ABSTRACT
The integrase strand transfer inhibitor (INSTI)-based regimens bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF), dolutegravir (DTG)+FTC/TAF, DTG/lamivudine (3TC), and DTG/rilpivirine (RPV) are all approved for treatment of HIV-infected patients, with various limitations. Here, time to in vitro viral breakthrough (VB) and resistance barrier using simulated human drug exposures at either full or suboptimal treatment adherence to each regimen were compared. At drug concentrations corresponding to full adherence and 1 missed dose (Cmin and Cmin−1), no VB occurred with any regimen. At Cmin−2, VB occurred only with DTG+3TC, with emergent resistance to both drugs. At Cmin−3, VB occurred with all regimens: 100% of DTG+3TC cultures had VB by day 12, and <15% of BIC+FTC+TAF, DTG+FTC+TAF, and DTG+RPV cultures had VB. Emergent reverse transcriptase (RT) or integrase (IN) resistance was seen with DTG+RPV and DTG+3TC but not with BIC+FTC+TAF or DTG+FTC+TAF. At Cmin−4, 100% VB occurred with DTG+3TC and DTG+FTC+TAF by day 12, while 94% VB occurred with DTG+RPV by day 25 and only 50% VB occurred with BIC+FTC+TAF by day 35. Emergent Cmin−4 drug resistance was seen with all regimens but at differing frequencies; DTG+RPV had the most cultures with resistance. Emergent resistance was consistent with clinical observations. Overall, under high adherence conditions, no in vitro VB or resistance development occurred with these INSTI-based regimens. However, when multiple missed doses were simulated in vitro, BIC+FTC+TAF had the highest forgiveness and barrier to resistance of all tested regimens. Compared to DTG+3TC and DTG+FTC+TAF, DTG+RPV had higher forgiveness but lower resistance barrier after several simulated missed doses.
KEYWORDS: bictegravir, forgiveness, resistance
INTRODUCTION
Antiretroviral therapy (ART) for treatment of HIV infection has significantly reduced morbidity and mortality for people living with HIV. However, ART use is lifelong, and there is a risk of treatment interruption or lapses in adherence over the course of taking these medications. Poor adherence is associated with loss of viral control and may lead to the development of drug resistance to one or more drugs in a patient’s regimen (1). “Forgiveness” refers to the ability to achieve or maintain viral suppression despite imperfect adherence to ART. This effect is regimen specific and depends on host, viral, and pharmacological factors (2).
Guidelines for initial treatment of HIV-1 infection primarily recommend three-drug combination ART that includes an integrase strand transfer inhibitor (INSTI) plus 2 nucleos(t)ide reverse transcriptase inhibitors (NRTIs). There are various exclusionary criteria when using different regimens, including preexisting resistance, level of viremia, and coinfection with hepatitis B virus, among others (3–6). Recommended regimens include the single-tablet regimen (STR) bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) and the regimens of dolutegravir (DTG) plus FTC/TAF (DTG+FTC/TAF) and dolutegravir/abacavir/lamivudine (DTG/ABC/3TC). More recently, two-drug combination ART has been explored, and some regimens have shown noninferiority compared to three-drug regimens in carefully selected populations (7–9). The INSTI plus 1 NRTI combination of DTG/lamivudine (3TC) is now included in the guidelines for a subset of patients, and the INSTI plus nonnucleoside reverse transcriptase inhibitor (NNRTI) combination of DTG/rilpivirine (RPV) is approved for patients switching ART regimens. In clinical trials, BIC/FTC/TAF, DTG/ABC/3TC, DTG+FTC/TAF, DTG/3TC, and DTG/RPV have all shown durable efficacy through at least 144 weeks in appropriately selected treatment-naive or virologically suppressed switch participants (10–13). However, infrequent cases of treatment failure and emergent drug resistance have been reported, mostly in people living with HIV (PLWH) with poor adherence and/or advanced HIV disease (12, 14–21). Given the long-term or lifelong nature of ART, it is important to understand the factors that may prevent virologic failure or resistance development.
Forgiveness can be used as a comparative descriptor of different antiretroviral (ARV) regimens; for example, it is known that boosted protease inhibitors (PIs) or NNRTIs are more forgiving of suboptimal adherence than unboosted PIs (22–24). Our group has developed an in vitro fixed-dose viral resistance breakthrough (VB) assay that uses clinically relevant drug concentrations in HIV-1-infected cells to evaluate regimen forgiveness and barrier to resistance (25, 26). Using clinical pharmacology information, we can simulate drug exposures at full adherence or suboptimal adherence to treatment in vitro. Previous work with this assay system has shown that the three-drug combination BIC+FTC+TAF has more forgiveness and a higher barrier to resistance than the two-drug combination DTG+3TC, demonstrated by less viral breakthrough and less drug resistance development. Here, we evaluated four ARV combinations in parallel, BIC+FTC+TAF, DTG+FTC+TAF, DTG+3TC, and DTG+RPV, to understand the relative time to in vitro viral breakthrough and resistance development in our experimental assay system.
RESULTS
Determination of cell culture equivalent physiologically relevant drug concentrations.
The pharmacokinetics of the approved INSTI-containing drug regimens BIC/FTC/TAF, DTG+FTC/TAF, DTG/3TC, and DTG/RPV have been studied in clinical trials. To translate in vivo drug concentrations to in vitro cell culture, clinical pharmacokinetic data were used and adjusted for human plasma protein binding where appropriate. For the INSTIs BIC and DTG and the NNRTI RPV, the median plasma drug concentrations at Cmin from participants in clinical trials are 2.61 μg/mL for BIC, 1.11 μg/mL for DTG, and 0.08 μg/mL for RPV (5,808 nM, 2,515 nM, and 218 nM, respectively) (27–29) (Table 1). These three drugs are highly protein bound but to different extents, and as such only a small fraction of the measured plasma drug concentration is free to enter target cells for inhibition of viral replication. An equilibrium dialysis assay was used to measure the fold change in drug concentration in human plasma and cell culture medium with 10% fetal bovine serum (FBS), and the resulting protein binding factors (PBF; human serum shifts) are 43.6 for BIC, 27.5 for DTG (30), and 32 for RPV (31) (internal data). The cell culture equivalent (CCE) Cmin, or the drug concentration in cell culture medium that represents the amount of drug in plasma free to enter target cells, is the clinical Cmin divided by the PBF and is 133 nM for BIC, 91 nM for DTG, and 6.8 nM for RPV. To determine drug concentrations simulating missed doses of daily ARV regimens, the following equation was used: Cmin−X = Cmin × (0.5[24×X/t1/2]); the pharmacologic in vivo half-lives for BIC, DTG, and RPV are 17.3 h, 14 h, and 50 h, respectively (27–29).
TABLE 1.
Cell culture drug concentrations simulating Cmin and Cmin after missing 1 to 4 consecutive doses
| Parameter | Value by antiretroviral drug |
|||||
|---|---|---|---|---|---|---|
| BIC | FTC | TAF | DTG | 3TC | RPV | |
| Clinical dosea (mg) | 50 | 200 | 25 | 50 | 300 | 25 |
| Mol wt (g/mol) | 449.4 | 247.2 | 534.5 | 419.4 | 229.3 | 366.4 |
| Clinical Cmin (μg/mL) | 2.61 | 0.096 | 0.008 | 1.11 | 0.042 | 0.08 |
| Clinical Cmin (nM) | 5808 | 388 | 15 | 2515 | 265 | 218 |
| Human serum shiftb | 43.6 | 1.0 | 1.0 | 27.5 | 1.0 | 32 |
| t1/2c (h) | 17 | 37 | 116 | 14 | 17.5 | 50 |
| CCEd Cmin, nM | 133 | 388 | 15 | 91 | 265 | 6.8 |
| CCE Cmin−1, nM | 50 | 248 | 13 | 28 | 102 | 4.9 |
| CCE Cmin−2, nM | 19 | 158 | 11 | 8.5 | 40 | 3.5 |
| CCE Cmin−3, nM | 7.1 | 101 | 9.8 | 2.6 | 15 | 2.5 |
| CCE Cmin−4, nM | 2.7 | 64.2 | 8.5 | 0.8 | 5.9 | 1.8 |
Clinical doses of BIC, FTC, and TAF in the single-tablet regimen of bictegravir/emtricitabine/tenofovir alafenamide and DTG, 3TC, and RPV in the single-tablet regimens of dolutegravir/lamivudine and dolutegravir/rilpivirine (27–29).
BIC and DTG data generated by standard equilibrium dialysis shift in human serum versus complete cell culture media (30). RPV data were generated internally and are comparable to reported serum shift (31).
Cell culture equivalent (CCE) dose is the clinical Cmin/human serum shift ratio; Cmin−X doses determined as Cmin × (0.5[24 × X/t1/2]).
To simulate in vivo drug concentrations in vitro for NRTIs, similar careful calculations were used. Since NRTIs have low protein binding, no protein binding adjustment is required for TAF, FTC, or 3TC. TAF is a prodrug of tenofovir that loads CD4+ T cells and is converted to the active metabolite tenofovir diphosphate (TFV-DP) (32). TFV-DP is highly charged and retained in cells with a relatively long intracellular half-life of 116 h (27, 33). In vitro experiments have determined the concentration of TFV-DP at Cmin in peripheral blood mononuclear cells (PBMCs) can be reached in cell culture by using 15 nM TAF (34). The active metabolite of FTC is FTC-triphosphate (FTC-TP), with an intracellular half-life of 37 h, and the active metabolite of 3TC is 3TC-triphosphate (3TC-TP), with an intracellular half-life of 17.5 h (35–37). The in vivo Cmin concentrations of FTC and 3TC are 96 nM and 42 nM, respectively, which result in 388 nM FTC-TP and 265 nM 3TC-TP (27–29).
Viral breakthrough of HIV-1 and resistance development with BIC+FTC+TAF, DTG+FTC+TAF, DTG+3TC, and DTG+RPV.
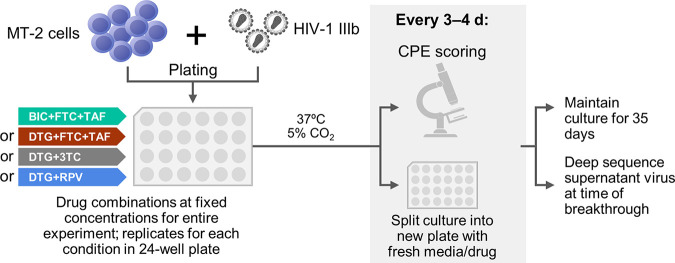
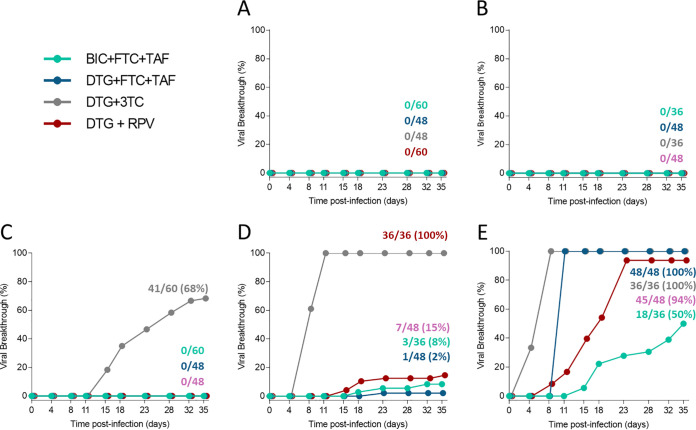
Viral breakthrough assays using the drug combinations of BIC+FTC+TAF, DTG+FTC+TAF, DTG+3TC, and DTG+RPV were performed in parallel at fixed drug concentrations simulating Cmin, Cmin minus one missed daily dose (Cmin−1), and Cmin−2, Cmin−3, and Cmin−4 consecutive missed daily doses. Briefly, MT-2 cells were infected with HIV-1 IIIB wild-type virus and maintained at single fixed drug concentrations in replicate cell cultures for up to 35 days; cultures were split every 3 to 4 days, and supernatant was harvested when viral breakthrough was observed by widespread cytopathic effect in the cell culture (Fig. 1). For each of the four drug combinations tested (BIC+FTC+TAF, DTG+FTC+TAF, DTG+3TC, and DTG+RPV), no viral breakthrough occurred at drug concentrations corresponding to full adherence and one missed dose (Cmin and Cmin−1) (Fig. 2A and B). At cell culture equivalent trough drug concentrations corresponding to two consecutive missed doses (Cmin−2), there was no viral breakthrough for BIC+FTC+TAF, DTG+FTC+TAF, or DTG+RPV (Fig. 2C). DTG+3TC had viral breakthrough starting at day 14 and had 41/60 cultures break through by the end of study (day 35). In these experiments, the supernatant virus was genotyped by next-generation sequencing, and resistance mutations present at ≥2% frequency were reported. In total, 13 of the DTG+3TC breakthrough cultures at the Cmin−2 drug concentration had emergent reverse transcriptase (RT) and/or integrase (IN) substitutions associated with drug resistance (Table 2). The most frequent substitution was M184V/I in RT in 4 cultures, present at 2.2% to 42.2% prevalence. This mutation confers high-level resistance to 3TC and FTC and hypersusceptibility to TAF and is the most frequent resistance mutation that emerges at virologic failure in PLWH treated with NRTIs (38–40). The S153F variant in IN was selected in 1 culture, and this mutation confers low-level reduced susceptibility to DTG and BIC and has been selected in PLWH and in resistance selections in vitro (30, 41, 42). Other drug-associated mutations selected were V75I in RT and L74M, G140E/R, or E157K in IN. These single mutations show no or minimal phenotypic resistance to 3TC or DTG but may increase drug resistance or viral fitness when combined with primary drug resistance mutations (41). The observation of these drug-associated mutations at lower levels by next-generation sequencing suggests that the virus is evolving to escape drug pressure. Evolution of patterns of resistance has been seen in patients; therefore, these in vitro developed mutations may further evolve to show phenotypic resistance (43, 44).
FIG 1.
In vitro viral breakthrough selections. MT-2 cells were bulk infected with HIV-1 IIIb strain and cultured in replicate on 24-well plates in the presence of fixed concentrations (Cmin, Cmin−1, Cmin−2, Cmin−3, or Cmin−4) of BIC+FTC+TAF, DTG+FTC+TAF, DTG+3TC, or DTG+RPV. Infected cultures were split every 3 to 4 days with fresh medium containing drugs and closely monitored for viral breakthrough by cytopathic effect (CPE) for up to 35 days of selection. Cell-free supernatants containing breakthrough virus were collected upon emergence and stored frozen for deep sequencing.
FIG 2.
Time to viral breakthrough. Time to viral breakthrough in MT-2 cells infected with wild-type HIV-1 IIIB strain is shown. Viral breakthrough selections for each drug combination were tested in replicate infected cultures in the presence of constant drug pressure for up to 35 days or until viral breakthrough was observed. The number of cultures with viral breakthrough by observed cytopathic effect was scored at each time point. Selections were performed at the following drug concentrations: simulated Cmin, the minimum drug exposures based on in vivo pharmacokinetics (A); simulated Cmin after missing 1 dose (Cmin−1) (B); Cmin after missing 2 consecutive doses (Cmin−2) (C); Cmin after missing 3 consecutive doses (Cmin−3) (D); and Cmin after missing 4 consecutive doses (Cmin−4) (E). Symbols have been slightly offset on the x axis to aid viewing.
TABLE 2.
Resistance
| In vitro drug concentration | Breakthrough frequency (resistance development) |
|||||||
|---|---|---|---|---|---|---|---|---|
| BIC+FTC+TAF |
DTG+FTC+TAF |
DTG+3TC |
DTG+RPV |
|||||
| VB (n/N; %) [first day of VB] | With resistance, Na | VB (n/N; %) [first day of VB]b | With resistance, Na | VB (n/N; %) [first day of VB] | With resistance, Na | VB (n/N; %) [first day of VB] | With resistance, Na | |
| C min | 0/60; 0 [NA] | 0 | 0/48; 0 [NA] | 0 | 0/60; 0 [NA] | 0 | 0/48; 0 [NA] | 0 |
| Cmin−1 | 0/36; 0 [NA] | 0 | 0/48; 0 [NA] | 0 | 0/36; 0 [NA] | 0 | 0/48; 0 [NA] | 0 |
| Cmin−2 | 0/60; 0 [NA] | 0 | 0/48; 0 [NA] | 0 | 41/60; 68 [14] | 13; RT, M184V/I (4), V75I (3); IN, G140E/R (2), E157K (2), L74M (1), S153F (1) | 0/48; 0 [NA] | 0 |
| Cmin−3 | 3/36; 8 [21] | 0 | 1/48; 2 [25] | 0 | 36/36; 100 [7] | 3; RT, none; IN, L74M (2), V72A (1), S153F (1) | 7/48; 15 [14] | 1; RT, M230I; IN, none |
| Cmin−4 | 18/36; 50 [15] | 3; RT, M184I (2); IN, G163R (1) | 48/48; 100 [11] | 6; RT, M184V (1), K219R (1); IN, Q148R (2), Q95R (1), H51Y (1), S153F (1) | 36/36; 100 [5] | 2; RT, none; IN, R263K (2), L74M (1) | 45/48; 94 [8] | 20; RT, E138K (8), K101E (3), M230I (2), V90I (2), V106I (1), Y181C (1), H221Y (1); IN, H51Y (2), R263K (1), M50I (1), Q95R (1), A128T (1), S153F (1), G163R (1) |
Reverse transcriptase (RT) substitutions are shown in plain text. Integrase (IN) substitutions are shown in italics. Some viral breakthrough supernatants had more than one emergent resistance mutation, as listed here: DTG+3TC Cmin−3, 1 L74M+S153F in IN; DTG+3TC Cmin−4, 1 L74M+R263K in IN; DTG+FTC+TAF Cmin−4, 1 M184V in RT + Q95R in IN; DTG+RPV Cmin−4, 1 V90I+V106I+E138K in RT, 1 Y181C in RT + H51Y in IN, 1 E138K in RT + H51Y in IN, 1 E138K in RT + Q95R in IN, and 1 E138K in RT + A128T in IN.
VB, viral breakthrough; NA, not applicable.
At Cmin−3 drug concentrations, viral breakthrough occurred for all four drug combinations tested (BIC+FTC+TAF, DTG+FTC+TAF, DTG+3TC, and DTG+RPV) but differed by time and extent of breakthrough (Fig. 2D). DTG+3TC had viral breakthrough first starting at day 7, with all cultures (36/36) breaking through by day 12 and IN resistance mutations in 3 of these cultures. BIC+FTC+TAF, DTG+FTC+TAF, and DTG+RPV had fewer cultures with breakthrough that were detected later than that for DTG+3TC. DTG+RPV breakthrough was first detected at day 14 and reached 7/48 cultures through day 35; BIC+FTC+TAF breakthrough was detected at day 21 and reached a maximum of 3/36 cultures; DTG+FTC+TAF had 1/48 cultures with breakthrough at day 25. The NNRTI resistance mutation M230I was detected in 1 DTG+RPV breakthrough culture, and no resistance was detected in the BIC+FTC+TAF or DTG+FTC+TAF breakthrough cultures.
At Cmin−4 drug concentrations for all regimens tested, viral breakthrough occurred earlier and to a greater extent than at higher drug concentrations (Fig. 2E). All DTG+3TC and DTG+FTC+TAF cultures (36/36 and 48/48, respectively) showed viral breakthrough by day 12. Two of the DTG+3TC cultures with breakthrough developed IN resistance, both with the R263K mutation. R263K confers about 2-fold resistance to both DTG and BIC and has been documented in rare cases of virologic failure (20, 42, 45–48). Six of the DTG+FTC+TAF breakthrough cultures developed RT or IN resistance, including 1 with M184V in RT and 2 with Q148R in IN; these mutations have been selected in patients receiving DTG-based therapy (49–51). The DTG+RPV selections had 94% (45/48 cultures) breakthrough by day 25. Twenty of the DTG+RPV breakthrough cultures developed resistance to RT or IN, with some cultures developing multiple mutations in one or both genes. Resistance mutations included the major RPV-associated mutations E138K, Y181C, and K101E as well as the IN mutations R263K and S153F. BIC+FTC+TAF was the combination associated with the slowest breakthrough, which was first detected at day 15 and reached 50% (18/36 cultures) by day 35. Three of the BIC+FTC+TAF breakthrough cultures developed resistance to components of the regimen; the mutations observed were the RT mutation M184I in 2 cultures and the IN mutation G163R in 1 culture. Overall, these four INSTI-containing regimens had no viral breakthrough at Cmin drug concentrations, as expected, but as multiple missed doses were simulated, the regimens had breakthrough and resistance development to different extents.
DISCUSSION
Major factors that lead to virologic failure and emergent drug resistance are high baseline viral loads, low baseline CD4+ T cell counts, and poor adherence to ART (52). Intermittent adherence using a structured treatment interruption strategy was attempted to decrease the exposure to HIV drugs with substantial toxicities; these interventions of antiretroviral regimens resulted in virologic rebound and resistance development and are not recommended in routine clinical care (53, 54). Some studies of less frequent dosing of currently recommended daily oral regimens are being conducted and may yield useful information on the risks to people with suboptimal adherence; however, these alternative dosing strategies should be undertaken with caution. Since clinical trials studying suboptimal adherence and potentially virologic failure could be risky for study participants, we have conducted studies simulating imperfect adherence and forgiveness of four treatment regimens in vitro.
This study evaluated the four regimens BIC/FTC/TAF, DTG+FTC/TAF, DTG/3TC, and DTG/RPV in viral breakthrough selection experiments to understand the relative time to in vitro viral breakthrough and resistance development. For each of the four ART regimens tested here, no viral breakthrough occurred at drug concentrations corresponding to full adherence or to one missed dose (Cmin and Cmin−1). This would be expected based on pharmacokinetic and virologic failure data from clinical trials. When drug concentrations were evaluated that corresponded to having missed two consecutive doses (Cmin−2), DTG+3TC was the only regimen that allowed viral breakthrough, with emergence of resistance mutations to RT or IN in some cultures. At Cmin−3 and Cmin−4 drug concentrations, all regimens allowed viral breakthrough; however, DTG+3TC consistently had the earliest and most extensive breakthrough. Interestingly, at Cmin−4, breakthrough occurred in 100% of DTG+FTC+TAF cultures, beginning at day 11. In contrast, breakthrough occurred in only 50% of BIC+FTC+TAF cultures, with the first breakthrough at day 15. This observation was seen with multiple independent experiments. The BIC/FTC/TAF and DTG+FTC/TAF regimens both demonstrate high efficacy in clinical trials, where frequent clinic visits and adherence counseling lead to higher adherence than in the real world (10, 55). However, there are pharmacokinetic, pharmacodynamic, and resistance differences between BIC and DTG that may have contributed to the difference seen in this study. BIC achieves higher drug exposures than DTG, has a longer effective plasma half-life, has more contacts with the IN-DNA target, has a longer dissociation half-life from the IN-DNA complexes, and has a more favorable resistance profile than DTG (30, 47, 56–59). At simulated Cmin−4, DTG+RPV broke through more slowly than DTG+3TC and DTG+FTC+TAF and did not achieve complete viral breakthrough by day 35 (end of selection experiment); however, these DTG+RPV breakthrough cultures developed more resistance than any of the other regimens. Cumulatively across all regimen conditions tested in this series, emergent RT and/or IN resistance was detected in 3/228 (1.3%) of BIC+FTC+TAF, 6/240 (2.5%) of DTG+FTC+TAF, 18/228 (7.9%) of DTG+3TC, and 21/240 (8.8%) of DTG+RPV breakthrough cultures. Both 2-drug combinations had more overall resistance development than the 3-drug combinations; this could be due to higher cumulative drug concentrations, more combinations with antiviral synergy, better activity against resistance mutations that emerge, and hypersusceptibility of the M184V resistance mutant to TAF.
The viral breakthrough trends observed with the four regimens studied here correlate with published clinical data. Overall, all the regimens have demonstrated high efficacy in clinical trials, and there have been no cases of virologic failure with resistance for participants taking BIC/FTC/TAF or DTG+FTC/TAF and few with DTG/3TC or DTG/RPV (7, 10, 12–15, 60–62). However, it is important to recognize that adherence in clinical trials is higher than that in real-world situations (63), and some of these clinical trials had limitations on baseline viral load, baseline resistance to study drugs, and/or hepatitis B virus coinfection. In one study looking at the impact of adherence on viral suppression with BIC- and DTG-containing triple therapy regimens, it was found that good adherence, above thresholds of 80% or 95%, independently predicted viral suppression at 6 months with either DTG/ABC/3TC or DTG multitablet combinations (DTG+FTC/TAF, DTG+FTC/TDF, and DTG+ABC+3TC) but not with BIC/FTC/TAF (64). In other words, better adherence resulted in greater viral suppression with the DTG-based regimens, but response to BIC/FTC/TAF did not depend on adherence. There are limited data on adherence and forgiveness with the two-drug regimens DTG/3TC or DTG/RPV, but the data cited here do suggest that BIC/FTC/TAF has higher forgiveness than other prescribed INSTI-containing regimens.
The resistance mutations observed in this study were also consistent with resistance observed clinically. The major mutations that emerge in PLWH with virologic failure are M184V/I in RT for FTC or 3TC, R263K for DTG or BIC or Q148R in IN for DTG, and K101E, E138K, Y181C, M230I, or H221Y in RT for RPV. Although development of resistance is low for all of these regimens, drug resistance can have severe consequences, and there have been reported cases of resistance in people that are consistent with those from our in vitro system. In clinical trials enrolling treatment-naive participants, noncoformulated DTG+3TC has selected for the M184V mutation in RT plus the R263K mutation in IN in 2 cases, both involving nonadherent trial participants (12, 14). DTG+RPV has selected for K101E, E138E/A, and M230L in RT in clinical trials (13, 65, 66) and E138Q and Y181C in RT in clinical practice (67). Resistance has been reported in treatment-naive individuals taking DTG+FTC+TDF in clinical practice with the mutations M184V/I in RT and G118R, E157Q, Q148K, and R263K in IN (68–71). In these cases, risk factors for resistance included advanced disease, comorbidities, high baseline viral load, and/or low baseline CD4 cell count and poor adherence. Rare cases of emergent resistance in clinical practice on BIC+FTC+TAF have seen emergent M184V/I in RT and H51Y, E138K, S147G, and R263K in IN (17–19). In these cases, risk factors for resistance included advanced disease, prior virologic failure on an INSTI-containing regimen, and/or poor adherence.
These in vitro models have limitations. There is not a direct translation of simulated missed doses in vitro to in vivo missed doses; this in vitro system is likely more sensitive to missed doses than in vivo, where the immune system would contribute to viral suppression and help keep HIV-1 in a latent state (72). In vivo, reactivation of virus may begin in the lymph node and take time to progress to detectable plasma viremia versus rapid spread and detection in culture. In addition, drug distribution in the body is heterogeneous and ARV penetration in tissues can be poor (73). Although we studied consecutive missed doses of drug here, suboptimal adherence can take many forms, including other patterns of missing doses of drug, administration with contraindicated medications that may decrease exposure, or insufficient food requirements. It is also important to recognize a major reason for poor adherence are side effects in PLWH, and although a drug combination may have high forgiveness or a high resistance barrier in this assay, the combination may have more side effects, which could lead to poor clinical adherence. In addition, drug concentrations here were kept constant for experimental consistency, whereas in vivo, drug concentrations reach a maximum concentration and then continually decline. For the 3TC and FTC drug concentrations, the cell culture clinical Cmin is below the 50% effective concentration (EC50) of these drugs in this system; however, synergy between drugs and hypersusceptibility of TAF to the M184V virus may have prevented some resistance development. Still, this model is one way to study missed doses and viral breakthrough in vitro in a controlled environment and may highlight differences between regimen potency under conditions simulating short periods of nonadherence.
Overall, there was no viral breakthrough or resistance development with these INSTI-based combinations under high-adherence conditions. When multiple missed doses were simulated in vitro, the drug combinations had different levels of forgiveness and barriers to resistance. BIC+FTC+TAF had the highest forgiveness and barrier to resistance. DTG+RPV had higher forgiveness but lower resistance barrier after multiple missed doses compared to DTG+3TC and DTG+FTC+TAF. These data suggest that higher drug levels, distinct resistance profiles, and antiviral synergy are more protective in individuals with suboptimal adherence and should be considered when choosing ARV regimens, particularly in the real world, where imperfect drug adherence is expected.
MATERIALS AND METHODS
Reagents, cell culture, and HIV strains.
BIC, FTC, 3TC, TAF, and RPV were synthesized at Gilead Sciences, Inc. (Foster City, CA, USA). DTG was purchased from Porton Pharma Solutions (Shanghai, China). All drug stocks were prepared in 100% dimethyl sulfoxide (DMSO), and potency in tissue culture was consistent with the literature values. The HTLV-1-transformed human T cell line MT-2 was obtained from the NIH AIDS Reagent Program (Germantown, MD, USA) (74, 75) and maintained at 37°C in 5% CO2 at densities below 1 × 106 cells/mL by serial passaging in RPMI cell culture medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics (Sigma-Aldrich, St. Louis, MO, USA). The laboratory-adapted HIV-1 IIIB strain (NIH AIDS Reagent Program) was used for infection (76).
Drug concentration determination.
The clinical minimum drug concentration (Cmin), defined as the steady-state trough plasma drug concentration, was obtained from individual drug package inserts for BIC/FTC/TAF (Biktarvy), DTG/3TC (Dovato), and DTG/RPV (Juluca) (27–29). A previously described standard equilibrium dialysis assay was used to directly measure the differences between free drugs in human plasma and cell culture medium (77). Briefly, 100% human plasma containing drug was added to one dialysis chamber, and cell culture medium (CCM; supplemented with 10% FBS) containing the same amount of drug was added to the second dialysis chamber. The chambers were then rotated for 3 h in a 37°C water bath, after which time the drug concentration in each chamber was determined by liquid chromatography-mass spectrometry (LC-MS). The resulting ratio, representing the fold difference in drug concentration in human plasma and CCM, identified the human plasma shift value for each drug. This shift was used to generate the cell culture equivalent (CCE) Cmin drug concentration (clinical Cmin/human plasma shift) that was used for BIC, DTG, and RPV. Determination of TAF Cmin concentration was described previously and generated by correlating intracellular tenofovir-diphosphate (TFV-DP) with its physiological concentration in peripheral blood mononuclear cells (PBMCs) from TAF-treated individuals (34). FTC and 3TC concentrations were not adjusted for protein binding and were set at their human plasma Cmin concentrations (35, 36). To simulate one, two, three, or four consecutive missed doses (Cmin−1, Cmin−2, Cmin−3, and Cmin−4), drug concentrations were adjusted by their plasma half-lives for BIC, DTG, and RPV and active metabolite half-lives for the NRTIs (TAF, FTC, and 3TC). Cmin−X doses was determined as Cmin × (0.5[24 × X/t1/2]) (27–29, 35, 36).
HIV-1 breakthrough selections in MT-2 cells.
MT-2 cells were infected with HIV-1 IIIB at a multiplicity of infection (MOI) of 0.05 for 3 h and plated in 24-well plates at 2 × 105 cells per well (Fig. 1). Experiments with each drug combination studied Cmin, Cmin−1, Cmin−2, Cmin−3, and Cmin−4 at fixed drug concentrations over the course of the experiment. Drugs were added 16 h after infection to a minimum of 12 replicate cultures at fixed concentrations equal to their CCE Cmin concentration or at concentrations adjusted for missing doses at 24, 48, 72, and 96 h (1, 2, 3, and 4 doses, respectively). Every 3 to 4 days, cells were diluted (1:5) into freshly prepared drug media and monitored for virus-induced cytopathic effects (CPE) over a period of 35 days. Cell-free viral supernatants were harvested from cultures showing >90% CPE and kept frozen at −80°C until further analyses.
Sequencing of breakthrough HIV-1 variants.
A next-generation sequencing/deep sequencing analysis of HIV-1 protease, RT, and IN was conducted on viral breakthrough samples. Sequencing used the DeepType HIV assay (Seq-IT) and Gilead-developed software to process and align data and identify substitutions present (78, 79). Resistance-associated substitutions were analyzed at a frequency cutoff of ≥2% prevalence.
Data availability.
Sequencing data related to this study have been deposited in NCBI under BioProject no. PRJNA812639.
ACKNOWLEDGMENTS
We thank Wade Blair, Derek Hansen, Christian Callebaut, and Nicolas Margot for thoughtful discussions. We acknowledge Martin Daeumer and Alex Thielen of Seq-IT for performing the next-generation sequencing.
REFERENCES
- 1.Nachega JB, Marconi VC, van Zyl GU, Gardner EM, Preiser W, Hong SY, Mills EJ, Gross R. 2011. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets 11:167–174. 10.2174/187152611795589663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuter J. 2008. Forgiveness of non-adherence to HIV-1 antiretroviral therapy. J Antimicrob Chemother 61:769–773. 10.1093/jac/dkn020. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). 2021. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 4.U.S. Department of Health & Human Services (DHHS), AIDSInfo. 2019. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. DHHS, Silver Spring, MD. [Google Scholar]
- 5.Saag MS, Gandhi RT, Hoy JF, Landovitz RJ, Thompson MA, Sax PE, Smith DM, Benson CA, Buchbinder SP, Del Rio C, Eron JJ, Jr, Fatkenheuer G, Gunthard HF, Molina JM, Jacobsen DM, Volberding PA. 2020. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA panel. JAMA 324:1651–1669. 10.1001/jama.2020.17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European AIDS Clinical Society (EACS). 2019. The EACS guidelines version 10. European AIDS Clinical Society, Brussels, Belgium. [Google Scholar]
- 7.Cahn P, Madero JS, Arribas JR, Antinori A, Ortiz R, Clarke AE, Hung CC, Rockstroh JK, Girard PM, Sievers J, Man C, Currie A, Underwood M, Tenorio AR, Pappa K, Wynne B, Fettiplace A, Gartland M, Aboud M, Smith K, GEMINI Study Team. 2019. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with hiv-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 393:143–155. 10.1016/S0140-6736(18)32462-0. [DOI] [PubMed] [Google Scholar]
- 8.Cahn P, Andrade-Villanueva J, Arribas JR, Gatell JM, Lama JR, Norton M, Patterson P, Madero JS, Sued O, Figueroa MI, Rolon MJ. 2014. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis 14:572–580. 10.1016/S1473-3099(14)70736-4. [DOI] [PubMed] [Google Scholar]
- 9.Raffi F, Babiker AG, Richert L, Molina JM, George EC, Antinori A, Arribas JR, Grarup J, Hudson F, Schwimmer C, Saillard J, Wallet C, Jansson PO, Allavena C, Van Leeuwen R, Delfraissy JF, Vella S, Chene G, Pozniak A, NEAT001/ANRS143 Study Group. 2014. Ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1: 96 week results from the NEAT001/ANRS143 randomised non-inferiority trial. Lancet 384:1942–1951. 10.1016/S0140-6736(14)61170-3. [DOI] [PubMed] [Google Scholar]
- 10.Orkin C, DeJesus E, Sax PE, Arribas JR, Gupta SK, Martorell C, Stephens JL, Stellbrink HJ, Wohl D, Maggiolo F, Thompson MA, Podzamczer D, Hagins D, Flamm JA, Brinson C, Clarke A, Huang H, Acosta R, Brainard DM, Collins SE, Martin H. 2020. Three-year outcomes of the fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide vs dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomised, double-blind non-inferiority trials. Lancet HIV 3:e389–e400. 10.1016/S2352-3018(20)30099-0. [DOI] [PubMed] [Google Scholar]
- 11.Walmsley S, Baumgarten A, Berenguer J, Felizarta F, Florence E, Khuong-Josses MA, Kilby JM, Lutz T, Podzamczer D, Portilla J, Roth N, Wong D, Granier C, Wynne B, Pappa K. 2015. Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 70:515–519. 10.1097/QAI.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahn P, Madero JS, Arribas JR, Antinori A, Ortiz R, Clarke AE, Hung CC, Rockstroh JK, Girard PM, Sievers J, Man CY, Urbaityte R, Brandon DJ, Underwood M, Pappa KA, Smith KY, Gartland M, Aboud M, van Wyk J, Wynne B. 2022. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy-naive adults with HIV-1 infection. AIDS 36:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Wyk J, Orkin C, Rubio R, Bogner J, Baker D, Khuong-Josses MA, Parks D, Angelis K, Kahl LP, Matthews J, Wang R, Underwood M, Wynne B, Nascimento MC, Vandermeulen K, Gartland M, Smith KY. 2020. Brief report: durable suppression and low rate of virologic failure 3 years after switch to dolutegravir + rilpivirine 2-drug regimen: 148-week results from the SWORD-1 and SWORD-2 randomized clinical trials. J Acquir Immune Defic Syndr 85:325–330. 10.1097/QAI.0000000000002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taiwo BO, Zheng L, Stefanescu A, Nyaku A, Bezins B, Wallis CL, Godfrey C, Sax PE, Acosta E, Haas D, Smith KY, Sha B, Van Dam C, Gulick RM. 2018. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)–infected participants with HIV-1 RNA <500 000 copies/mL. Clin Infect Dis 66:1689–1697. 10.1093/jac/dky564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Workowski K, Orkin C, Sax P, Hagins D, Koenig E, Stephens J, Wohl A, Lazzarin A, Gupta S, Huang H, Acosta R, Hindman J, Brainard D, Collins S, Martin H. 2021. Four-year outcomes of B/F/TAF in treatment-naïve adults. Poster 2268 HIV Medicine, 22(SUPPL 2):32–33. 10.1111/hiv.13131. [DOI] [Google Scholar]
- 16.Cevik M, Orkin C, Sax PE. 2020. Emergent resistance to dolutegravir among INSTI-naive patients on first-line or second-line antiretroviral therapy: a review of published cases. Open Forum Infect Dis 7:ofaa202. 10.1093/ofid/ofaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanig T, Marcelin AG, Calvez V. Selection of integrase inhibitor (INI) resistance mutations in an INI experienced patient treated by bictegravir. Poster PE13/7.
- 18.Lozano AB, Chueca N, de Salazar A, Fernandez-Fuertes E, Collado A, Fernandez JM, Alvarez M, Garcia F. 2020. Failure to bictegravir and development of resistance mutations in an antiretroviral-experienced patient. Antiviral Res 179:104717. 10.1016/j.antiviral.2020.104717. [DOI] [PubMed] [Google Scholar]
- 19.Braun P, Wiesmann F, Naeth G, Knechten H, Stoll M. 2020. Development of integrase inhibitor resistance under firstline treatment with bictegravir. Poster 125. http://www.hivglasgow.org/wp-content/uploads/2020/11/P125_Braun.pdf.
- 20.Chamberlain N, Mena L, Brock JB. 2021. Case report: emergent resistance in a treatment-naive person with human immunodeficiency virus under bictegravir-based therapy. Open Forum Infect Dis 8:ofab297. 10.1093/ofid/ofab297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribera E. 2018. New dual combination of dolutegravir-rilpivirine for switching to maintenance antiretroviral therapy. AIDS Rev 20:179–186. 10.24875/AIDSRev.M18000026. [DOI] [PubMed] [Google Scholar]
- 22.Maggiolo F, Ravasio L, Ripamonti D, Gregis G, Quinzan G, Arici C, Airoldi M, Suter F. 2005. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis 40:158–163. 10.1086/426595. [DOI] [PubMed] [Google Scholar]
- 23.Gulick RM. 2006. Adherence to antiretroviral therapy: how much is enough? Clin Infect Dis 43:942–944. 10.1086/507549. [DOI] [PubMed] [Google Scholar]
- 24.Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, Zingman BS. 2007. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95%. J Acquir Immune Defic Syndr 45:4–8. 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- 25.Mulato A, Hansen D, Thielen A, Porter D, Stepan G, White K, Daeumer M, Cihlar T, Yant SR. 2016. Rapid in vitro evaluation of antiretroviral barrier to resistance at therapeutic drug levels. AIDS Res Hum Retroviruses 32:1237–1247. 10.1089/AID.2016.0071. [DOI] [PubMed] [Google Scholar]
- 26.Mulato A, Acosta R, Chang S, Martin R, Yant SR, Cihlar T, White K. 2021. Simulating HIV breakthrough and resistance development during variable adherence to antiretroviral treatment. J Acquir Immune Defic Syndr 86:369–377. 10.1097/QAI.0000000000002562. [DOI] [PubMed] [Google Scholar]
- 27.Gilead Sciences Inc. 2021. BIKTARVY (bictegravir, emtricitabine, and tenofovir alafenamide) tablets, for oral use. Gilead Sciences Inc, Foster City, CA. [Google Scholar]
- 28.GlaxoSmithKline. 2019. DOVATO (dolutegravir and lamivudine) tablets, for oral use. GlaxoSmithKline, Research Triangle Park, NC. [Google Scholar]
- 29.GlaxoSmithKline. 2021. JULUCA (dolutegravir and rilpivirine) tablets, for oral use. GlaxoSmithKline, Research Triangle Park, NC. [Google Scholar]
- 30.Tsiang M, Jones GS, Goldsmith J, Mulato A, Hansen D, Kan E, Tsai L, Bam RA, Stepan G, Stray KM, Niedziela-Majka A, Yant SR, Yu H, Kukolj G, Cihlar T, Lazerwith SE, White KL, Jin H. 2016. Antiviral activity of bictegravir (gs-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother 60:7086–7097. 10.1128/AAC.01474-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Medicines Agency (EMA). 2011. Assessment report: edurant. International non-proprietary name: rilpivirine. Procedure no. EMEA/H/C/002264. European Medicines Agency, Amsterdam, the Netherlands. [Google Scholar]
- 32.Lee WA, He G-X, Eisenberg E, Cihlar T, Swaminathan S, Mulato A, Cundy KC. 2005. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother 49:1898–1906. 10.1128/AAC.49.5.1898-1906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Custodio J, West SK, Lutz J, Vu A, Xiao D, Collins S, Das M, Kearney BP, Mathias A. 2017. Twice daily administration of tenofovir alafenamide in combination with rifampin: potential for tenofovir alafenamide use in HIV-TB coinfection. Presentation. https://www.natap.org/2017/EACS/EACS_47.htm.
- 34.Callebaut C, Liu Y, Babusis D, Ray A, Miller M, Kitrinos K. 2017. Viability of primary osteoblasts after treatment with tenofovir alafenamide: lack of cytotoxicity at clinically relevant drug concentrations. PLoS One 12:e0169948. 10.1371/journal.pone.0169948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickinson L, Yapa HM, Jackson A, Moyle G, Else L, Amara A, Khoo S, Back D, Karolia Z, Higgs C, Boffito M. 2015. Plasma tenofovir, emtricitabine, and rilpivirine and intracellular tenofovir diphosphate and emtricitabine triphosphate pharmacokinetics following drug intake cessation. Antimicrob Agents Chemother 59:6080–6086. 10.1128/AAC.01441-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuen GJ, Lou Y, Bumgarner NF, Bishop JP, Smith GA, Otto VR, Hoelscher DD. 2004. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob Agents Chemother 48:176–182. 10.1128/AAC.48.1.176-182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Else LJ, Jackson A, Puls R, Hill A, Fahey P, Lin E, Amara A, Siccardi M, Watson V, Tjia J, Emery S, Khoo S, Back DJ, Boffito M. 2012. Pharmacokinetics of lamivudine and lamivudine-triphosphate after administration of 300 milligrams and 150 milligrams once daily to healthy volunteers: results of the ENCORE 2 study. Antimicrob Agents Chemother 56:1427–1433. 10.1128/AAC.05599-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schinazi RF, Lloyd RM, Jr, Nguyen M-HH, Cannon DL, McMillan A, Ilksoy N, Chu CK, Liotta DC, Bazmi HZ, Mellors JW. 1993. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother 37:875–881. 10.1128/AAC.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tisdale M, Kemp SD, Parry NR, Larder BA. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3'-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA 90:5653–5656. 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wainberg MA, Moisi D, Oliveira M, Toni TD, Brenner BG. 2011. Transmission dynamics of the M184V drug resistance mutation in primary HIV infection. J Antimicrob Chemother 66:2346–2349. 10.1093/jac/dkr291. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, Foster SA, Hazen RJ, Miki S, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A, Fujiwara T. 2011. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother 55:813–821. 10.1128/AAC.01209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andreatta K, Chang S, Martin R, Willkom M, White KL. 2018. Integrase inhibitor resistance selections initiated with drug resistant HIV-1. Poster 546. https://www.natap.org/2018/CROI/croi_134.htm.
- 43.Winters MA, Lloyd RM, Jr, Shafer RW, Kozal MJ, Miller MD, Holodniy M. 2012. Development of elvitegravir resistance and linkage of integrase inhibitor mutations with protease and reverse transcriptase resistance mutations. PLoS One 7:e40514. 10.1371/journal.pone.0040514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennings PS, Kryazhimskiy S, Wakeley J. 2014. Loss and recovery of genetic diversity in adapting populations of HIV. PLoS Genet 10:e1004000. 10.1371/journal.pgen.1004000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quashie PK, Mesplede T, Han YS, Oliveira M, Singhroy DN, Fujiwara T, Underwood MR, Wainberg MA. 2012. Characterization of the R263K mutation in HIV-1 integrase that confers low-level resistance to the second-generation integrase strand transfer inhibitor dolutegravir. J Virol 86:2696–2705. 10.1128/JVI.06591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mesplede T, Quashie PK, Osman N, Han Y, Singhroy DN, Lie Y, Petropoulos CJ, Huang W, Wainberg MA. 2013. Viral fitness cost prevents HIV-1 from evading dolutegravir drug pressure. Retrovirology 10:22. 10.1186/1742-4690-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SJ, Zhao XZ, Burke TR, Jr, Hughes SH. 2018. Efficacies of cabotegravir and bictegravir against drug-resistant HIV-1 integrase mutants. Retrovirology 15:37. 10.1186/s12977-018-0420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, Richmond G, Buendia CB, Fourie J, Ramgopal M, Hagins D, Felizarta F, Madruga J, Reuter T, Newman T, Small CB, Lombaard J, Grinsztejn B, Dorey D, Underwood M, Griffith S, Min S, Extended SAILING Study Team. 2013. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 382:700–708. 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 49.Naeger LK, Harrington P, Komatsu T, Deming D. 2016. Effect of dolutegravir functional monotherapy on HIV-1 virological response in integrase strand transfer inhibitor resistant patients. Antivir Ther 21:481–488. 10.3851/IMP3033. [DOI] [PubMed] [Google Scholar]
- 50.Oldenbuettel C, Wolf E, Ritter A, Noe S, Heldwein S, Pascucci R, Wiese C, Von Krosigk A, Jaegel-Guedes E, Jaeger H, Balogh A, Koegl C, Spinner CD. 2016. Dolutegravir monotherapy as treatment de-escalation in hiv-infected adults with virological control: dolumono cohort results. Antivir Ther 22:169–172. 10.3851/IMP3082. [DOI] [PubMed] [Google Scholar]
- 51.Blanco JL, Rojas J, Paredes R, Negredo E, Mallolas J, Casadella M, Clotet B, Gatell JM, de Lazzari E, Martinez E, Team DS. 2018. Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother. 10.1093/jac/dky093. [DOI] [PubMed] [Google Scholar]
- 52.McCluskey SM, Siedner MJ, Marconi VC. 2019. Management of virologic failure and HIV drug resistance. Infect Dis Clin North Am 33:707–742. 10.1016/j.idc.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gulick RM. 2002. Structured treatment interruption in patients infected with HIV: a new approach to therapy. Drugs 62:245–253. 10.2165/00003495-200262020-00001. [DOI] [PubMed] [Google Scholar]
- 54.Montaner J, Harris M, Hogg R. 2005. Structured treatment interruptions: a risky business. Clin Infect Dis 40:601–603. 10.1086/427707. [DOI] [PubMed] [Google Scholar]
- 55.Achieng L, Musangi H, Billingsley K, Onguit S, Ombegoh E, Bryant L, Mwiindi J, Smith N, Keiser P. 2013. The use of pill counts as a facilitator of adherence with antiretroviral therapy in resource limited settings. PLoS One 8:e67259. 10.1371/journal.pone.0067259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White KL, Osman N, Cuadra-Foy E, Brenner BG, Shivakumar D, Campigotto F, Tsiang M, Morganelli PA, Novikov N, Lazerwith SE, Jin H, Niedziela-Majka A. 2021. Long dissociation of bictegravir from HIV-1 integrase-DNA complexes. Antimicrob Agents Chemother 65:e02406-20. 10.1128/AAC.02406-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cook NJ, Li W, Berta D, Badaoui M, Ballandras-Colas A, Nans A, Kotecha A, Rosta E, Engelman AN, Cherepanov P. 2020. Structural basis of second-generation HIV integrase inhibitor action and viral resistance. Science 367:806–810. 10.1126/science.aay4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliveira M, Ibanescu RI, Anstett K, Mesplede T, Routy JP, Robbins MA, Brenner BG, Montreal Primary H, the Montreal Primary HIV (PHI) Cohort Study Group. 2018. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology 15:56. 10.1186/s12977-018-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith SJ, Zhao XZ, Passos DO, Lyumkis D, Burke TR, Jr, Hughes SH. 2020. HIV-1 integrase inhibitors that are active against drug-resistant integrase mutants. Antimicrob Agents Chemother 64:e00611-20. 10.1128/AAC.00611-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daar ES, DeJesus E, Ruane P, Crofoot G, Oguchi G, Creticos C, Rockstroh JK, Molina JM, Koenig E, Liu YP, Custodio J, Andreatta K, Graham H, Cheng A, Martin H, Quirk E. 2018. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV 5:e347–e356. 10.1016/S2352-3018(18)30091-2. [DOI] [PubMed] [Google Scholar]
- 61.Molina JM, Ward D, Brar I, Mills A, Stellbrink HJ, Lopez-Cortes L, Ruane P, Podzamczer D, Brinson C, Custodio J, Liu H, Andreatta K, Martin H, Cheng A, Quirk E. 2018. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV 5:e357–e365. 10.1016/S2352-3018(18)30092-4. [DOI] [PubMed] [Google Scholar]
- 62.van Wyk J, Ajana F, Bisshop F, De Wit S, Osiyemi O, Portilla J, Routy JP, Wyen C, Ait-Khaled M, Nascimento MC, Pappa KA, Wang R, Wright J, Tenorio AR, Wynne B, Aboud M, Gartland MJ, Smith KY. 2020. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose two-drug regimen versus continuing a tenofovir alafenamide-based three- or four-drug regimen for maintenance of virologic suppression in adults with HIV-1: phase 3, randomized, non-inferiority TANGO study. Clin Infect Dis 71:1920–1929. 10.1093/cid/ciz1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. 2019. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. PPA 13:475–490. 10.2147/PPA.S192735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sax PE, Althoff KN, Eron JJ, Radtchenko J, Diaz-Cuervo H, Mounzer K, Ramgopal M, Santiago S, Elion RA. 2020. Impact of adherence on viral suppression with bictegravir- and dolutegravir (DTG)-containing triple therapy in clinical practice. Poster P029. https://www.natap.org/2020/GLASGOW/GLASGOW_16.htm.
- 65.Llibre JM, Hung CC, Brinson C, Castelli F, Girard PM, Kahl LP, Blair EA, Angelis K, Wynne B, Vandermeulen K, Underwood M, Smith K, Gartland M, Aboud M. 2018. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 391:839–849. 10.1016/S0140-6736(17)33095-7. [DOI] [PubMed] [Google Scholar]
- 66.Aboud M, Orkin C, Podzamczer D, Bogner JR, Baker D, Khuong-Josses MA, Parks D, Angelis K, Kahl LP, Blair EA, Adkison K, Underwood M, Matthews JE, Wynne B, Vandermeulen K, Gartland M, Smith K. 2019. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV 6:e576–e587. 10.1016/S2352-3018(19)30149-3. [DOI] [PubMed] [Google Scholar]
- 67.Capetti AF, Cossu MV, Sterrantino G, Barbarini G, Di Giambenedetto S, De Socio GV, Orofino G, Di Biagio A, Celesia BM, Rusconi S, Argenteri B, Rizzardini G. 2018. Dolutegravir plus rilpivirine as a switch option in cART-experienced patients: 96-week data. Ann Pharmacother 52:740–746. 10.1177/1060028018761600. [DOI] [PubMed] [Google Scholar]
- 68.Lepik KJ, Harrigan PR, Yip B, Wang L, Robbins MA, Zhang WW, Toy J, Akagi L, Lima VD, Guillemi S, Montaner JSG, Barrios R. 2017. Emergent drug resistance with integrase strand transfer inhibitor-based regimens. AIDS 31:1425–1434. 10.1097/QAD.0000000000001494. [DOI] [PubMed] [Google Scholar]
- 69.Pena MJ, Chueca N, D'Avolio A, Zarzalejos JM, Garcia F. 2019. Virological failure in HIV to triple therapy with dolutegravir-based firstline treatment: rare but possible. Open Forum Infect Dis 6:ofy332. 10.1093/ofid/ofy332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fulcher JA, Du Y, Zhang TH, Sun R, Landovitz RJ. 2018. Emergence of integrase resistance mutations during initial therapy containing dolutegravir. Clin Infect Dis 67:791–794. 10.1093/cid/ciy228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lubke N, Jensen B, Huttig F, Feldt T, Walker A, Thielen A, Daumer M, Obermeier M, Kaiser R, Knops E, Heger E, Sierra S, Oette M, Lengauer T, Timm J, Haussinger D. 2019. Failure of dolutegravir first-line ART with selection of virus carrying R263K and G118R. N Engl J Med 381:887–889. 10.1056/NEJMc1806554. [DOI] [PubMed] [Google Scholar]
- 72.Siliciano RF, Greene WC. 2011. HIV latency. Cold Spring Harb Perspect Med 1:a007096. 10.1101/cshperspect.a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, Beilman GJ, Khoruts A, Thorkelson A, Schmidt TE, Anderson J, Perkey K, Stevenson M, Perelson AS, Douek DC, Haase AT, Schacker TW. 2014. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci USA 111:2307–2312. 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haertle T, Carrera CJ, Wasson DB, Sowers LC, Richman DD, Carson DA. 1988. Metabolism and anti-human immunodeficiency virus-1 activity of 2-halo-2’,3’-dideoxyadenosine derivatives. J Biol Chem 263:5870–5875. 10.1016/S0021-9258(18)60646-5. [DOI] [PubMed] [Google Scholar]
- 75.Harada S, Koyanagi Y, Yamamoto N. 1985. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229:563–566. 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 76.Shi C, Mellors JW. 1997. A recombinant retroviral system for rapid in vivo analysis of human immunodeficiency virus type 1 susceptibility to reverse transcriptase inhibitors. Antimicrob Agents Chemother 41:2781–2785. 10.1128/AAC.41.12.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mo H, Yang C, Wang K, Wang Y, Huang M, Murray B, Qi X, Sun SC, Deshpande M, Rhodes G, Miller MD. 2011. Estimation of inhibitory quotient using a comparative equilibrium dialysis assay for prediction of viral response to hepatitis C virus inhibitors. J Viral Hepat 18:338–348. 10.1111/j.1365-2893.2010.01314.x. [DOI] [PubMed] [Google Scholar]
- 78.Svarovskaia ES, Dvory-Sobol H, Parkin N, Hebner C, Gontcharova V, Martin R, Ouyang W, Han B, Xu S, Ku K, Chiu S, Gane E, Jacobson IM, Nelson DR, Lawitz E, Wyles DL, Bekele N, Brainard D, Symonds WT, McHutchison JG, Miller MD, Mo H. 2014. Infrequent development of resistance in genotype 1–6 hepatitis C virus-infected subjects treated with sofosbuvir in phase 2 and 3 clinical trials. Clin Infect Dis 59:1666–1674. 10.1093/cid/ciu697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Donaldson EF, Harrington PR, O'Rear JJ, Naeger LK. 2015. Clinical evidence and bioinformatics characterization of potential hepatitis C virus resistance pathways for sofosbuvir. Hepatology 61:56–65. 10.1002/hep.27375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequencing data related to this study have been deposited in NCBI under BioProject no. PRJNA812639.