Abstract
Renibacterium salmoninarum is a genospecies that is an obligate pathogen of salmonid fish and is capable of intracellular survival. Conventional typing systems have failed to differentiate isolates of R. salmoninarum. We used two methods to assess the extent of molecular variation which was present in isolates from different geographic locations. In one analysis we investigated possible polymorphisms in a specific region of the genome, the intergenic spacer (ITS) region between the 16S and 23S rRNA genes. In the other analysis we analyzed differences throughout the genome by using randomly amplified polymorphic DNA (RAPD). We amplified the spacer region of 74 isolates by using PCR and performed a DNA sequence analysis with 14 geographically distinct samples. The results showed that the 16S-23S ribosomal DNA spacer region of R. salmoninarum is highly conserved and suggested that only a single copy of the rRNA operon is present in this slowly growing pathogen. DNA sequencing of the spacer region showed that it was the same length in all 14 isolates examined, and the same nucleotide sequence, sequevar 1, was obtained for 11 of these isolates. Two other sequevars were found. No tRNA genes were found. We found that RAPD analysis allows reproducible differentiation between isolates of R. salmoninarum obtained from different hosts and different geographic regions. By using RAPD analysis it was possible to differentiate between isolates with identical ITS sequences.
Phylogenetically, Renibacterium salmoninarum is a member of the Micrococcus-Arthrobacter subdivision of the actinomycetes, a heterogeneous group of bacteria typified by high G+C contents (5, 22, 27, 38). R. salmoninarum is a slowly growing, fastidious organism with a narrow temperature range for optimal growth (10 to 20°C) and is an obligate pathogen of salmonid fish. This organism is distributed in much of the Northern Hemisphere and Chile and usually causes a chronic, systemic, granulomatous infection, bacterial kidney disease (BKD), which can be fatal under the appropriate conditions (14). The pathogen survives intracellularly and can be transmitted vertically within an ovum, as well as horizontally between cohabiting fish. There is no effective vaccine or chemotherapy. Furthermore, the presence of subclinical infections complicates attempts to control the disease through eradication programs.
The epidemiology of BKD, particularly the interactions which occur between wild and farmed salmonids, is unclear. This is mainly because attempts to differentiate between isolates of R. salmoninarum so far have been unsuccessful. This bacterium appears to possess remarkable biochemical uniformity, and no reliable serological means of distinguishing between isolates has been found (8, 19). A recent study of 40 isolates of R. salmoninarum from North America in which multilocus enzyme electrophoresis was used indicated that the level of genetic diversity was low (39). The lengthy periods required for growth of the bacterium (often 6 weeks or more) and the consequent degradation of antigenic or enzymically active components cause problems for studies which rely on the use of such components.
There are a variety of DNA-based methods available for differentiating between isolates, strains, and species of bacteria. The 16S-23S rRNA intergenic spacer (ITS) has proven to be useful for such differentiation in many cases (6, 17, 25, 28). The ITS appears to have a higher evolutionary rate than either 16S ribosomal DNA (rDNA) or 23S rDNA (28, 30) has, and there are variations in the ITS length and nucleotide sequence which make it possible to distinguish between closely related bacterial species and, sometimes, between strains and isolates (21). Incomplete 16S rRNA gene sequences of two isolates of R. salmoninarum have been determined (22, 29), but there have been no previous studies of either the 23S rRNA gene or the ITS of R. salmoninarum.
An alternative to using species-specific DNA sequences for isolate or strain differentiation involves a PCR-based method, randomly amplified polymorphic DNA (RAPD) analysis. Usually with this method, short random primers are used to rapidly detect genomic polymorphisms under low-stringency conditions (43, 45). RAPD analysis is widely used for differentiating between bacterial isolates (26, 42) and relies upon small quantities of genomic DNA, which makes it ideally suited to the study of slowly growing and fastidious organisms. We investigated the ITS and also performed a RAPD analysis of the R. salmoninarum genome in order to assess the potential of these methods for examining the molecular variability between isolates.
MATERIALS AND METHODS
Bacterial isolates.
Seventy-four isolates of R. salmoninarum were used in this study. Table 1 shows the isolate designations, countries of origin, and sources of isolation and the GenBank accession numbers for the 16S-23S rRNA ITS sequences which were determined. R. salmoninarum was cultured in SKDM broth supplemented with 5% spent culture broth at 15°C (4, 15). The specificity control species (Table 2) were cultured on nutrient agar at 25°C.
TABLE 1.
R. salmoninarum isolates used in this study
| Isolate | Geographic origin | Sourcea | Isolate | Geographic origin | Sourcea |
|---|---|---|---|---|---|
| 970083-88 | Southern England | Rainbow trout (f) | F-120-87(P-2) | Iceland | Atlantic salmon (f) |
| 970083-102 | Southern England | Rainbow trout (f) | F-130-87(P-4) | Iceland | Rainbow trout (f) |
| 980106 #1.1.5b | Southern England | Rainbow trout (f) | F-138-87(0-78) | Iceland | Atlantic salmon (f) |
| 980036-150 | Wales | Rainbow trout (f) | F-260-87(P-16) | Iceland | Atlantic salmon (f) |
| 980036-87 | Wales | Rainbow trout (f) | F-273-87(P-19) | Iceland | Atlantic salmon (f) |
| 970419-1.2.3b | Southern England | Atlantic salmon (w) | F-283-87(P-10) | Iceland | Atlantic salmon (f) |
| 970153-19b | Southern England | Grayling(w) | F-358-87(P-13) | Iceland | Atlantic salmon (w) |
| A6 | Southern England | Rainbow trout (f) | S-182-90(P-7)c | Iceland | Atlantic salmon (f) |
| A80 | Southern England | Rainbow trout (f) | Iwatec | Japan | Coho salmon |
| W2 | Northern England | Rainbow trout (f) | Siletz | Oregon | Coho salmon (f) |
| W6b | Northern England | Rainbow trout (f) | Marion Forksb | Oregon | Chinook salmon (f) |
| WMV1 | Southern England | Rainbow trout (f) | Little Goose | Washington | Chinook salmon (f) |
| MT409 | Scotland | Unknown | CCM6205 | Washington | Coho salmon (f) |
| MT417b | Scotland | Atlantic salmon (f) | 84-019-OC | Washington | Chinook salmon (w) |
| MT239 | Scotland | Atlantic salmon (f) | SS-ChS-94-1 | Oregon | Chinook salmon |
| MT426 | Scotland | Unknown | Cow ChS94 P22 | Washington | Chinook salmon (f) |
| NCIMB1111 | Scotland | Atlantic salmon (w) | Idaho 91-126 | Idaho | Sockeye salmon (f) |
| NCIMB1112 | Scotland | Atlantic salmon (w) | RFL-643.94 #1 | Washington | Sockeye salmon (f) |
| NCIMB1113 | Scotland | Atlantic salmon (w) | CCM6206 | Oregon | Chinook salmon (f) |
| NCIMB1114 | Scotland | Atlantic salmon (w) | Round Butte | Oregon | Chinook salmon (f) |
| NCIMB1115 | Scotland | Atlantic salmon (w) | NCIMB2196 | Wyoming | Brook trout (f) |
| NCIMB1116 | Scotland | Atlantic salmon (w) | ATCC 33209b | Oregon | Chinook salmon (f) |
| MT420 | Scotland | Atlantic salmon (f) | D-6 | Oregon | Coho salmon |
| MT452 | Scotland | Rainbow trout (f) | Cole River | Oregon | Unknown |
| MT1363 | Scotland | Rainbow trout (f) | Looking Glass | Oregon | Unknown |
| MT410 | Scotland | Unknown | AcF6-1d | Northwest Territories, Canada | Arctic charr (w) |
| FT-10 | Scotland | Atlantic salmon | Rs 9b | Sweden | Rainbow trout |
| BA99 | Scotland | Atlantic salmon | Rs 19 | Sweden | Atlantic salmon |
| DR143b | Alberta, Canada | Brook trout (f) | Rs 61 | Sweden | Arctic char |
| DR384 | British Columbia, Canada | Coho salmon (f) | Rs 116 | Sweden | Grayling |
| 3784 | British Columbia, Canada | Sockeye salmon (f) | Rs 122 | Sweden | Rainbow trout (f) |
| 980002 | British Columbia, Canada | Chinook salmon (f) | Rs 125 | Sweden | Rainbow trout |
| 960023 | British Columbia, Canada | Coho salmon (f) | Rs 126 | Sweden | Rainbow trout |
| 960046 | British Columbia, Canada | Coho salmon (f) | 3015-86b | Norway | Atlantic salmon |
| GC96-1 | British Columbia, Canada | Sockeye salmon (w) | 4451-86 | Norway | Atlantic salmon |
| DR-128 | British Columbia, Canada | Rainbow trout(w) | DK98951714 | Denmark | Rainbow trout (f) |
| RS-TSA | Nova Scotia, Canada | Atlantic salmon (f) | BY1996b | Alaska | Chinook salmon (f) |
Isolates were obtained from wild fish (w) or from farmed (captive reared) fish (f). The complete histories of some isolates are not known.
SV1 isolates whose 16S-23S rRNA ITS region was sequenced. The sequences have been deposited in the GenBank database under accession no. AF093461, AF093462, AF093465, AF093466, AF093467, AF093468, AF093469, AF093470, AF093471, AF093472, and AF093473.
SV2 isolates whose 16S-23S rRNA ITS region was sequenced. The sequences have been deposited in the GenBank database under accession no. AF093463 and AF093474.
SV3 isolate whose 16S-23S rRNA ITS region was sequenced. The sequence has been deposited in the GenBank database under accession no. AF093464.
TABLE 2.
Reference organisms used
| Species or group | Strain or sourcea |
|---|---|
| Arthrobacter aurescens | NCIMB 8912 |
| Arthrobacter globiformis | NCIMB 8907 |
| Arthrobacter nicotianae | NCIMB 9458 |
| Bacillus cereus | NCIMB 11925 |
| Brevibacterium linens | NCIMB 8546 |
| Enterobacter aerogenes | UPCC |
| Enterococcus faecalis | UPCC |
| Listeria innocua | UPCC |
| Listeria ivanovi | UPCC |
| Listeria seeligeri | UPCC |
| Micrococcus luteus | NCTC 7563 |
| Micrococcus roseus | NCIMB 9149 |
| Rhodococcus equi | UPCC |
| Staphylococcus aureus | UPCC |
| Aeromonas hydrophila | UPCC |
| Aeromonas salmonicida | UPCC |
| Escherichia coli | UPCC |
| Escherichia coli B | UPCC |
| Escherichia coli enteropathogenic | UPCC |
| Klebsiella edwardii | NCTC 10896 |
| Klebsiella pneumoniae | NCIMB 418 |
| Pseudomonas aeruginosa | ATCC 9027 |
| Pseudomonas fluorescens | NCIMB 1953 |
| Serratia marcescens | NCIMB 11783 |
| Vibrio anguillarum | UPCC |
| Vibrio parahaemolyticus | UPCC |
| Yersinia ruckeri | UPCC |
UPCC, University of Plymouth Culture Collection.
DNA preparation and amplification of the ITS and specific R. salmoninarum genes.
Genomic DNA was isolated by using a Puregene D-6000 DNA isolation kit according to the instructions of the manufacturer (Gentra Systems Inc.). DNA extracted by this method was electrophoresed on 1.2% agarose gels. Images of each gel were captured with a Kodak model DC40 digital camera, and the DNA concentration was determined for each isolate by using Kodak Digital Science 1D Image Analysis software.
PCR amplification was performed with a DNA thermal cycler (Perkin-Elmer). The primers used to amplify the ITS sequence were selected from region 2 of the 16S rRNA gene sequence for R. salmoninarum and from two highly conserved regions corresponding to regions 5 and 7 of the 23S rRNA gene sequence (21) for Micrococcus luteus obtained from the GenBank database (29, 36). All of the primers (Table 3), including those used for amplification of the R. salmoninarum msa, hly, and rsh genes (9, 16, 20), were chosen by using Amplify software (13). Each 50-μl reaction mixture contained 1 U of Taq polymerase (Boehringer Mannheim), reaction buffer (Boehringer Mannheim), 1.5 mM MgCl2, 24 pmol of each primer, each deoxynucleoside triphosphate at a concentration of 0.2 mM, and 10 ng of bacterial DNA. The reaction mixtures were overlaid with mineral oil (Sigma), incubated at 96°C for 2 min, and then subjected to 25 cycles consisting of 96°C for 30 s, 65°C for 30 s, and 72°C for 90 s. Amplification products were analyzed on 1.5 and 2% agarose gels.
TABLE 3.
Primers used to amplify the R. salmoninarum msa, hly, and rsh genes and the 16S-23S rDNA spacer region
| Gene or region | Size of PCR prod-uct (bp) | Primer
|
|
|---|---|---|---|
| Designation | Sequence (5′-3′) | ||
| msa | 487 | Rs57+127 | TCGCAAGGTGAAGGGAATTCTTCC |
| Rs57−611 | GGTTTGTCTCCAAAGGAGACTTGC | ||
| 297 | Rs57+155 | CAACAGTACAAGGCTTCAGCAGTG | |
| Rs57−449 | CCGAAACCTACGTTTAGAGTCGTC | ||
| hly | 542 | RsMP+338 | ATCGGCTCAGACTAGCGCCATAAT |
| RsMP−877 | GCTTCAAGATCGATGACCTTCGAG | ||
| 336 | RsMP+487 | TTACTGAGGTCCTTGATGGTCAGG | |
| RsMP−820 | CGATCGGTGCGGTCATTCAAGATA | ||
| rsh | 572 | RsH+231 | TCCGGTCATCATGCTTTCTTCGCT |
| RsH−800 | ATTGCCACCAAGCTGAAGTACCTG | ||
| 374 | RsH+401 | TGCCCAATCTGAAGACAGCGACTA | |
| RsH−772 | GGTCGATAATGCTCGTCATGCCTA | ||
| 16S-23S spacer | 751 | RS+1002 | CCGTCCAAGTCACGAAAGTTGGTA |
| ML−1329 | ATCGCAGATTCCCACGTCCTTCTT | ||
| 895 | RS+1002 | CCGTCCAAGTCACGAAAGTTGGTA | |
| ML−1469 | GTGGGTACTGAGATGTTTCAGTTC | ||
RAPD PCR.
The RAPD analysis was performed with 19 isolates, and two separate methods were employed. First, a Ready-To-Go RAPD Analysis Beads kit (Pharmacia Biotech) containing six distinct random 10-mer primers, including primer P1 (GGTGCGGGAA), primer P2 (GTTTCGCTCC), primer P3 (GTAGACCCGT), primer P4 (AAGAGCCCGT), primer P5 (AACGCGCAAC), and primer P6 (CCCGTCAGCA), was used according to the manufacturer’s instructions. Each 25-μl reaction mixture contained 25 pmol of primer and 2.5 or 10 ng of template DNA. The reactions were performed in a Perkin-Elmer thermal cycler by using one cycle consisting of 95°C for 5 min and then 45 cycles consisting of 95°C for 1 min, 36°C for 1 min, and 72°C for 2 min.
Second, the method described by Atienzar et al. (3) was used. Briefly, the following two primers were selected from the 10 primers in a kit obtained from Operon Technologies Inc.: primer OPA9 (GGGTAACGCC) and primer OPB1 (GTTTCGCTCC). Each 25-μl reaction mixture contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 5.11 mM MgCl2, 0.1% Triton X-100, 0.1% gelatin, each deoxynucleoside triphosphate at a concentration of 0.33 mM, 2 μM primer, 2.5 μg of bovine serum albumin, 2.8 U of Taq DNA polymerase (Immunogen International), and 2.5 or 10 ng of template DNA. The reactions were performed in a Perkin-Elmer thermal cycler by using one cycle consisting of 95°C for 5 min, 39 cycles consisting of 95°C for 1 min, 50°C for 1 min, and 74°C for 1 min, and one cycle consisting of 95°C for 1 min, 50°C for 1 min, and 74°C for 10 min. The PCR products were analyzed on 1.2% agarose gels in Tris-borate-EDTA buffer. Images of each gel were captured with a Kodak model DC40 digital camera, and the DNA profile was analyzed by using Kodak Digital Science 1D Image Analysis software.
Sequence analysis.
PCR products spanning the ITS were sequenced directly by a cycle sequencing method and were aligned by workers at MWG-Biotech Ltd., Milton Keynes, United Kingdom. The R. salmoninarum sequences were compared with the sequences of other organisms obtained from the GenBank database by using the gapped BLAST program (1) and the GeneStream align program (IGH, Montpellier, France) (33).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the nucleotide sequences determined in this study are shown in Table 1.
RESULTS
Amplification of specific R. salmoninarum genes.
In order to confirm the identity of the DNA extracted from R. salmoninarum cultures, six sets of primers were designed to amplify known regions of three R. salmoninarum genes. For each of the 74 isolates of R. salmoninarum tested in the six PCR a single band of the appropriate size was amplified (Table 3). No amplification products were obtained from PCR mixtures containing template DNA derived from the specificity control species or from any of the interspersed negative controls.
Amplification of the 16S-23S rDNA spacer region.
The ITS of 74 isolates of R. salmoninarum were amplified by using primers for highly conserved sequences near the 3′ end of the 16S rRNA gene and the 5′ end of the 23S rRNA gene. Primers RS+1002 and ML−1329 amplified a 751-bp fragment, while primers RS+1002 and ML−1469 amplified a 895-bp fragment. In every case only a single band was detected with the primers that were used. In addition, for each primer set no size differences were detected on 1.5 or 2% agarose gels.
Sequencing of the ITS from total PCR products and sequence analysis.
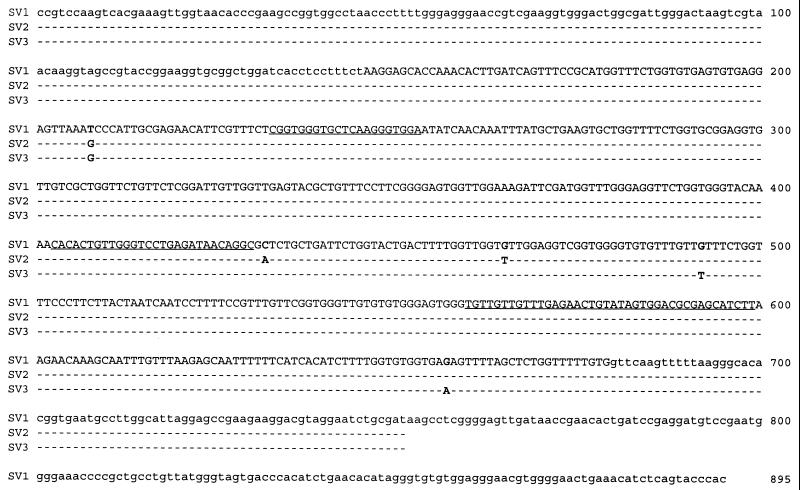
The complete 16S-23S rDNA spacer region sequences of 14 R. salmoninarum isolates were determined by directly sequencing PCR-amplified products. PCR products were amplified with primers RS+1002 and ML−1329, which bind to highly conserved regions 2 and 5 of the 16S-23S rRNA operon (21). Only a single unambiguous sequence was obtained for each PCR product generated. We found that all of the isolates possessed ITS sequences that were the same length, 534 bp. Furthermore, 11 isolates had the same nucleotide sequence, which was designated sequevar 1 (SV1) (Fig. 1). These 11 isolates were obtained from a broad geographic area, which included the mainland United States, Alaska, Canada, Sweden, England, Scotland, and Norway, and from a variety of host salmonid fish species, including chinook salmon, Atlantic salmon, rainbow trout, brook trout, and grayling. Only three isolates possessed spacer regions whose sequences differed from this sequence. The sequences of isolates S-182-90 and Iwate, obtained from Atlantic salmon from Iceland and coho salmon from Japan, respectively, exhibited three identical single-base differences, and the ITS sequence of these organisms was designated sequevar 2 (SV2). Sequevar 3 (SV3), the ITS sequence of isolate AcF6-1 obtained from Arctic char from the Northwest Territories of Canada, also exhibited three single-base differences, one of which was also found in the ITS sequences of S-182-90 and Iwate (Fig. 1). In order to confirm that the ITS sequences obtained by PCR amplification with primers RS+1002 and ML−1329 each represented a single homogeneous copy of the 16S-23S rRNA ITS region, we sequenced the PCR product amplified with primers RS+1002 and ML−1469 from the genome of type strain ATCC 33209. Primer ML−1469 binds deeper in the 23S rRNA gene than primer ML–1329 in highly conserved region 7 (21). The single unambiguous sequence obtained in this way exactly matched the sequences obtained for ATCC 33209 and the 10 other SV1 isolates by using primers RS+1002 and ML−1329.
FIG. 1.
SV1, SV2, and SV3 of the 16S-23S rRNA ITS of R. salmoninarum. The isolates with each sequevar are identified in Table 1. The sequence of the region from nucleotide 1 to nucleotide 750 was determined for 14 isolates by using PCR-amplified products obtained with primers RS+1002 and ML−1329. The sequence for nucleotides 1 to 895 was confirmed for type strain ATCC 33209 by using PCR-amplified products obtained with primers RS+1002 and ML−1469. The uppercase letters represent the 534-bp ITS sequence. The lowercase letters for nucleotides 1 to 145 represent the 3′ end of the R. salmoninarum 16S rRNA gene (22, 29), while the final 216 bp represents the 5′ end of the R. salmoninarum 23S rRNA gene. The three regions that are substantially the same in members of the actinomycetes are underlined.
The R. salmoninarum ITS exhibited 34 to 47% identity with the 16S-23S rDNA spacer region sequences of actinomycetes in the GenBank database. Three regions that were approximately 20, 27, and 35 bp long (Fig. 1) were found to be highly conserved in a number of other members of the actinomycetes, including Bifidobacterium sp., Brevibacterium sp., Kitasatosporia sp., Rhodococcus erythropolis, Streptomyces sp., Microtetraspora sp., and Streptosporangium sp. Sequences for members of the genera Arthrobacter and Micrococcus, two genera which are closely related to R. salmoninarum, were not available in the database and hence were not included in the comparison.
RAPD analysis as a means of differentiating isolates.
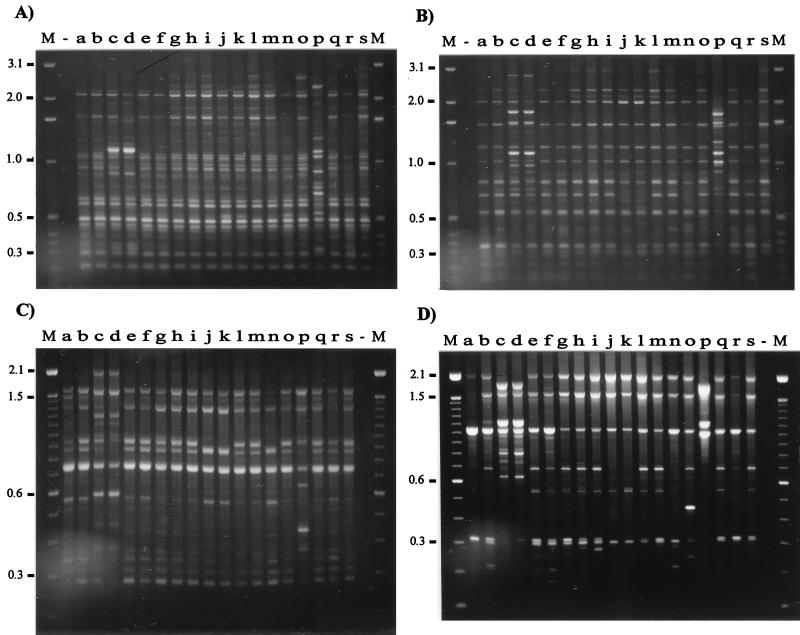
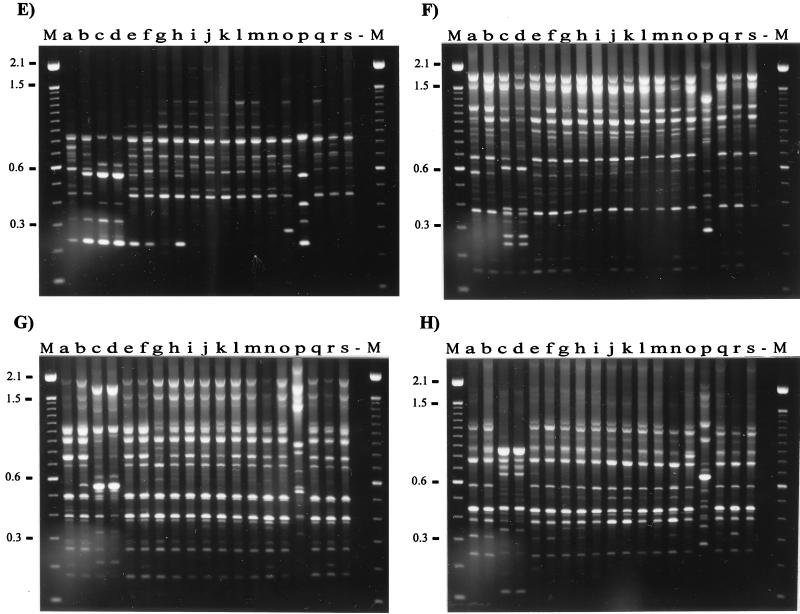
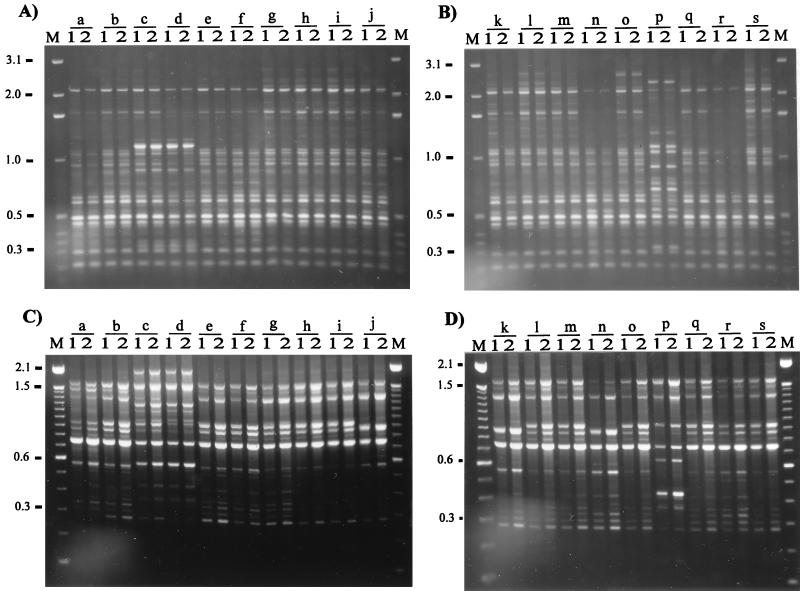
We observed that with all of the primers the geographic origins of 19 isolates were reflected in the RAPD band patterns. Using eight random primers and two RAPD methods, we discerned three arbitrary groups of isolates visually (Fig. 2). Group 1 contained isolates from Canada (Fig. 2, lanes a, b, l, and r), Scotland (lanes h, i, m, q, and s), and England (lanes e and f), as well as two isolates from the United States isolates (lanes g and o); group 2 contained isolates from Iceland (lanes j, k, and n); and group 3 contained the other isolates from the United States (lanes c, d, and p). None of the isolates produced identical RAPD patterns with the eight primers, and in most cases, using two or three primers revealed differences between isolates. We chose primers which consistently gave a distinct and reproducible band pattern for each isolate tested. However, primers P2, P3, P4, P5, and P6 gave the clearest and most discriminatory patterns for each isolate regardless of origin. When these primers were used, it was possible to identify differences between isolates from the same country; e.g., primers P2 and P6 discriminated between Icelandic isolates, while primers P2, P3, and P4 revealed differences between English isolates. Differences in RAPD fingerprints could not be attributed to the presence of plasmid DNA. We previously examined DNA extracts of more than 70 R. salmoninarum isolates and found no evidence of plasmid DNA (unpublished data). In order to assess the reproducibility and variation of RAPD fingerprinting, we performed PCR reamplification analyses by using all of the primers, DNA extracted from R. salmoninarum cultures on different occasions, and two DNA template concentrations, as recommended by Welsh et al. (44). Small differences in the quality and concentrations of two templates can lead to spurious differences in the RAPD pattern; therefore, every experiment should include at least two concentrations of genomic DNA for each individual. The results obtained with two primers, primers OPA9 and P1, are presented in Fig. 3. DNA fingerprints were very reproducible; the only discrepancies were confined to the presence or absence of faint bands. The intensities of these faint bands would render them below the limit for inclusion in any analysis of DNA fingerprints.
FIG. 2.
RAPD fingerprints of 19 isolates of R. salmoninarum from a variety of geographic areas and a variety of host species. The DNA fingerprints were obtained by PCR amplification with primers OPA9 (A), OPB1 (B), P1 (C), P2 (D), P3 (E), P4 (F), P5 (G), and P6 (H). Lane a, isolate DR384; lane b, isolate DR128; lane c, isolate ATCC 33209; lane d, isolate Round Butte; lane e, isolate W2; lane f, isolate W6; lane g, isolate Little Goose; lane h, isolate MT1363; lane i, isolate BA99; lane j, isolate S-182-90; lane k, isolate F-273-87; lane l, isolate RS-TSA; lane m, isolate MT410; lane n, isolate F-138-87; lane o, isolate NCIMB2196; lane p, isolate Marion Forks; lane q, isolate MT417; lane r, isolate DR143; lane s, isolate MT420; lane −, water control. Lanes M contained markers (1-kb DNA ladder [Gibco BRL] in panels A and B and 100-bp DNA ladder [Gibco BRL] in panels C to H). The molecular sizes (in kilobases) are indicated on the left.
FIG. 3.
Reproducibility of RAPD fingerprinting. The DNA fingerprints for DNA extracted on separate occasions with two different concentrations of template were obtained after PCR amplification with primers as described in the Legend to Fig. 2. For the contents of lanes a to s see the legend to Fig. 2. Lanes 1, 2.5 ng of DNA template; lanes 2, 10 ng of DNA template. Only the results obtained with the following two primers are shown: primer OPA9 (A and B) and primer P1 (C and D). Lane M contained markers (see the legend to Fig. 2). The molecular sizes (in kilobases) are indicated on the left.
DISCUSSION
The widespread distribution of R. salmoninarum in the United Kingdom, many European countries, Japan, North America, and Chile and the variety of salmonid host species in these regions suggested the possibility that the genetic diversity of isolates in these areas may be reflected by the number, length, and sequence of the 16S-23S rRNA ITS region. While inter- and intrageneric relationships may be elucidated by examining 16S and 23S rDNA sequences, the ITS has provided information on intraspecific relationships in other bacteria (17, 28, 30, 40). Three distinct ITS sequences (sequevars) were obtained from 14 R. salmoninarum isolates. Isolates from Iceland, Japan, and the Canadian Northwest Territories which had three single-base substitutions in the ITS exhibited some divergence from the highly conserved SV1 which was present in isolates from the United States, the United Kingdom, mainland Europe, and Alberta, Canada. It may be that in areas of the world which could be regarded as relatively isolated from the mainstream intensive salmonid culture areas of North America and Europe the bacterium has diverged from this pattern. It is interesting that the sole Alaskan isolate was an SV1 isolate. BKD has been reported in wild and farmed fish from a number of Alaskan river systems (11, 32), and it seems likely that Alaskan salmon have been exposed to the sequevar of R. salmoninarum carried by salmon from the Pacific coast of Canada or the United States at some stage during their oceanic migrations.
This study provides no evidence that there are multiple copies of the rRNA operon in R. salmoninarum. A single unambiguous nucleotide sequence was obtained for all of the isolates examined, and 11 of the isolates possessed spacer regions that had the same nucleotide sequence. The presence of a nucleotide sequence generated from a highly conserved region deeper in the 23S rRNA gene confirmed these results. Typical tRNA genes were not found in the ITS region of R. salmoninarum. Furthermore, we found no evidence that there were multiple amplicons in PCR mixtures when we used two sets of primers for highly conserved regions of the 16S and 23S rRNA genes. However, absolute proof that there is a single rRNA operon would require direct sequencing from the genome. We concluded that R. salmoninarum probably has a single copy of the rRNA operon, a finding which is consistent with what has been described for a number of other slowly growing organisms (2, 7, 18, 37, 40) and is a further indication of the conservative genetic composition of this obligate pathogen. Generally, our findings suggest that the 16S-23S rDNA spacer region is of limited use for routine discrimination between R. salmoninarum isolates but may offer some clues as to geographic origins.
The lack of a way to differentiate between isolates of R. salmoninarum has constrained epidemiological studies of BKD. In particular, development of a means of contact tracing would allow BKD outbreaks to be traced back to the source of infection and would help resolve some of the difficulties associated with investigation of the interactions between farmed and wild salmonid fish. We used two methods to do this, examination of ITS variation and RAPD analysis, which have been used successfully in studies of other bacteria. Our work shows that compared with ITS variation, RAPD analysis is a better method for discriminating between isolates of R. salmoninarum. In our study, R. salmoninarum isolates from a variety of sources, some with identical 16S-23S spacer region DNA sequences, could be distinguished on the basis of RAPD patterns generated by two different methods. RAPD analysis has provided a reliable and reproducible method for molecular typing and genetic characterization of a variety of microorganisms (23, 24, 34, 41). This method is particularly useful for examining the genomic diversity among strains of bacteria which are indistinguishable by other molecular methods. For example, RAPD analysis of strains of Bacillus cereus revealed a remarkable diversity which was not revealed by rRNA or tRNA ITS-targeted PCR (10). A number of factors have been identified as influencing the outcome of RAPD fingerprinting (12, 31, 35). In our studies, using eight primers and two different methods for PCR amplification of purified DNA template produced RAPD fingerprints which were reproducible with two different DNA concentrations and with DNA extracted on different occasions. In every case, RAPD fingerprints distinguished the same groups of isolates.
So far, R. salmoninarum has defied attempts to find a reproducible way to differentiate between isolates. This study is the first study which revealed the genetic diversity within the species by using a DNA-based method for differentiating between isolates from a wide variety of sources and therefore represents a substantial advance in our understanding of a fastidious intracellular pathogen which is capable of surviving within its host in very low numbers. We are extending our investigations of R. salmoninarum by using RAPD analysis in conjunction with other molecular typing methods as part of a coordinated program to examine farm and wild R. salmoninarum isolates from the United Kingdom and other sources. This work should result in a wide-ranging analysis of isolate differences.
In conclusion, R. salmoninarum is a highly conserved genospecies. The molecular variation in the sequence of the 16S-23S rDNA spacer region of isolates from widely separated environments is extremely limited. RAPD analysis is a reliable and reproducible technique for discriminating between isolates of R. salmoninarum and should facilitate epidemiological studies of this pathogen.
ACKNOWLEDGMENTS
This study was funded by project FC1103 of the Ministry for Agriculture, Fisheries and Food U.K.
We thank the following individuals for providing isolates of R. salmoninarum: Gavin Barker and Edel Chambers, CEFAS Laboratory, Weymouth, England; Joyce Petrie, SOEAFD, Marine Laboratory, Aberdeen, Scotland; Craig Banner, Department of Microbiology, Oregon State University, Corvallis; Brian Souter, Department of Fisheries and Oceans, Freshwater Institute, Winnipeg, Manitoba, Canada; Trevor Evelyn, Dorothee Kieser, and Gina Prosperi-Porta, Department of Fisheries and Oceans, Nanaimo, British Columbia, Canada; Sigridur Gudmundsdottir, Institute for Experimental Pathology, University of Iceland, Reykjavik, Iceland; Eva Jansson and Eva Saker, National Veterinary Institute, Uppsala, Sweden; Ted Meyers and Sally Short, Alaska Department of Fish and Game, Southeast Fish Pathology, Juneau; Ole Eske Heuer, Danish Veterinary Laboratory, Aarhus, Denmark; Dougie A. McIntosh, Instituto Oswaldo Cruz, Rio de Janeiro, Brazil; and Steve G. Griffiths, Research and Productivity Council of New Brunswick, Frederickton, New Brunswick, Canada.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amikam D, Glaser G, Razin S. Mycoplasmas (Mollicutes) have a low number of rRNA genes. J Bacteriol. 1984;158:376–378. doi: 10.1128/jb.158.1.376-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atienzar F, Child P, Evenden A, Jha A, Savva D, Walker C, Depledge M. Application of the arbitrarily primed polymerase chain reaction for the detection of DNA damage. Mar Environ Res. 1998;46:331–335. [Google Scholar]
- 4.Austin B, Embley T M, Goodfellow M. Selective isolation of Renibacterium salmoninarum. FEMS Microbiol Lett. 1983;17:111–114. [Google Scholar]
- 5.Banner C R, Rohovec J S, Fryer J L. A new value for mol percent guanine + cytosine of DNA for the salmonid fish pathogen Renibacterium salmoninarum. FEMS Microbiol Lett. 1991;79:57–60. doi: 10.1016/0378-1097(91)90527-h. [DOI] [PubMed] [Google Scholar]
- 6.Barry T, Colleran G, Glennon M, Dunican L K, Gannon F. The 16S/23S ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Applic. 1991;1:51–56. doi: 10.1101/gr.1.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Bercovier H, Kafri O, Sela S. Mycobacteria possess a surprisingly small number of ribosomal RNA genes in relation to the size of their genome. Biochem Biophys Res Commun. 1986;136:1136–1141. doi: 10.1016/0006-291x(86)90452-3. [DOI] [PubMed] [Google Scholar]
- 8.Bruno D W, Munro A L S. Uniformity in the biochemical properties of Renibacterium salmoninarum isolates obtained from several sources. FEMS Microbiol Lett. 1986;33:247–250. [Google Scholar]
- 9.Chien M-S, Gilbert T L, Huang C, Landolt M L, O’Hara P J, Winton J R. Molecular cloning and sequence analysis of the gene coding for the 57-kDa major soluble antigen of the salmonid fish pathogen Renibacterium salmoninarum. FEMS Microbiol Lett. 1992;96:259–266. doi: 10.1016/0378-1097(92)90414-j. [DOI] [PubMed] [Google Scholar]
- 10.Daffonchio D, Borin S, Frova G, Manachini P L, Sorlini C. PCR fingerprinting of whole genomes: the spacers between the 16S and 23S rRNA genes and of intergenic tRNA gene regions reveal a different intraspecific genomic variability of Bacillus cereus and Bacillus licheniformis. Int J Syst Bacteriol. 1998;48:107–116. doi: 10.1099/00207713-48-1-107. [DOI] [PubMed] [Google Scholar]
- 11.Didier A J., Jr . Bacterial kidney disease. In: Dietrich R A, editor. Alaskan wildlife diseases. Fairbanks: University of Alaska; 1981. pp. 361–367. [Google Scholar]
- 12.Ellsworth D L, Rittenhouse K D, Honeycutt R L. Artifactual variation in randomly amplified polymorphic DNA banding patterns. BioTechniques. 1993;14:214–217. [PubMed] [Google Scholar]
- 13.Engels W R. Contributing software to the Internet: the Amplify program. Trends Biochem Sci. 1993;18:448–450. doi: 10.1016/0968-0004(93)90148-g. [DOI] [PubMed] [Google Scholar]
- 14.Evelyn T P T. Bacterial kidney disease—BKD. In: Inglis V, Roberts R J, Bromage N R, editors. Bacterial diseases of fish. Oxford, United Kingdom: Blackwell; 1993. pp. 177–195. [Google Scholar]
- 15.Evelyn T P T, Prosperi-Porta L, Ketcheson J E. Two new techniques for obtaining consistent results when growing Renibacterium salmoninarum on KDM2 culture medium. Dis Aquat Org. 1990;9:209–212. [Google Scholar]
- 16.Evenden A J. The use of gene cloning techniques in the study of the fish pathogen Renibacterium salmoninarum. Ph.D. thesis. Plymouth, United Kingdom: University of Plymouth; 1993. [Google Scholar]
- 17.Forsman P, Tilsala-Timisjarvi A, Alatossava T. Identification of staphylococcal and streptococcal causes of bovine mastitis using 16S-23S rRNA spacer regions. Microbiology. 1997;143:3491–3500. doi: 10.1099/00221287-143-11-3491. [DOI] [PubMed] [Google Scholar]
- 18.Frothingham R, Wilson K H. Sequence-based differentiation of strains in the Mycobacterium avium complex. J Bacteriol. 1993;175:2818–2825. doi: 10.1128/jb.175.10.2818-2825.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodfellow M, Embley T M, Austin B. Numerical taxonomy and emended description of Renibacterium salmoninarum. J Gen Microbiol. 1985;131:2739–2752. [Google Scholar]
- 20.Grayson T H, Evenden A J, Gilpin M L, Martin K L, Munn C B. A gene from Renibacterium salmoninarum encoding a product which shows homology to bacterial zinc-metalloproteases. Microbiology. 1995;141:1331–1341. doi: 10.1099/13500872-141-6-1331. [DOI] [PubMed] [Google Scholar]
- 21.Gurtler V, Stanisich V A. New approaches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology. 1996;142:3–16. doi: 10.1099/13500872-142-1-3. [DOI] [PubMed] [Google Scholar]
- 22.Gutenberger S K, Giovannoni S J, Field K G, Fryer J L, Rohovec J S. A phylogenetic comparison of the 16S rRNA sequence of the fish pathogen, Renibacterium salmoninarum, to Gram-positive bacteria. FEMS Microbiol Lett. 1991;77:151–156. doi: 10.1016/0378-1097(91)90543-j. [DOI] [PubMed] [Google Scholar]
- 23.Haase A, Smith-Vaughan H, Melder A, Wood Y, Janmaat A, Gilfedder J, Kemp D, Currie B. Subdivision of Burkholderia pseudomallei ribotypes into multiple types by random amplified polymorphic DNA analysis provides new insights into epidemiology. J Clin Microbiol. 1995;33:1687–1690. doi: 10.1128/jcm.33.7.1687-1690.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen B M, Damgaard P H, Eilenberg J, Pedersen J C. Molecular and phenotypic characterization of Bacillus thuringiensis isolated from leaves and insects. J Invertebr Pathol. 1998;71:106–114. doi: 10.1006/jipa.1997.4712. [DOI] [PubMed] [Google Scholar]
- 25.Jensen M A, Webster J A, Straus N. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl Environ Microbiol. 1993;59:945–952. doi: 10.1128/aem.59.4.945-952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kersulyte D, Woods J P, Keath E J, Goldman W E, Berg D E. Diversity among clinical isolates of Histoplasma capsulatum detected by polymerase chain reaction with arbitrary primers. J Bacteriol. 1992;174:7075–7079. doi: 10.1128/jb.174.22.7075-7079.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch C, Rainey F A, Stackebrandt E. 16S rDNA studies on members of Arthrobacter and Micrococcus: an aid for their future taxonomic restructuring. FEMS Microbiol Lett. 1994;123:167–172. [Google Scholar]
- 28.Leblond-Bourget N, Philippe H, Mangin I, Decaris B. 16S rRNA and 16S to 23S internal transcribed spacer sequence analyses reveal inter- and intraspecific Bifidobacterium phylogeny. Int J Syst Bacteriol. 1996;46:102–111. doi: 10.1099/00207713-46-1-102. [DOI] [PubMed] [Google Scholar]
- 29.Magnusson H B, Fridjonsson O H, Andresson O S, Benediktsdottir E, Gudmundsdottir S, Andresdottir V. Renibacterium salmoninarum, the causative agent of bacterial kidney disease in salmonid fish, detected by nested reverse transcription-PCR of 16S rRNA sequences. Appl Environ Microbiol. 1994;60:4580–4583. doi: 10.1128/aem.60.12.4580-4583.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauel, M. J., S. J. Giovannoni, and J. L. Fryer. Phylogenetic analysis of Piscirickettsia salmonis by 16S, internal transcribed spacer (ITS) and 23S ribosomal DNA sequencing. Dis. Aquat. Org., in press. [DOI] [PubMed]
- 31.Meunier J R, Grimont P A. Factors affecting reproducibility of random amplified polymorphic DNA fingerprinting. Res Microbiol. 1993;144:373–379. doi: 10.1016/0923-2508(93)90194-7. [DOI] [PubMed] [Google Scholar]
- 32.Meyers T R, Short S, Farrington C, Lipson K, Geiger H J, Gates R. Establishment of a negative-positive threshold optical density value for the enzyme-linked immunosorbent assay (ELISA) to detect soluble antigen of Renibacterium salmoninarum in Alaskan Pacific salmon. Dis Aquat Org. 1993;16:191–197. [Google Scholar]
- 33.Myers E, Miller W. Optimal alignments in linear space. CABIOS. 1988;4:11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- 34.Nakao H, Popovic T. Use of random amplified polymorphic DNA for rapid molecular subtyping of Corynebacterium diphtheriae. Diagn Microbiol Infect Dis. 1998;30:167–172. doi: 10.1016/s0732-8893(97)00237-x. [DOI] [PubMed] [Google Scholar]
- 35.Penner G A, Bush A, Wise R. Reproducibility of random amplified polymorphic DNA (RAPD) analysis among laboratories. PCR Methods Applic. 1993;2:341–345. doi: 10.1101/gr.2.4.341. [DOI] [PubMed] [Google Scholar]
- 36.Regensburger A, Ludwig W, Frank R, Blocker H, Schleifer K H. Complete nucleotide sequence of a 23S ribosomal RNA gene from Micrococcus luteus. Nucleic Acids Res. 1988;16:2344. doi: 10.1093/nar/16.5.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sela S, Clark-Curtiss J E, Bercovier H. Characterization and taxonomic implications of the rRNA genes of Mycobacterium leprae. J Bacteriol. 1989;171:70–73. doi: 10.1128/jb.171.1.70-73.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stackebrandt E, Wehmeyer U, Nader H, Fiedler F. Phylogenetic relationship of the fish pathogenic Renibacterium salmoninarum to Arthrobacter, Micrococcus and related taxa. FEMS Microbiol Lett. 1988;50:117–120. [Google Scholar]
- 39.Starliper C E. Genetic diversity of North American isolates of Renibacterium salmoninarum. Dis Aquat Org. 1996;27:207–213. [Google Scholar]
- 40.Van der Giessen J W B, Haring R M, van der Zeijst B A M. Comparison of the 23S ribosomal RNA genes and the spacer region between the 16S and 23S rRNA genes of the closely related Mycobacterium avium and Mycobacterium paratuberculosis and the fast-growing Mycobacterium phlei. Microbiology. 1994;140:1103–1108. doi: 10.1099/13500872-140-5-1103. [DOI] [PubMed] [Google Scholar]
- 41.Wang G, van Dam A P, Spanjaard L, Dankert J. Molecular typing of Borrelia burgdorferi sensu lato by randomly amplified polymorphic DNA fingerprinting analysis. J Clin Microbiol. 1998;36:768–776. doi: 10.1128/jcm.36.3.768-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G, Wittam W T S, Berg C M, Berg D M. RAPD (arbitrary primer) PCR is more sensitive than multilocus enzyme electrophoresis for distinguishing related bacterial strains. Nucleic Acids Res. 1993;21:5930–5933. doi: 10.1093/nar/21.25.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh J, Ralph D, McClelland M. DNA and RNA fingerprinting using arbitrarily primed PCR. In: Innis M A, Gelfand D H, Sninsky J J, editors. PCR strategies. London, United Kingdom: Academic Press; 1995. pp. 249–275. [Google Scholar]
- 45.Williams J G K, Kubelik A R, Livak K J, Rafalski J A, Tingley S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]