 29.055 | 55.2045 | 1.26 | NA |
| 23 | Male | 15.04/45 | 11.11 | 280 | 13.504 | 11.204 | 21.2876 | 0.83 | 16 |
| 24 | Female | 19.98/48 | 10.42 | 280 | 8.845 | 12.011 | 22.8209 | 1.36 | NA |
| 25 | Female | 18.03/45 | 11.11 | 300 | 19.414 | 19.836 | 37.6884 | 1.02 | NA |
| 26 | Male | 17.99/52 | 9.62 | 315 | 24.241 | 13.826 | 26.2694 | 0.57 | 16 |
| 27 | Male | 19.27/59 | 8.47 | 330 | 8.871 | 12.769 | 24.2611 | 1.44 | 16 |
| 28 | Male | 20.68/67 | 7.46 | 420 | 14.421 | 15.808 | 30.0352 | 1.10 | NA |
Bone concentrations are reported in micrograms per gram by multiplying by a conversion factor of 1.9 g/mL for bone density (17). NA, not applicable.
Currently, there is no well-defined critical concentration to define cycloserine resistance. Our previous study showed that the tentative epidemiological cutoff (ECOFF) for cycloserine was 32 μg/mL (19), while a proposed clinical susceptibility breakpoint of cycloserine was 64 μg/mL at a dose of 750 mg twice daily in Monte Carlo experiments (15). In this study, each isolate recovered from an individual patient. Finally, 12 of 28 patients were determined to be culture positive and performed the MICs test. All the MICs of these isolates against cycloserine were uniform at 16 μg/mL, which therefore could be considered susceptible strains. Deshpande et al. performed a hollow-fiber study and reported that the efficacy of cycloserine was driven by the percentage of time that the concentration persisted above the MIC (%T>MIC). A target value for %T>MIC of 30% has been proposed and was associated with a 1.0-log10 CFU/mL kill, while a %T>MIC of 64% is linked to 80% maximal kill (20). In this study, concentrations of cycloserine that exceeded 16 μg/mL were observed in 53.6% (15/28) of plasma samples and 28.6% (8/28) of bone samples. Our study demonstrated an even plasma concentration within the 2- to 7-h sampling interval. Taken together with the long reported half-life of cycloserine (about 18 h, as reported by the WHO prequalification team [21]), we boldly assume that about half of our enrolled patients were able to achieve a target exposure of a %T>MIC of 30% with the 500-mg/day dosage, whereas the target exposure of a %T>MIC of 64% remained unattainable. This speculation means that the 500-mg/day dosage of cycloserine in the enrolled patients was only mildly effective but not enough to achieve full efficacy. However, this speculated outcome is much better than the analysis according to the recommended plasma concentration range. A patient-based systematic clinical trial is needed to address this ambiguity.
In conclusion, cycloserine demonstrated good efficiency of penetration into bone, which makes it a valuable candidate drug for the treatment of osteoarticular TB, whereas the 500-mg/day dosage generally could not acquire the desired concentration for effective treatment.
ACKNOWLEDGMENTS
This study was supported by the Natural Science Fund of China (82072328), the Beijing Hospitals Authority Youth Programme (QML20211602), the Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20181602), and the Tongzhou “Yun He” Talent Project (YHLD2019001).
We have no conflicts of interest to disclose.
Contributor Information
Shibing Qin, Email: qinshibing2019@163.com.
Hairong Huang, Email: huanghairong@tb123.org.
REFERENCES
- 1.World Health Organization. 2021. Global tuberculosis report 2020. World Health Organization, Geneva, Switzerland. https://www.who.int/news-room/fact-sheets/detail/tuberculosis. [Google Scholar]
- 2.Solovic I, Jonsson J, Korzeniewska-Koseła M, Chiotan DI, Pace-Asciak A, Slump E, Rumetshofer R, Abubakar I, Kos S, Svetina-Sorli P, Haas W, Bauer T, Sandgren A, van der Werf MJ. 2013. Challenges in diagnosing extrapulmonary tuberculosis in the European Union, 2011. Euro Surveill 18:20432. doi: 10.2807/ese.18.12.20432-en. [DOI] [PubMed] [Google Scholar]
- 3.Wang G, Dong W, Lan T, Fan J, Tang K, Li Y, Yan G, Jiang G, Ma Y, Shang Y, Qin S, Huang H. 2018. Diagnostic accuracy evaluation of the conventional and molecular tests for spinal tuberculosis in a cohort, head-to-head study. Emerg Microbes Infect 7:109. doi: 10.1038/s41426-018-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang Y, An J, Shu W, Huo F, Chu N, Gao M, Qin S, Huang H, Chen X, Xu S. 2019. Epidemiology of extrapulmonary tuberculosis among inpatients, China, 2008-2017. Emerg Infect Dis 25:457–464. doi: 10.3201/eid2503.180572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agashe VM, Johari AN, Shah M, Anjum R, Romano C, Drago L, Sharma HK, Benzakour T. 2020. Diagnosis of osteoarticular tuberculosis: perceptions, protocols, practices, and priorities in the endemic and non-endemic areas of the world—a WAIOT view. Microorganisms 8:1312. doi: 10.3390/microorganisms8091312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upadhyay M, Patel J, Kundnani V, Ruparel S, Patel A. 2020. Drug sensitivity patterns in Xpert-positive spinal tuberculosis: an observational study of 252 patients. Eur Spine J 29:1476–1482. doi: 10.1007/s00586-020-06305-x. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. 2019. WHO consolidated guidelines on drug-resistant tuberculosis treatment. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 8.World Health Organization. 2018. Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 9.Evangelopoulos D, Prosser GA, Rodgers A, Dagg BM, Khatri B, Ho MM, Gutierrez MG, Cortes T, de Carvalho LPS. 2019. Comparative fitness analysis of D-cycloserine resistant mutants reveals both fitness-neutral and high-fitness cost genotypes. Nat Commun 10:4177. doi: 10.1038/s41467-019-12074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsultan A, Peloquin CA. 2014. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 74:839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 11.Court R, Centner CM, Chirehwa M, Wiesner L, Denti P, de Vries N, Harding J, Gumbo T, Maartens G, McIlleron H. 2021. Neuropsychiatric toxicity and cycloserine concentrations during treatment for multidrug-resistant tuberculosis. Int J Infect Dis 105:688–694. doi: 10.1016/j.ijid.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu H, Guo S-C, Liu Z-Q, Wang B, Fu L, Chu N-H, Lu Y. 2018. Therapeutic drug monitoring of cycloserine and linezolid during anti-tuberculosis treatment in Beijing, China. Int J Tuberc Lung Dis 22:931–936. doi: 10.5588/ijtld.17.0648. [DOI] [PubMed] [Google Scholar]
- 13.Hung W-Y, Yu M-C, Chiang Y-C, Chang J-H, Chiang C-Y, Chang C-C, Chuang H-C, Bai K-J. 2014. Serum concentrations of cycloserine and outcome of multidrug-resistant tuberculosis in Northern Taiwan. Int J Tuberc Lung Dis 18:601–606. doi: 10.5588/ijtld.13.0268. [DOI] [PubMed] [Google Scholar]
- 14.Baron H, Epstein IG, Mulinos MG, Nair KG. 1955. Absorption, distribution, and excretion of cycloserine in man. Antibiot Annu 3:136–140. [PubMed] [Google Scholar]
- 15.Deshpande D, Alffenaar J-WC, Köser CU, Dheda K, Chapagain ML, Simbar N, Schön T, Sturkenboom MGG, McIlleron H, Lee PS, Koeuth T, Mpagama SG, Banu S, Foongladda S, Ogarkov O, Pholwat S, Houpt ER, Heysell SK, Gumbo T. 2018. d-Cycloserine pharmacokinetics/pharmacodynamics, susceptibility, and dosing implications in multidrug-resistant tuberculosis: a Faustian deal. Clin Infect Dis 67:S308–S316. doi: 10.1093/cid/ciy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Galiën R, Boveneind-Vrubleuskaya NV, Peloquin C, Skrahina A, Touw DJ, Alffenaar JC. 2020. Pharmacokinetic modeling, simulation, and development of a limited sampling strategy of cycloserine in patients with multidrug-/extensively drug-resistant tuberculosis. Clin Pharmacokinet 59:899–910. doi: 10.1007/s40262-020-00860-8. [DOI] [PubMed] [Google Scholar]
- 17.Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. 2019. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis 81:128–136. doi: 10.1016/j.ijid.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Alghamdi WA, Alsultan A, Al-Shaer MH, An G, Ahmed S, Alkabab Y, Banu S, Barbakadze K, Houpt E, Kipiani M, Mikiashvili L, Schmidt S, Heysell SK, Kempker RR, Cegielski JP, Peloquin CA. 2019. Cycloserine population pharmacokinetics and pharmacodynamics in patients with tuberculosis. Antimicrob Agents Chemother 63:e00055-19. doi: 10.1128/AAC.00055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Zeng X, Shi W, Hu Y, Nie W, Chu N, Huang H. 2018. Validation of cycloserine efficacy in treatment of multidrug-resistant and extensively drug-resistant tuberculosis in Beijing, China. Antimicrob Agents Chemother 62:e01824-17. doi: 10.1128/AAC.01824-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chirehwa MT, Court R, de Kock M, Wiesner L, de Vries N, Harding J, Gumbo T, Maartens G, Warren R, Denti P, McIlleron H. 2020. Population pharmacokinetics of cycloserine and pharmacokinetic/pharmacodynamic target attainment in multidrug-resistant tuberculosis patients dosed with terizidone. Antimicrob Agents Chemother 64:e01381-20. doi: 10.1128/AAC.01381-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. 2015. Notes on the design of bioequivalence study: cycloserine. World Health Organization, Geneva, Switzerland. [Google Scholar]