ABSTRACT
Drug resistance mutations tend to disrupt key physiological processes and frequently carry fitness costs, which are a central determinant of the rate of spread of these mutations in natural populations. Head-to-head competition assays provide a standard approach to measuring fitness for malaria parasites. These assays typically use a standardized culture medium containing RPMI 1640, which has a 1.4- to 5.5-fold higher concentration of amino acids than human blood. In this rich medium, we predict that fitness costs will be underestimated because resource competition is weak. We tested this prediction using an artemisinin-sensitive parasite edited to contain kelch-C580Y or R561H mutations conferring resistance to artemisinin or synonymous control mutations. We examined the impact of these single amino acid mutations on fitness, using replicated head-to-head competition experiments conducted in media containing (i) normal RPMI, (ii) modified RPMI with reduced amino acid concentration, (iii) RPMI containing only isoleucine, or (iv) 3-fold diluted RPMI. We found a significant 1.3- to 1.4-fold increase in fitness costs measured in modified and isoleucine-only media relative to normal media, while fitness costs were 2.5-fold higher in diluted media. We conclude that fitness costs are strongly affected by media composition and will be significantly underestimated in normal RPMI. Several components differed between media, including pABA and sodium bicarbonate concentrations, so we cannot directly determine which is responsible. Elevated fitness costs in nature will limit spread of artemisinin (ART) resistance but will also promote evolution of compensatory mutations that restore fitness and can be exploited to maximize selection in laboratory experiments.
KEYWORDS: resource limitation, Plasmodium falciparum, artemisinin resistance, fitness costs, compensation, artemisinin, nutrient limitation, compensatory mutation, competition
INTRODUCTION
Drug resistance mutations typically carry a fitness cost: pathogens carrying these mutations tend to be outcompeted by wild-type pathogens when they are in direct competition, but they have a fitness advantage over wild-type parasites when exposed to drug treatment (1, 2). Such fitness costs occur because drug resistance mutations interfere with key metabolic pathways; for example, in the case of the malaria parasite Plasmodium falciparum, mutations conferring resistance to chloroquine in pfcrt reduce peptide transport capacity (3), kelch13 mutations underlying artemisinin resistance reduce levels of endocytosis (4), and dihydrofolate reductase mutations reduce efficiency of folate synthesis (5, 6). Fitness costs associated with chloroquine resistance provide an explanation for the rapid disappearance of drug resistance (pfcrt) alleles from Malawi and other countries after removal of chloroquine as first-line treatment (7, 8). Fitness costs may also help explain why resistance alleles have historically evolved in low-transmission regions such as Southeast Asia and South America, rather than in sub-Saharan Africa where most parasites are found (9). In such low-transmission regions, most infections contain single-parasite genotypes (10), so direct competition between drug-resistant and drug-sensitive parasites is minimized, allowing emergence and transmission of drug-resistant mutations to new hosts (11). Fitness costs are also an important driver of compensatory changes that ameliorate fitness (12, 13); if costs are high, then there is a strong selection for compensatory changes, while if costs are low, such compensatory changes are less likely to emerge (2, 14).
Fitness costs are now widely measured in the laboratory in studies of antimalarial drug resistance (15–19). This is typically done using competition experiments in which resistant and sensitive parasites have competed in culture, and their proportions are monitored over time to calculate selection coefficients, which measure the relative fitness advantage of each generation (19). For example, parasite clones with a selection coefficient of −0.1 produced 10% less offspring each generation than the competing parasite. A concern is that the magnitude of fitness costs determined using competition-based assays are likely to be dependent on the medium used. Limitation of critical resources (e.g., nutrients, space, light) determines the strength of competition between organisms (20). Therefore, the strength of competition and size of fitness costs measured are expected to depend on the degree of resource limitation (21, 22). There is strong precedent for the role of nutrient limitation in mediating bacterial competition (23–25). Furthermore, comparisons of nonisogenic artemisinin-resistant and -sensitive parasites from Cambodia have suggested that fitness costs are exacerbated in conditions of amino acid starvation (26).
RPMI 1640, a nutrient-rich medium designed to maximize growth of multiple cell types, forms the basis of malaria culture media. Red blood cells, and a lipid source—either human serum or a bovine serum substitute (AlbuMAX)—are then added to RPMI to make complete media. The resultant complete medium contains much higher levels of many nutrients than human blood (27–29). For example, amino acids are present at concentrations 1.4- to 5.5-fold (mean, 2.6-fold) greater than observed in human blood. Of these amino acids, isoleucine is critical for malaria parasite growth (30) because other amino acids can be obtained from hemoglobin digestion (31). However, isoleucine is found at a 5.5-fold greater concentration in RPMI than in human blood (Table S1 in the supplemental material) (27, 28).
These experiments were designed to investigate the impact of medium composition on measurement of fitness costs of antimalarial drug resistance, with a focus on amino acid composition. We expect that the strength of competition between parasites in competitive growth assays will depend on resource limitation: competition will be greatest when resources such as isoleucine are limited, while when resources are abundant, competition will be minimized. We also expect that metabolically impaired drug-resistant parasites will be disproportionately affected compared with wild-type parasites when nutrients are limited but will suffer minimal costs when nutrients are plentiful. We predicted that fitness costs associated with drug resistance will be underestimated when measured in media containing normal RPMI compared with media containing amino levels more comparable to that found in human blood. We examined two different artemisinin (ART) resistance mutations in this work, kelch-C580Y, the predominant ART-resistant (ART-R) mutation in much of Southeast Asia (32), and kelch-R561H, which is circulating at lower levels in Southeast Asia but has recently arisen in Rwandan populations (33–35).
RESULTS
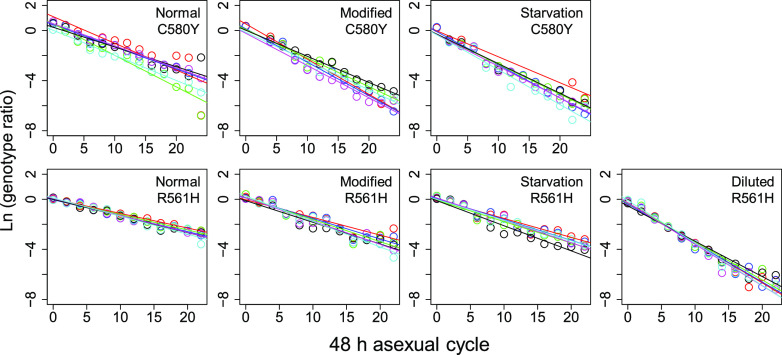
The experimental design is summarized in Fig. 1. The final data set comprised fitness cost measures for the R561H mutation in 4 different medium types and for the C580Y mutation in 3 different medium types (we did not conduct competition using 3-fold diluted RPMI for this mutation). Each of these 7 competition assays had 6 replicates, with 15 aliquots collected at 4-day intervals over 2 months. The amplicon sequence data used to quantify frequencies of the competing parasites are summarized in Table S2 in the supplemental material. We calculated allele frequencies from 5,885 ± 3,421 reads per sampling time point after excluding time points with <100 reads. We then estimated relative fitness of the competing parasites, by measuring the slope of the best fit line of ln (parasite ratio) against time measured in asexual cycles (Fig. 2).
FIG 1.
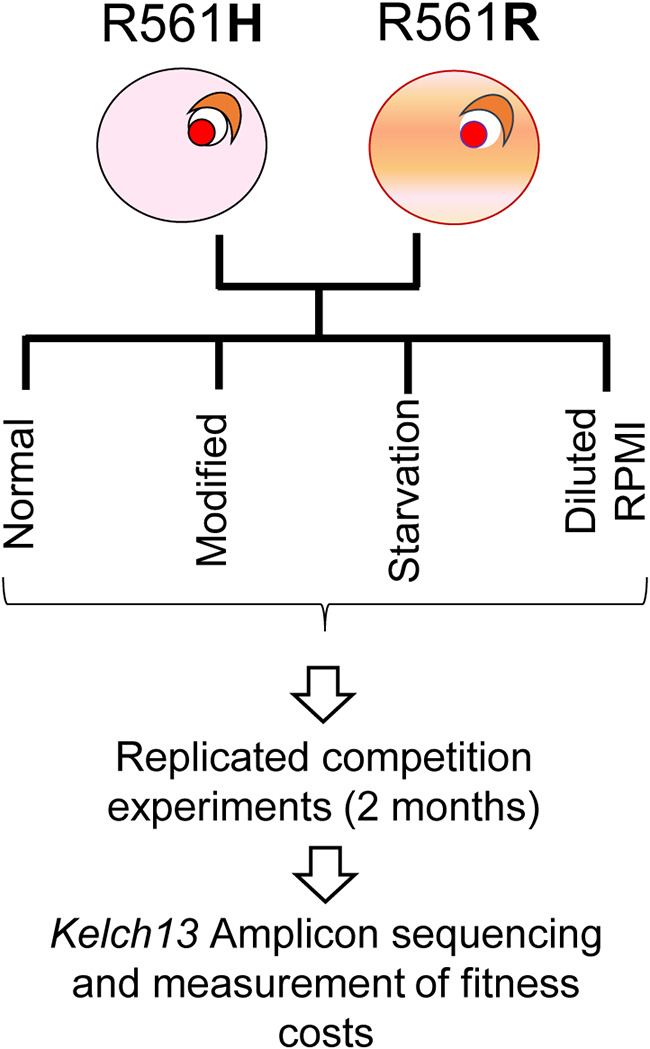
Overview of project design. CRISPR modified parasites with either wild type or kelch13-R561H were mixed in 50:50 proportions, and replicated competition experiments were conducted in 4 different media types over 2 months. We used amplicon sequencing to determine frequencies of competing parasites and determine selection coefficients. There experiments were also conducted with wild type and C580Y.
FIG 2.
Trajectory of competing parasites observed in replicate experiments. The plots show the natural log of the parasite ratio against time between competing parasites. The results shown are for the ART-R mutant (C580Y or R561H) compared to the isogenic ART-S clones (C580C or R561R) for each variant amino acid. The slope for the least-squares fit provides an estimate of the selection coefficient (s). Each color represents one replicate.
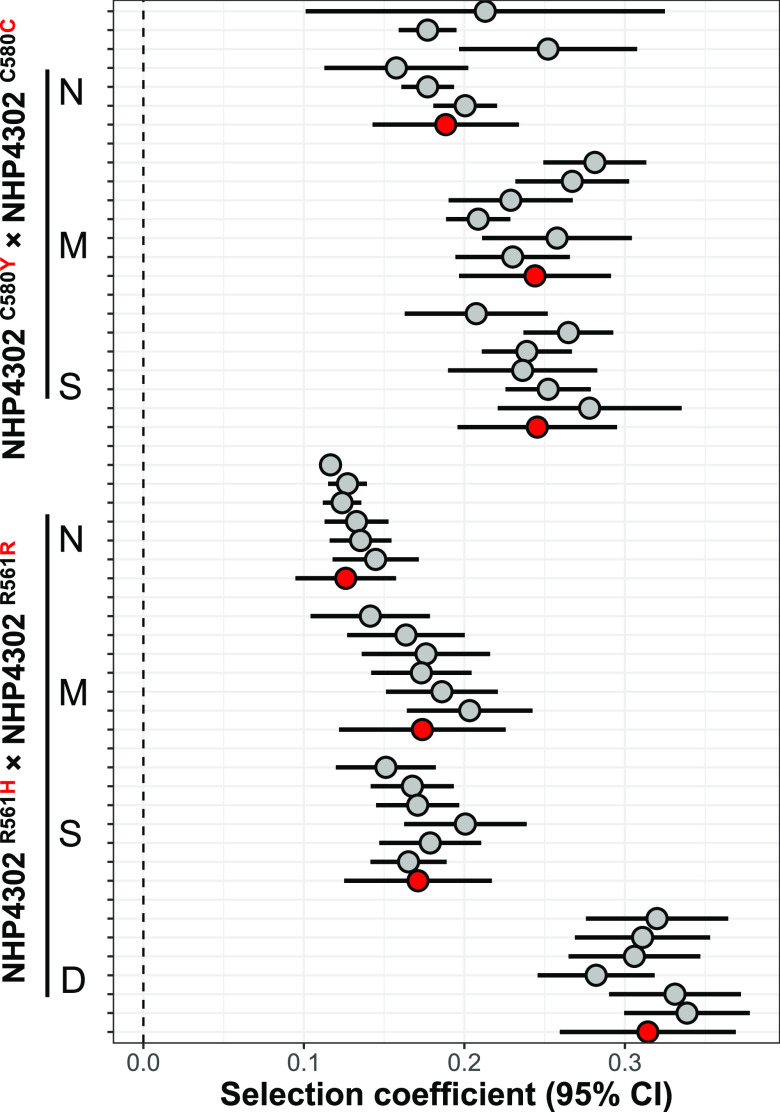
We found a significant impact of media composition on fitness costs for both R561H and C580Y (Fig. 3, Table 1, and Table 2). Fitness costs were lowest in media containing normal RPMI but were 1.3-fold higher in both modified (t test, P = 0.0192) and starvation media (t test, P = 0.0148) for C580Y. We observed parallel changes for R561H, with a 1.38-fold increase in fitness costs in modified media (t test, P = 9.01 × 10−04) and a 1.5-fold increase in starvation media (t test, 3.10 × 10−04) (Fig. 2 and Table 2). We did not see any significant difference between modified and starvation media for either R561H (t test, P = 0.89) or C580Y (t test, P = 0.97).
FIG 3.
Outcome of competition experiments in different media. The plot shows selection coefficients (s) with 95% confidence intervals for competition experiments conducted in different media. Six replicate competition experiments were conducted for each set. Grey points shows selection coefficients for each replicate, while the red points show meta-analysis results for each experimental comparison. The “winning” parasites (NHP4302C580C or NHP4302R561R) are shown on the right for each comparison.
TABLE 1.
Calculation and summary for selection coefficients of all competition experimentsa
| Competition expt | Medium | Assay ID | s | SE | R 2 | n | P |
|---|---|---|---|---|---|---|---|
| NHP4302C580Y (ART-R) vs NHP4302C580C (ART-S) | Standard | A.1 | 0.2130 | 0.0495 | 0.6734 | 9 | 1.97E-03 |
| A.2 | 0.1771 | 0.0081 | 0.9797 | 10 | 8.61E-10 | ||
| A.3 | 0.2521 | 0.0245 | 0.9217 | 9 | 2.82E-06 | ||
| A.4 | 0.1576 | 0.0201 | 0.8601 | 10 | 1.41E-05 | ||
| A.5 | 0.1771 | 0.0074 | 0.9830 | 10 | 3.50E-10 | ||
| A.6 | 0.2005 | 0.0089 | 0.9807 | 10 | 6.67E-10 | ||
| A.meta | 0.1884 | 0.0456 | 0.8013 | 68 | 1.01E-33 | ||
| Modified | B.1 | 0.2813 | 0.0101 | 0.9962 | 3 | 1.01E-04 | |
| B.2 | 0.2672 | 0.0159 | 0.9659 | 10 | 1.16E-08 | ||
| B.3 | 0.2289 | 0.0171 | 0.9522 | 9 | 3.01E-07 | ||
| B.4 | 0.2086 | 0.0088 | 0.9841 | 9 | 2.11E-09 | ||
| B.5 | 0.2577 | 0.0206 | 0.9455 | 9 | 5.46E-07 | ||
| B.6 | 0.2301 | 0.0155 | 0.9652 | 8 | 4.07E-07 | ||
| B.meta | 0.2440 | 0.0473 | 0.9271 | 58 | 9.39E-33 | ||
| Starvation | C.1 | 0.2074 | 0.0160 | 0.9766 | 4 | 2.07E-04 | |
| C.2 | 0.2648 | 0.0126 | 0.9780 | 10 | 1.29E-09 | ||
| C.3 | 0.2389 | 0.0126 | 0.9730 | 10 | 3.60E-09 | ||
| C.4 | 0.2363 | 0.0167 | 0.9803 | 4 | 1.46E-04 | ||
| C.5 | 0.2522 | 0.0119 | 0.9781 | 10 | 1.24E-09 | ||
| C.6 | 0.2780 | 0.0249 | 0.9399 | 8 | 3.65E-06 | ||
| C.meta | 0.2454 | 0.0497 | 0.9418 | 56 | 3.35E-32 | ||
| NHP4302R561H (ART-R) vs NHP4302R561R (ART-S) | Standard | A.1 | 0.1166 | 0.0030 | 0.9934 | 10 | 3.01E-12 |
| A.2 | 0.1272 | 0.0054 | 0.9821 | 10 | 4.50E-10 | ||
| A.3 | 0.1238 | 0.0054 | 0.9815 | 9 | 5.44E-10 | ||
| A.4 | 0.1328 | 0.0088 | 0.9620 | 9 | 1.07E-07 | ||
| A.5 | 0.1353 | 0.0081 | 0.9753 | 7 | 6.93E-07 | ||
| A.6 | 0.1447 | 0.0121 | 0.9352 | 10 | 2.90E-07 | ||
| A.meta | 0.1261 | 0.0313 | 0.9558 | 66 | 2.74E-42 | ||
| Modified | B.1 | 0.1414 | 0.0167 | 0.8778 | 10 | 7.08E-06 | |
| B.2 | 0.1636 | 0.0164 | 0.9083 | 10 | 1.66E-06 | ||
| B.3 | 0.1761 | 0.0177 | 0.9169 | 9 | 3.68E-06 | ||
| B.4 | 0.1732 | 0.0140 | 0.9385 | 10 | 2.23E-07 | ||
| B.5 | 0.1860 | 0.0156 | 0.9341 | 10 | 3.15E-07 | ||
| B.6 | 0.2033 | 0.0173 | 0.9389 | 9 | 9.14E-07 | ||
| B.meta | 0.1738 | 0.0519 | 0.8974 | 68 | 9.06E-29 | ||
| Starvation | C.1 | 0.1511 | 0.0140 | 0.9213 | 10 | 7.71E-07 | |
| C.2 | 0.1676 | 0.0116 | 0.9542 | 10 | 5.08E-08 | ||
| C.3 | 0.1709 | 0.0116 | 0.9559 | 10 | 4.19E-08 | ||
| C.4 | 0.2006 | 0.0171 | 0.9322 | 10 | 3.64E-07 | ||
| C.5 | 0.1787 | 0.0130 | 0.9694 | 6 | 9.09E-06 | ||
| C.6 | 0.1652 | 0.0107 | 0.9601 | 10 | 2.54E-08 | ||
| C.meta | 0.1711 | 0.0460 | 0.9157 | 66 | 7.91E-33 | ||
| Diluted | D.1 | 0.3200 | 0.0199 | 0.9629 | 10 | 1.76E-08 | |
| D.2 | 0.3110 | 0.0189 | 0.9643 | 10 | 1.44E-08 | ||
| D.3 | 0.3059 | 0.0181 | 0.9694 | 9 | 4.04E-08 | ||
| D.4 | 0.2821 | 0.0161 | 0.9715 | 9 | 2.92E-08 | ||
| D.5 | 0.3312 | 0.0182 | 0.9737 | 9 | 2.03E-08 | ||
| D.6 | 0.3387 | 0.0173 | 0.9771 | 9 | 1.09E-08 | ||
| D.meta | 0.3143 | 0.0548 | 0.9648 | 66 | 7.86E-39 |
Selection coefficients (s) (mean ± standard error) were measured as in Fig. 2. For each combination, we conducted six independent competition assays (assay ID). The final three columns report the correlation coefficient (R2), the number of time points at which genotype frequencies were measured (n), and the statistical significance (P) for a test of the null hypothesis that the slope is not different from zero. The winning parasite clone is shown on the right for each comparison. Time points were not included in the analysis for three reasons, (i) where the relationship between ln (p/q) and time was nonlinear, (ii) where one of the competing parasites had spread to fixation, or (iii) where we obtained insufficient reads (<100) from a particular sample to accurately measure allele frequencies due to slight unevenness in pooling of amplicons (see Materials and Methods).
TABLE 2.
Pairwise comparison of fitness costs (s) of drug resistance mutations measured in different mediaa
| Mutation and medium | Standard | Modified | Starvation | Diluted |
|---|---|---|---|---|
| C580Y | ||||
| Standard | 0.019 | 0.015 | NDb | |
| Modified | 1.295 | 0.967 | ND | |
| Starvation | 1.303 | 1.006 | ND | |
| Diluted | ND | ND | ND | |
| R561H | ||||
| Standard | 9.01E-04 | 3.10E-04 | 1.94E-09 | |
| Modified | 1.38 | 0.89 | 3.18E-07 | |
| Starvation | 1.36 | 0.98 | 1.04E-07 | |
| Diluted | 2.49 | 1.81 | 1.84 |
Upper triangle shows significance of t tests comparing fitness cost measured in different medium types. Light gray shading, P < 0.05; dark gray shading, P < 0.001. The lower triangle shows fold change in fitness costs measured in different media. Results are summarized graphically in Fig. 3, while all replicates are compared statistically in Table 1.
ND, not determined.
We found the largest fitness cost differences between normal RPMI and 3-fold diluted RPMI. In this medium, R561H showed a fitness cost of 0.31 ± 0.05 compared with 0.13 ± 0.03 in normal RPMI (t test, P = 1.94 × 10−09). This represents a 2.49-fold difference in fitness costs depending on medium composition used (Table 2).
DISCUSSION
Nutrient limitation increases fitness costs in P. falciparum.
These experiments were designed to test the prediction that fitness costs are dependent on the nutrient composition of parasite growth media. We observed a modest (1.29- to 1.38-fold) but significant increase in selection costs when parasites were grown in media containing reduced amino acid composition designed to mimic that found in human blood. This was observed for both C580Y and R561H mutations, providing strong support for this hypothesis.
Malaria parasites are able to synthesize all amino acids except for isoleucine (30, 31); hence, we would expect that isoleucine concentrations might be of particular importance. However, as artemisinin-resistant parasites show reduced endocytosis of hemoglobin compared to wild-type parasites (4), access to amino acids synthesized from products of hemoglobin digestion may be also limited in ART-R parasites. Interestingly, we found that starvation media, containing isoleucine only in concentrations mimicking normal blood, also resulted in comparable increases in fitness costs to that observed in modified media containing 7 amino acids. These results are consistent with isoleucine levels being sufficient to cause the observed changes in fitness costs.
Threefold diluted RPMI contains slightly higher isoleucine concentrations than the modified and starvation media (Table S1), so it would be expected to have a less pronounced impact on fitness measures if isoleucine is solely responsible. However, diluted media had a massive (2.5-fold) impact on fitness coefficients, suggesting that limitation of other RPMI components impacts fitness coefficients. We used Hanks’ balanced salt solution as a diluent for RPMI. This matches the salt concentration of RPMI 1640 but lacks several trace elements and has a lower concentration of NaHCO3. We suggest two possible reasons for the elevated fitness costs. (i) One or more of these trace elements are critical for Plasmodium growth. A likely candidate is para-aminobenzoic acid (pABA), which is required for folate biosynthesis. As folate is needed for pyrimidine synthesis and methionine metabolism, pABA is critical for malaria parasite growth (36, 37). Previous studies have shown that pABA limits malaria parasite growth rates (37). Furthermore, manipulation of pABA levels alters the outcome of competition between pyrimethamine-resistant and -sensitive malaria parasites (Plasmodium chabaudi) in rodent malaria parasites, providing an in vivo demonstration of the importance of nutrient limitation in determining competitive outcomes (21, 22). (ii) The lower level of NaHCO3 in diluted (10.7 nM) versus normal RPMI (23.8 nM) could reduce buffering capacity of the media and influence relative fitness of parasites in competition.
Bunditvorapoom et al. (26) observed that differences in fitness were exacerbated in low-amino acid conditions in comparisons between two resistant and two sensitive parasites isolated from Cambodian patients. Ring-stage ART-susceptible (ART-S), but not ART-R, parasites were able to mature to trophozoites under low-amino acid conditions, while both ART-S and ART-R parasites made the ring-to-trophozoite transition under standard culture conductions. Our results confirm and extend this interesting observation by quantifying the impact of amino acid starvation on fitness in isogenic CRISPR-edited parasites.
Fitness costs are widely measured in the laboratory to understand why particular resistance mutations are spreading in nature. These studies are particularly powerful when combined with CRISPR because single mutations can be examined on the same genetic background (16–19). An important point is that fitness costs are likely to be underestimated by studies using normal RPMI because this is a rich medium designed to allow growth of multiple cell types or microbe species. We can obtain more accurate estimates of relative fitness using RPMI with reduced amino acid levels. However, we note that multiple aspects of media used in malaria culture (lipid source, immunity, movement) differ from that observed in human blood (27–29), so translating fitness cost measured in the laboratory to the situation within patients is problematic.
Comparisons with prior data.
We previously examined fitness costs of NHP4302C580Y (selection coefficient [s] = 0.15) and NHP4302R561H (s = 0.084) in normal RPMI 1640 with AlbuMAX (19). The results from the current study are consistent in showing higher fitness costs for NHP4302C580Y (s = 0.19) than NHP4302R561H (s = 0.13); however, the fitness costs are marginally higher in both cases. This results from improvements in our statistical analysis: we now analyze data only where the slope of the relationship between ln (proportion of resistant alleles) against time shows a good fit to a linear model. Reanalysis of the raw data from Nair et al. (19) using our new scripts (see Materials and Methods) provides revised fitness cost estimates (NHP4302C580Y, s = 0.163; NHP4302R561H, s = 0.124) that are comparable with those from the current study.
As the central aim of this project was to examine fitness costs associated with C580Y and R561H mutations in the absence of drug pressure, we did not conduct ring-stage assays (RSA) to examine survival following dihydroartemisinin (DHA) treatment in this study. However, Table S3 lists prior RSA data collected from parasites in which C580Y or R561H mutations were added or removed using CRISPR or zinc finger approaches.
Practical consequences of resource limitation.
The dramatic effects of resource limitation on competitive interactions between parasites can be effectively harnessed for experimental work. We provide three examples.
Measuring fitness costs in malaria parasites typically requires 1 to 2 months of coculture to resolve competitive outcomes. Resource limitation allows significant shortening of these experiments because fitness costs are magnified, and one of the two competitors will reach fixation. For example, in our experiments using the kelch-R561H resistance mutation conferring artemisinin resistance, parasites bearing the NHP4302R561H allele were still present on day 50 in normal media but were eliminated by day 26 in diluted media. Hence, we can shorten these experiments and better resolve small differences in fitness.
We have recently utilized malaria parasite genetic crosses to generate large-progeny pools, which can then be monitored over time to examine relative fitness of different parasite alleles (38). We have previously conducted these experiments in media containing normal RPMI. We expect that conducting such experiments in diluted RPMI will result in much more rapid detection of differentially selected alleles. Similar approaches are being used to evaluate the fitness consequences of piggyBac insertions in large-scale malaria mutagenesis libraries (39, 40). Again, these assays could be made more stringent by the use of diluted media to enhance competition.
Compensatory mutations, which reduce the fitness costs of deleterious resistance alleles, are thought to play a major role in resistance evolution (2, 12). In the case of pyrimethamine resistance, copy number amplification of GTP-cyclohydrolase I, the gene at the base of the folate biosynthesis cycle, compensates for reduced efficiency of dihydrofolate reductase later in this biochemical pathway (41, 42). However, rather few other examples are known in malaria. Magnifying the fitness costs of drug resistance alleles using nutrient limitation can accelerate evolution of compensatory changes in laboratory evolution studies. We are currently conducting evolution experiments using diluted media aimed at evolving compensatory changes following the introduction of CRISPR-generated drug resistance alleles.
MATERIALS AND METHODS
Parasites.
We used four parasite clones in this work, NHP4302C580Y, NHP4302C580C, NHP4302R561H, and NHP4302R561R. These were all generated using CRISPR/Cas9 editing by introducing nonsynonymous substitutions of key amino acids determining ART resistance (NHP4302C580Y and NHP4302R561H) or control synonymous mutations in the same codons, NHP4302C580C and NHP4302R561R (19). The original parasite used for editing (NHP4302) was cloned from a parasite isolate recovered from a patient visiting the Wang Pha clinic run by the Shoklo Malaria Research Unit (SMRU) in 2008. This isolate had a wild-type kelch13 allele and cleared rapidly from the infected patient’s blood following treatment (clearance rate half-life [T1/2P], 1.98 h) (19).
Medium composition.
These experiments measured the fitness costs of isogenic parasites with or without drug resistance mutations in the kelch13 locus, using media that differed in composition. We cultured asexual blood-stage parasites in culture medium (RPMI 1640 supplemented with 2 mM l-glutamine, 25 mM HEPES, and 50 g/L gentamicin, 0.1 M hypoxanthine, and 0.4% AlbuMAX as a serum source) at 2% hematocrit and maintained them at 37°C with 5% O2, 5% CO2, and 90% N2. However, the composition of the RPMI differed in these experiments (Table S1 in the supplemental material). In brief, the four medium types used were (i) normal RPMI 1640; (ii) modified RPMI containing reduced levels of amino acids that approximates that found in human blood (28); (iii) starvation RPMI that contained isoleucine only at the same levels as in modified media. Isoleucine is the only essential amino acid for P. falciparum growth; all others can be obtained from hemoglobin digestion (30, 31); and (iv) diluted RPMI that contained a 3-fold dilution of RPMI in Hanks’ basal salt solution (HBSS). HBSS contains salts at the same concentration as RPMI 1640 but lacks amino acids and trace elements found in RPMI and has lower levels of NaHCO3 (4.167 nM). Diluted RPMI therefore shows 3-fold lower levels of amino acids and trace elements than normal media and lower concentrations of NaHCO3 (10.714 nM) than normal RPMI (23.810 nM). Red blood cells from different donors may have a large influence on P. falciparum growth rates (43); we therefore used a single donor (O-positive [O+]) for all experiments.
Competition experiments.
We conducted two sets of competition experiments, (i) NHP4302C580Y (ART-R) against NHP4302C580C (ART-S), and (ii) NHP4302R561H (ART-R) against NHP4302R561R (ART-S). These experiments were conducted in the four medium types, with 6 replicates per experiment. In brief, cultures of the two parasite lines for comparison were (i) synchronized to 80% late schizonts using MACS purification columns (Miltenyi Biotec), (ii) diluted to 0.1% density and grown overnight to generate ring-stage parasites, and (iii) mixed in equal proportions at a density of 0.1%. We ran 6 replicates of each competition experiment in the wells of a 6-well plate. These were then maintained for 2 months, with removal of aliquots (80 μL packed red cells) and dilution down to 0.1% from each culture at 4-day intervals.
Selection coefficient estimation.
We used amplicon sequencing to determine frequencies of the two competing parasites in each experiment (19). In brief, we amplified a 249-bp region of the kelch13 locus from each sampling time point of the competition experiments. These were then Illumina sequenced (2 × 250-bp reads) to high read depth on a MiSeq. We used oligonucleotides with individual barcodes to allow pooling of PCR products from multiple different reactions for sequencing (Read1 sequence primer, TATGGTAATTGTACGTCAAATGGTAGAATTTATTGTATTGGGGGATATGATGGC; Read2 sequence primer, AGTCAGCCAGCCAGGCATATGGAAATTGTTC; index sequence primer, AATGATACGGCGACCACCGAGATCTACACGCT). We counted the number of reads from the two competing parasite lines to determine their frequency within each culture. We then plotted the natural log (Freq [resistant alleles]/Freq [sensitive alleles]) over time in asexual cycles (1 asexual cycle, 48 h) and measured the slope of the best-fit line to give the selection coefficient. Scripts used are provided in GitHub (https://github.com/emilyli0325/Amplicon.analysis.git); these have been updated from those used in reference 19. We remove data points (i) that only contains reads from one allele (allele frequency equals 0 or 1) and all data after those points; (ii) when we use Cook's distance (44) to detect outliers that do not fit the linear regression with a cutoff of 4/n; the first remaining data point was then defined as starting time point, asexual cycle 0; and (iii) where there were <100 reads, providing inaccurate measures of allele frequency.
Statistical analysis.
We analyzed the slopes from the 6 replicate cultures using the R program metaphor to conduct a random-effects analysis to determine the impact of medium type on fitness costs.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grant R37 AI048071 (to T.A.). Work at Texas Biomedical Research Institute was conducted in facilities constructed with support from Research Facilities Improvement Program grant C06 RR013556 from the National Center for Research Resources.
S.N., X.L., and T.A. designed the experiments. S.N. conducted experiments with assistance from M.M.-W. and M.F. X.L. performed all the next-generation sequencing (NGS) analysis and data curation. S.N., X.L., and T.A. wrote the original manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Andersson DI, Levin BR. 1999. The biological cost of antibiotic resistance. Curr Opin Microbiol 2:489–493. 10.1016/S1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 2.Durão P, Balbontín R, Gordo I. 2018. Evolutionary mechanisms shaping the maintenance of antibiotic resistance. Trends Microbiol 26:677–691. 10.1016/j.tim.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Shafik SH, Cobbold SA, Barkat K, Richards SN, Lancaster NS, Llinás M, Hogg SJ, Summers RL, McConville MJ, Martin RE. 2020. The natural function of the malaria parasite's chloroquine resistance transporter. Nat Commun 11:3922. 10.1038/s41467-020-17781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnbaum J, Scharf S, Schmidt S, Jonscher E, Hoeijmakers WAM, Flemming S, Toenhake CG, Schmitt M, Sabitzki R, Bergmann B, Fröhlke U, Mesén-Ramírez P, Blancke Soares A, Herrmann H, Bártfai R, Spielmann T. 2020. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science 367:51–59. 10.1126/science.aax4735. [DOI] [PubMed] [Google Scholar]
- 5.Sirawaraporn W, Sathitkul T, Sirawaraporn R, Yuthavong Y, Santi DV. 1997. Antifolate-resistant mutants of Plasmodium falciparum dihydrofolate reductase. Proc Natl Acad Sci USA 94:1124–1129. 10.1073/pnas.94.4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costanzo MS, Hartl DL. 2011. The evolutionary landscape of antifolate resistance in Plasmodium falciparum. J Genet 90:187–190. 10.1007/s12041-011-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Mu J, Li G, Chen P, Guo X, Fu L, Chen L, Su X, Wellems TE. 2005. Decreased prevalence of the Plasmodium falciparum chloroquine resistance transporter 76T marker associated with cessation of chloroquine use against P. falciparum malaria in Hainan, People's Republic of China. Am J Trop Med Hyg 72:410–414. 10.4269/ajtmh.2005.72.410. [DOI] [PubMed] [Google Scholar]
- 8.Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, Djimdé AA, Kouriba B, Taylor TE, Plowe CV. 2003. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis 187:1870–1875. 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 9.Ross LS, Fidock DA. 2019. Elucidating mechanisms of drug-resistant Plasmodium falciparum. Cell Host Microbe 26:35–47. 10.1016/j.chom.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nkhoma SC, Nair S, Al-Saai S, Ashley E, McGready R, Phyo AP, Nosten F, Anderson TJ. 2013. Population genetic correlates of declining transmission in a human pathogen. Mol Ecol 22:273–285. 10.1111/mec.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerqueira GC, Cheeseman IH, Schaffner SF, Nair S, McDew-White M, Phyo AP, Ashley EA, Melnikov A, Rogov P, Birren BW, Nosten F, Anderson TJC, Neafsey DE. 2017. Longitudinal genomic surveillance of Plasmodium falciparum malaria parasites reveals complex genomic architecture of emerging artemisinin resistance. Genome Biol 18:78. 10.1186/s13059-017-1204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Björkman J, Nagaev I, Berg OG, Hughes D, Andersson DI. 2000. Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science 287:1479–1482. 10.1126/science.287.5457.1479. [DOI] [PubMed] [Google Scholar]
- 13.Levin BR, Perrot V, Walker N. 2000. Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genetics 154:985–997. 10.1093/genetics/154.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartl DL, Clark AG, Sinauer A. 2018. Principles of population genetics. Sinauer Associates, Inc. Publishers, Sunderland, MA. [Google Scholar]
- 15.Duvalsaint M, Conrad MD, Tukwasibwe S, Tumwebaze PK, Legac J, Cooper RA, Rosenthal PJ. 2021. Balanced impacts of fitness and drug pressure on the evolution of PfMDR1 polymorphisms in Plasmodium falciparum. Malar J 20:292. 10.1186/s12936-021-03823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabryszewski SJ, Dhingra SK, Combrinck JM, Lewis IA, Callaghan PS, Hassett MR, Siriwardana A, Henrich PP, Lee AH, Gnädig NF, Musset L, Llinás M, Egan TJ, Roepe PD, Fidock DA. 2016. Evolution of fitness cost-neutral mutant PfCRT conferring P. falciparum 4-aminoquinoline drug resistance is accompanied by altered parasite metabolism and digestive vacuole physiology. PLoS Pathog 12:e1005976. 10.1371/journal.ppat.1005976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross LS, Dhingra SK, Mok S, Yeo T, Wicht KJ, Kümpornsin K, Takala-Harrison S, Witkowski B, Fairhurst RM, Ariey F, Menard D, Fidock DA. 2018. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat Commun 9:3314. 10.1038/s41467-018-05652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straimer J, Gnädig NF, Stokes BH, Ehrenberger M, Crane AA, Fidock DA. 2017. Plasmodium falciparum K13 mutations differentially impact ozonide susceptibility and parasite fitness in vitro. mBio 8:e00172-17. 10.1128/mBio.00172-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair S, Li X, Arya GA, McDew-White M, Ferrari M, Nosten F, Anderson TJC. 2018. Fitness costs and the rapid spread of kelch13-C580Y substitutions conferring artemisinin resistance. Antimicrob Agents Chemother 62:e00605-18. 10.1128/AAC.00605-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilman D. 1982. Resource competition and community structure. Monogr Popul Biol 17:1–296. [PubMed] [Google Scholar]
- 21.Wale N, Sim DG, Jones MJ, Salathe R, Day T, Read AF. 2017. Resource limitation prevents the emergence of drug resistance by intensifying within-host competition. Proc Natl Acad Sci USA 114:13774–13779. 10.1073/pnas.1715874115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wale N, Sim DG, Read AF. 2017. A nutrient mediates intraspecific competition between rodent malaria parasites in vivo. Proc Biol Sci 284:20171067. 10.1098/rspb.2017.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letten AD, Hall AR, Levine JM. 2021. Using ecological coexistence theory to understand antibiotic resistance and microbial competition. Nat Ecol Evol 5:431–441. 10.1038/s41559-020-01385-w. [DOI] [PubMed] [Google Scholar]
- 24.Paulander W, Maisnier-Patin S, Andersson DI. 2009. The fitness cost of streptomycin resistance depends on rpsL mutation, carbon source and RpoS (sigmaS). Genetics 183:539–546. 10.1534/genetics.109.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen A, Aarestrup FM, Olsen JE. 2009. The in vitro fitness cost of antimicrobial resistance in Escherichia coli varies with the growth conditions. FEMS Microbiol Lett 299:53–59. 10.1111/j.1574-6968.2009.01734.x. [DOI] [PubMed] [Google Scholar]
- 26.Bunditvorapoom D, Kochakarn T, Kotanan N, Modchang C, Kümpornsin K, Loesbanluechai D, Krasae T, Cui L, Chotivanich K, White NJ, Wilairat P, Miotto O, Chookajorn T. 2018. Fitness loss under amino acid starvation in artemisinin-resistant Plasmodium falciparum isolates from Cambodia. Sci Rep 8:12622. 10.1038/s41598-018-30593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeRoux M, Lakshmanan V, Daily JP. 2009. Plasmodium falciparum biology: analysis of in vitro versus in vivo growth conditions. Trends Parasitol 25:474–481. 10.1016/j.pt.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Desai SA. 2013. Insights gained from P. falciparum cultivation in modified media. ScientificWorldJournal 2013:363505. 10.1155/2013/363505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown AC, Guler JL. 2020. From circulation to cultivation: Plasmodium in vivo versus in vitro. Trends Parasitol 36:914–926. 10.1016/j.pt.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Istvan ES, Gluzman IY, Gross J, Goldberg DE. 2006. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc Natl Acad Sci USA 103:8840–8845. 10.1073/pnas.0601876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Istvan ES, Dharia NV, Bopp SE, Gluzman I, Winzeler EA, Goldberg DE. 2011. Validation of isoleucine utilization targets in Plasmodium falciparum. Proc Natl Acad Sci USA 108:1627–1632. 10.1073/pnas.1011560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson TJ, Nair S, McDew-White M, Cheeseman IH, Nkhoma S, Bilgic F, McGready R, Ashley E, Pyae Phyo A, White NJ, Nosten F. 2017. Population parameters underlying an ongoing soft sweep in Southeast Asian malaria parasites. Mol Biol Evol 34:131–144. 10.1093/molbev/msw228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uwimana A, Umulisa N, Venkatesan M, Svigel SS, Zhou Z, Munyaneza T, Habimana RM, Rucogoza A, Moriarty LF, Sandford R, Piercefield E, Goldman I, Ezema B, Talundzic E, Pacheco MA, Escalante AA, Ngamije D, Mangala JN, Kabera M, Munguti K, Murindahabi M, Brieger W, Musanabaganwa C, Mutesa L, Udhayakumar V, Mbituyumuremyi A, Halsey ES, Lucchi NW. 2021. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis 21:1120–1128. 10.1016/S1473-3099(21)00142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Straimer J, Gandhi P, Renner KC, Schmitt EK. 2021. High prevalence of P. falciparum K13 mutations in Rwanda is associated with slow parasite clearance after treatment with artemether-lumefantrine. J Infect Dis. 10.1093/infdis/jiab352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uwimana A, Legrand E, Stokes BH, Ndikumana JM, Warsame M, Umulisa N, Ngamije D, Munyaneza T, Mazarati JB, Munguti K, Campagne P, Criscuolo A, Ariey F, Murindahabi M, Ringwald P, Fidock DA, Mbituyumuremyi A, Menard D. 2020. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med 26:1602–1608. 10.1038/s41591-020-1005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller IB, Hyde JE. 2013. Folate metabolism in human malaria parasites–75 years on. Mol Biochem Parasitol 188:63–77. 10.1016/j.molbiopara.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Kicska GA, Ting LM, Schramm VL, Kim K. 2003. Effect of dietary p-aminobenzoic acid on murine Plasmodium yoelii infection. J Infect Dis 188:1776–1781. 10.1086/379373. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Kumar S, McDew-White M, Haile M, Cheeseman IH, Emrich S, Button-Simons K, Nosten F, Kappe SHI, Ferdig MT, Anderson TJC, Vaughan AM. 2019. Genetic mapping of fitness determinants across the malaria parasite Plasmodium falciparum life cycle. PLoS Genet 15:e1008453. 10.1371/journal.pgen.1008453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, Wang C, Otto TD, Oberstaller J, Liao X, Adapa SR, Udenze K, Bronner IF, Casandra D, Mayho M, Brown J, Li S, Swanson J, Rayner JC, Jiang RHY, Adams JH. 2018. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 360:eaap7847. 10.1126/science.aap7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bronner IF, Otto TD, Zhang M, Udenze K, Wang C, Quail MA, Jiang RH, Adams JH, Rayner JC. 2016. Quantitative insertion-site sequencing (QIseq) for high throughput phenotyping of transposon mutants. Genome Res 26:980–989. 10.1101/gr.200279.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kümpornsin K, Modchang C, Heinberg A, Ekland EH, Jirawatcharadech P, Chobson P, Suwanakitti N, Chaotheing S, Wilairat P, Deitsch KW, Kamchonwongpaisan S, Fidock DA, Kirkman LA, Yuthavong Y, Chookajorn T. 2014. Origin of robustness in generating drug-resistant malaria parasites. Mol Biol Evol 31:1649–1660. 10.1093/molbev/msu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nair S, Miller B, Barends M, Jaidee A, Patel J, Mayxay M, Newton P, Nosten F, Ferdig MT, Anderson TJ. 2008. Adaptive copy number evolution in malaria parasites. PLoS Genet 4:e1000243. 10.1371/journal.pgen.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebel E, Kuypers F, Lin C, Petrov D, Egan E. 2020. Common host variation drives malaria parasite fitness in healthy human red cells. Elife 10:e69808. 10.7554/eLife.69808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cook RD. 1977. Detection of influential observation in linear regression. Technometrics 19:15–18. 10.1080/00401706.1977.10489493. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download aac.01529-21-s0003.xlsx, XLSX file, 0.01 MB (15.6KB, xlsx)
Table S2. Download aac.01529-21-s0001.xlsx, XLSX file, 0.05 MB (50.1KB, xlsx)
Table S3. Download aac.01529-21-s0002.xlsx, XLSX file, 0.01 MB (14.5KB, xlsx)