ABSTRACT
Brazil ranks second among countries for new cases and first for relapse cases of leprosy worldwide. The Mycobacterium leprae Resistance Surveillance Plan was established. We aimed to present the results of a 2-year follow-up of the National Surveillance Plan in Brazil. A cross-sectional study of leprosy cases was performed to investigate antimicrobial resistance (AMR) in Brazil from October 2018 to September 2020. Molecular screening targeting genes related to dapsone (folP1), rifampin (rpoB), and ofloxacin resistance (gyrA) was performed. During the referral period, 63,520 active leprosy patients were registered in Brazil, and 1,183 fulfilled the inclusion criteria for molecular AMR investigation. In total, only 16 (1.4%) patients had genetic polymorphisms associated with AMR. Of these, 8 (50%) had cases of leprosy relapse, 7 (43.8%) had cases of suspected therapeutic failure with standard treatment, and 1 (6.2%) was a case of new leprosy presentation. M. leprae strains with AMR-associated mutations were found for all three genes screened. Isolates from two patients showed simultaneous resistance to dapsone and rifampin, indicating multidrug resistance (MDR). No significant relationship between clinical variables and the presence of AMR was identified. Our study revealed a low frequency of AMR in Brazil. Isolates were resistant mainly to dapsone, and a very low number of isolates were resistant to rifampin, the main bactericidal agent for leprosy, or presented MDR, reinforcing the importance of the standard World Health Organization multidrug therapy. The greater frequency of AMR among relapsed patients supports the need to constantly monitor this group.
KEYWORDS: Brazil, drug resistance, M. leprae, sequence analysis, DNA, surveillance, leprosy
INTRODUCTION
Leprosy is a chronic infectious disease caused by Mycobacterium leprae and Mycobacterium lepromatosis (1). The average annual number of new cases over the last 10 years has remained stable worldwide. In 2019, 202,185 new cases and 3,897 relapse cases were reported in more than 120 countries, demonstrating the maintenance of the transmission chain (2). The monitoring of antimicrobial resistance (AMR) in M. leprae isolated from patients with new and relapsed cases has been recommended by the World Health Organization (WHO), with the purpose of evaluating the effectiveness of multidrug therapy (MDT). Current guidelines are focused mainly on the investigation of resistance to rifampin, the backbone drug in the WHO-MDT regimen (3). AMR can result in persistent infection and ineffective treatment regimens, hindering leprosy control measures (4).
Recent studies in China (5), France (6), and India (7) reported AMR proportions of 25%, 11.2%, and 7% among leprosy isolates, respectively. Despite these reports, it is still unknown whether AMR affects the maintenance of disease burden. The 12-dose regimen of WHO-MDT is highly effective (8), and it has been successful in the treatment of cases of isolated resistance to dapsone (9). Brazil is among the 23 countries with the highest burdens of the disease, and in 2019, it was responsible for 13.7% of new cases and 43.5% of relapse cases worldwide (2).
The importance of investigating resistant M. leprae strains in Brazil was highlighted after the publication of study results obtained from sentinel surveillance in 19 countries between 2009 and 2015. The proportion of rifampin resistance found in Brazil seemed alarming, at 9.1%, compared to the registered global proportion of 3.8% (10). Previous studies in Brazil showed 5% rifampin resistance and 2% multidrug resistance (MDR) (11, 12). Most resistant cases were identified through research conducted by tertiary reference centers (11–13) and may not represent the true national epidemiological scenario.
The main objective of the present study is to present the data from a 2-year follow-up of the M. leprae Resistance Surveillance Plan elaborated by the National Leprosy Program at the Brazilian Ministry of Health (MH). The plan covers all Brazilian states where biopsy samples are collected from primary and retreatment cases as indicated by the WHO. We also aimed to investigate possible clinical characteristics related to AMR.
RESULTS
Characteristics of the investigated patients.
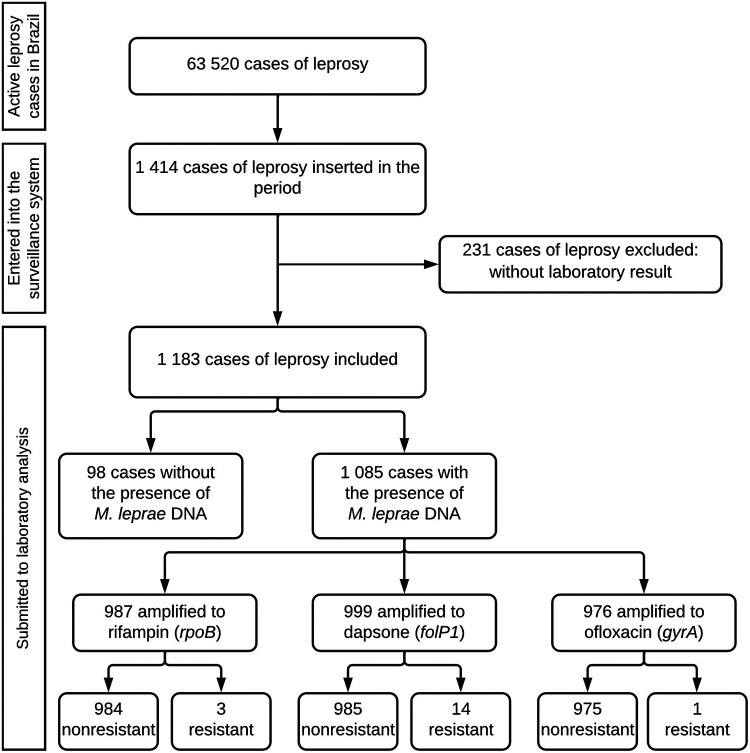
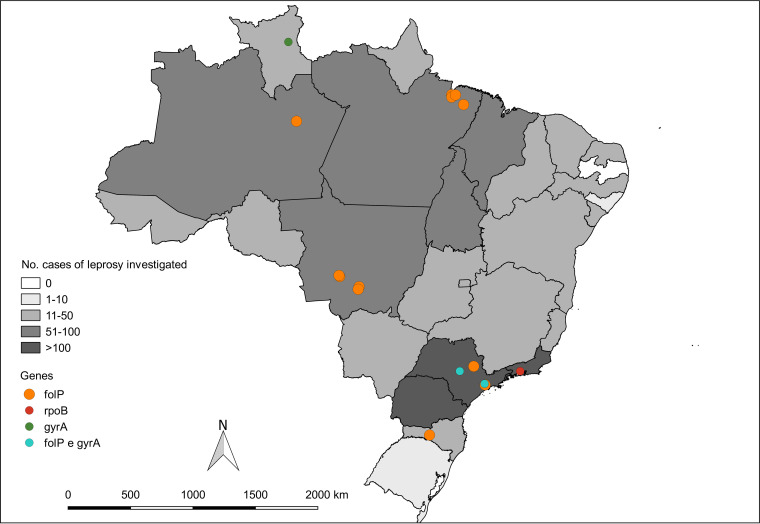
From October 2018 to September 2020, 63,520 active leprosy patients were registered in Brazil, and isolates from 1,414 (2.23%) cases of leprosy were investigated for AMR. Among these, 1,183 (83.66%) were included in this study, and 16 (1.35%) cases had AMR (Fig. 1). In the demographic analysis, there was a predominance of 860 males (72.69%), with a female/male sex ratio of 1:3. A total of 887 (74.98%) patients were using MDT, and 421 (35.59%) presented active reactional states during AMR investigation. The demographic and clinical characteristics of the leprosy patients are detailed in Table 1. The investigation was performed in 26 Brazilian states, among which 3 states reported more than 100 cases investigated. Resistant cases were diagnosed in 7 states, with higher diagnosis numbers in three states: Pará (4 cases), Mato Grosso (4 cases), and São Paulo (4 cases) (Fig. 2).
FIG 1.
Flowchart of leprosy cases investigated for AMR in Brazil between October 2018 and September 2020.
TABLE 1.
Characteristics of patients with leprosy investigated for AMR from October 2018 to September 2020 in Brazila
| Variable | Total (n = 1,183) | New case (n = 344) | Relapse (n = 305) | Suspected treatment failure (n = 534) |
|---|---|---|---|---|
| Age, yrs, median (IQR) | 47 (37–49) | 48 (35–49) | 50 (39–61) | 46 (37–59) |
| Sex, male, n (%) | 860 (72.7) | 241 (70.1) | 217 (71.1) | 402 (75.3) |
| Region | ||||
| North, n (%) | 309 (26.1) | 79 (23.0) | 79 (25.9) | 151 (28.3) |
| Northeast, n (%) | 192 (16.2) | 65 (18.9) | 35 (11.5) | 92 (17.2) |
| Southeast, n (%) | 407 (34.4) | 137 (39.8) | 125 (41.0) | 145 (27.1) |
| South, n (%) | 138 (11.7) | 31 (9.0) | 46 (15.1) | 61 (11.4) |
| Midwest, n (%) | 137 (11.6) | 32 (9.3) | 20 (6.5) | 85 (16.0) |
| Clinical form, WHO-multibacillary, n (%) | 1017 (86.0) | 223 (64.8) | 278 (91.1) | 516 (96.6) |
| Reaction, yes, n (%) | 421 (35.6) | 90 (26.2) | 102 (33.4) | 229 (42.9) |
| Treatment regimen, WHO-MDT, n (%) | 887 (75.0) | 220 (64.0) | 260 (85.2) | 407 (76.2) |
| Presence of M. leprae DNA, yes, n (%) | 1085 (91.7) | 321 (93.3) | 273 (89.5) | 491 (92.0) |
| Bacilloscopic index, median (IQR) | 3.00 (2.00–4.25) | 3.50 (2.25–4.75) | 2.00 (0.66–3.95) | 3.00 (1.75–4.00) |
MDT, multidrug therapy; WHO, World Health Organization; presence of M. leprae DNA detected by PCR; IQR, interquartile range.
FIG 2.
Map of the states of Brazil that investigated cases of leprosy for AMR and samples with drug resistance between October 2018 and September 2020.
In the subgroup analysis, isolates from patients being treated for suspected therapeutic failure were the most investigated isolates, with 534 (45.14%) patients in this group; the mean time of treatment at the time of the investigation was 12 months, and the mean positive bacilloscopic index (BI) was 2.80+. New multibacillary cases were the second most investigated subgroup, with 344 (29.08%) patients, of whom 283 (82.27%) had a positive BI, with an average of 3.60+. Finally, among 305 (25.78%) relapse cases investigated, 230 (75.41%) had information about the date of the previous treatment, with an average time of 12 years between the last treatment and the current treatment, and the mean positive BI was 2.80+. Of the 33 paucibacillary cases investigated for AMR, 24 (72.7%) presented a positive PCR result, with amplification of at least one of the three genes evaluated, and of those, 14 (58.33%) were clinically tuberculoid, 10 (41.66%) were classified as relapse, and 9 (37.50%) were classified as suspected therapeutic failure.
AMR findings and characteristics of patients with resistant infection.
Among the 1,085 cases positive for the presence of M. leprae DNA, amplification was successful for rpoB in 987 (90.96%) samples, for folP1 in 999 (92.07%) samples, and for gyrA in 976 (89.95%) samples. The proportion of isolates with dapsone resistance was 1.2%, representing 12 cases. Another two cases (0.2%) concomitantly had resistance to rifampin and dapsone. We also identified one case of isolated rifampin resistance and one case of isolated ofloxacin resistance (0.1% each).
Table 2 shows the characteristics for each resistant strain. Resistant M. leprae strains were found more frequently in patients with leprosy relapse. In this subgroup, the frequency of resistance to rifampin alone was 1/273 (0.36%), that of dapsone resistance alone was 5/273 (1.83%), that of ofloxacin resistance alone was 1/273 (0.36%), and that of MDR (dapsone and rifampin) was 1/273 (0.36%). The average time between initial cure and relapse in AMR patients was 8.5 years. Among the eight relapse-resistant cases, four patients experienced treatment irregularity, two patients were lost to follow-up, and two patients possibly received inappropriate paucibacillary treatment.
TABLE 2.
Characteristics of leprosy cases with antimicrobial resistance from October 2018 to September 2020 in Brazila
| No. | Case | Resistanceb | Genotype | Region | Age | Sex | Clinical form, WHO | BI | Treatment | Reaction | Relapse data |
Suspected treatment failure data |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time of relapse (yrs) | Previous treatment/no. doses | Outcome of previous treatment | Entry/yr | Current treatment/no. doses | |||||||||||
| 1 | Relapse | Rifampin + dapsone | rpoB TCG 456 TTG (Ser-Leu) folP1 CCC 55 CGC (Pro-Arg) | Southeast | 61 | F | MB | NA | Substitutive | NA | NA | MDT-MB irregular/9 | Abandonment | ||
| 2 | Relapse | Dapsone | folP1 CCC 55 CGC (Pro-Arg) | Southeast | 53 | F | MB | 0.0 | MDT | No | 7 | MDT-PB regular/6 | Cure | ||
| 3 | Relapse | Dapsone | folP1 CCC 55 CGC (Pro-Arg) | North | 28 | F | MB | 4.0 | MDT | Yes | NA | MDT-MB irregular/8 | Abandonment | ||
| 4 | Relapse | Dapsone | folP1 CCC 55 CGC (Pro-Arg) | Midwest | 42 | M | MB | NA | MDT | No | 12 | MDT-MB regular/12 | Cure | ||
| 5 | Relapse | Dapsone | folP1 ACC 53 GCC (Thr-Ala) | South | 80 | M | MB | NA | MDT | No | 6 | MDT-MB regular/24 | Cure | ||
| 6 | Relapse | Rifampin | rpoB TCG 456 ATG (Ser-Met) | Southeast | 24 | M | MB | 2.75 | MDT | No | 9 | MDT-PB irregular/6 | Cure | ||
| 7 | Relapse | Dapsone | folP1 ACC 53 ATC (Tre-Ile) | North | 80 | F | MB | 4.0 | MDT | NA | NA | NA | NA | ||
| 8 | Relapse | Ofloxacin | gyrA GCA 91 GTA (Ala-Val) | North | 37 | M | MB | 3.25 | MDT | Yes | NA | MDT-MB irregular/12 | Cure | ||
| 9 | Suspected treatment failure | Rifampin + dapsone | rpoB TCG 456 TTG (Ser-Leu) folP1 CCC 55 CGC (Pro-Arg) | Southeast | 36 | M | MB | NA | MDT | No | New case/2018 | MDT-MB/17 | |||
| 10 | Suspected treatment failure | Dapsone | folP1 CCC 55 CGC (Pro-Arg) | Midwest | 51 | F | MB | NA | MDT | No | New case/2018 | MDT-MB/17 | |||
| 11 | Suspected treatment failure | Dapsone | folP1 CCC 55 CGC (Pro-Arg) | Midwest | 28 | M | MB | NA | MDT | Yes | Reentry/2019 | MDT-MB/15 | |||
| 12 | Suspected treatment failure | Dapsone | folP1 ACC 53 GCC (Thr-Ala) | Midwest | 51 | M | MB | 1.0 | Substitutive | Yes | New case/2015 | MDT-MB/24 | |||
| 13 | Suspected treatment failure | Dapsone | folP1 CCC 55 CGC (Pro-Arg) | North | 75 | F | MB | 4.0 | MDT | No | Reentry/2019 | MDT-MB/17 | |||
| 14 | Suspected treatment failure | Dapsone | folP1 CCC 55 CGC (Pro-Arg) | North | 24 | F | MB | 4.0 | Substitutive | No | New case/2017 | MDT-MB/NA | |||
| 15 | Suspected treatment failure | Dapsone | folP1 CCC 55 CGC (Pro-Arg) | North | 44 | F | MB | 3.3 | MDT | Yes | New case/2017 | MDT-MB/NA | |||
| 16 | New case | Dapsone | folP1 CCC 55 CGC (Pro-Arg) | Southeast | 59 | M | MB | 2.0 | MDT | Yes | |||||
M, male; F, female; WHO, World Health Organization; MB, multibacillary; BI, bacilloscopic index; NA, not available; MDT, multidrug therapy; substitutive, treatment regimen with ofloxacin, minocycline, and clarithromycin.
Resistance inferred from mutations in drug resistance-determining regions that have been proven to cause resistance.
AMR was detected in seven patients being treated for suspected therapeutic failure; of these, 6/491 (1.22%) had dapsone resistance alone, and 1/491 (0.20%) had MDR (dapsone and rifampin). All of these patients were in their second cycle of treatment. Among new cases, only 1/321 (0.31%) had isolated dapsone resistance (Table 2).
Bivariate and multivariate analysis.
In the subgroup analysis, patients with leprosy relapse presented a greater proportion of resistant cases than patients suspected of treatment failure and new multibacillary cases, with an odds ratio (OR) of 3.03 (95% confidence interval [CI] of 1.13 to 8.16; P = 0.043); however, in the multivariate analysis, this association was not confirmed: OR = 8.12 (95% CI = 1.35 to 154.86; P = 0.054). No other significant association was observed (Table 3).
TABLE 3.
Demographic and clinical characteristics of patients with leprosy and the results of univariate and multivariate risk factor analyses
| Variable | n a | Resistant | Nonresistant | OR (95% Cl) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| Age (yrs), n (%) | |||||||
| >50b | 462 | 8 (1.7) | 454 (98.3) | 0.74 (0.28–1.98)e | 0.726 | 0.55 (0.08–3.32) | 0.525 |
| ≤50 | 623 | 8 (1.3) | 615 (98.7) | ||||
| Sex, n (%) | |||||||
| Female | 286 | 8 (2.79) | 278 (97.21) | 2.85 (1.06–7.65)e | 0.060 | 2.21 (0.71–6.50) | 0.147 |
| Male | 799 | 8 (1.00) | 791 (99.00) | ||||
| Region, n (%) | |||||||
| North | 282 | 6 (2.13) | 276 (97.87) | 1.72 (0.62–4.79)c,e | 0.441 | ||
| Northeast | 166 | 0 (0.00) | 166 (100.00) | 1.000 | |||
| Southeast | 368 | 5 (1.36) | 363 (98.64) | 0.88 (0.30–2.56)c,f | 1.000 | ||
| South | 135 | 1 (0.74) | 134 (99.26) | 0.47 (0.06–3.55)c,f | 0.708 | ||
| Midwest | 134 | 4 (2.99) | 130 (97.01) | 2.41 (0.77–7.58)c,f | 0.124 | ||
| Case, n (%) | |||||||
| Relapse | 273 | 8 (2.93) | 265 (97.07) | 3.03 (1.13–8.16)d,e | 0.043 | 8.12 (1.35–154.86) | 0.054 |
| Suspected treatment failure | 491 | 7 (1.43) | 484 (98.57) | 0.94 (0.35–2.54)d,e | 1.000 | 4.86 (0.84–91.71) | 0.141 |
| New case | 321 | 1 (0.31) | 320 (99.69) | 0.16 (0.02–1.19)d,f | 0.049 | ||
| Clinical form, WHO, n (%) | |||||||
| Multibacillary | 942 | 16 (1.70) | 926 (98.30) | 1.000 | |||
| Paucibacillary | 24 | 0 (0.00) | 24 (100.00) | ||||
| Reaction, n (%) | |||||||
| Yes | 389 | 6 (1.54) | 383 (98.46) | 1.11 (0.38–3.24)e | 1.000 | 0.93 (0.30–2.74) | 0.899 |
| No | 577 | 8 (1.39) | 569 (98.61) |
Analysis performed only with patients with the presence of M. leprae DNA detected by PCR. Data are expressed as absolute frequency (n) and percentage (%) with tailed P values for categorical variables, chi-square test or Fisher’s exact test, and for numerical variables, Mann–Whitney U.
Cutoff at 50 years old. Variables with low variability were dropped from the multivariate analysis.
Compared to all the others in the group.
Compared to all the others in the group.
Categorical variables determined by chi-square test.
Categorical variables determined by Fisher’s exact test.
DISCUSSION
The strategies indicated by the WHO to ensure the success of therapeutic schemes for leprosy include the early detection and monitoring of AMR (14). The present study shows a low frequency of AMR, especially for rifampin, the primary drug in WHO-MDT. In addition, we did not identify any significant relationship between clinical characteristics and the occurrence of AMR. This is the first study conducted in Brazil that analyzed national data and a large number of samples. The present data are in line with studies conducted 10 years ago indicating low rates of rifampin resistance (11, 12).
In our study, AMR was more prevalent in isolates from relapse cases, which has also been observed in other countries (15–17). However, the frequency of AMR in this subgroup was lower than that reported by the WHO surveillance network between 2010 and 2015, with 10% for rifampin, 13% for dapsone, and 2% for ofloxacin among the 254 cases investigated in Brazil (10). The frequency of AMR was also lower than that in the studies by da Silva Rocha et al. (11), with a 1.08% rate of resistance to rifampin and a 3.26% rate of MDR, and Contreras et al. (12), with a 2.5% rate of rifampin resistance, performed in Brazil. These differences can be explained mainly by different study periods, coverage areas, sample sizes, and selection criteria. Small samples can result in overestimated effect sizes. However, AMR surveillance in this subgroup is important, as recurrence due to antimicrobial-resistant leprosy can maintain an increased frequency of primary resistance.
An important finding is the number of leprosy cases with suspected therapeutic failure investigated for AMR; interestingly, the frequency of AMR in this subgroup was the second highest found. The concept of therapeutic failure in leprosy has been discussed in Brazil for years. Due to the replication time of M. leprae, it is likely that these are cases of primary resistance and mainly due to dapsone, since irrespective of discussion in the 1980s, it seems that dapsone-resistant strains can survive. This finding indicates that an improved MDT scheme replacing dapsone with minocycline, for example, could be used.
As expected, isolated dapsone resistance was the most frequently observed type of resistance. This result was similar to findings in Malaysia (18), France (6), and the Ivory Coast (19) but different from findings in China (20) and India (16), where rifampin resistance was more frequent. The frequency of isolated resistance to rifampin and ofloxacin was considered very low, in contrast to results reported in India (7); however, our findings were similar to those in studies by Wahyuni et al. (17) and Chen et al. (5) Our results support the effectiveness of disease control actions according to the WHO-MDT regimen (8). We found two cases of MDR (rifampin plus dapsone). This number was lower than the 12 reported in the study conducted by Rosa et al. (21) in a cluster area located in a hyperendemic state in northern Brazil. These data indicate a peculiarity of the population of a former colony that has not been observed in the rest of the country.
The frequency of primary resistance was not high in this study. This result differs from the findings of research conducted in the northern region of Brazil in Manaus (12), which reported a primary resistance frequency of 2.30% (3/126), and in Pará (21), which reported a primary resistance frequency of 31.50% (6/19). The national AMR surveillance program aims to contribute information to support the establishment of national guidelines (22, 23). Treatment irregularity, loss to follow-up, possible inappropriate treatment regimens, and prolonged treatment periods were characteristics of resistant cases in the relapse and therapeutic failure subgroups. Notably, of the total cases reported during the study period, only 1% were new multibacillary cases and 9.60% were relapse cases.
In the genotype analysis, the mutation identified most frequently in cases of resistant leprosy was in codon 55 (Pro-Arg), which confers dapsone resistance. This mutation has also been reported as the most prevalent mutation in previous studies (11, 21). This result suggests that there is probably persistence of this genotype during disease transmission in Brazil, demonstrating the need to monitor dapsone resistance mutations and perform more consistent investigations of detected primary and index cases. The multivariate analysis model used showed that none of the demographic or clinical variables influenced the occurrence of drug resistance. The same results were found in studies in Colombia (24), China (25), and India (26). This may indicate that AMR is more related to the inherent characteristics of the bacillus and that it is probably influenced by factors such as irregular use of antimicrobials (27). Leprosy relapse was associated with resistance detection in the multivariate analysis, but the CI was considerably wide. However, these patients should be constantly monitored for AMR.
A limitation of this study is the poor quality of some of the records in the surveillance system; some of these had a high proportion of blank and/or incomplete fields for important variables. The lack of completeness can also hinder the guidance of national management strategies based on the real-world scenario of AMR. Reduced power was related to few instances of resistance in M. leprae. We also identified other important limitations, such as the absence of data regarding previous or concomitant treatment for other chronic infections, the short period of operation of the National Surveillance Plan, and the fact that different mechanisms of resistance related to other genes have not yet been identified (28). Further studies are needed on AMR in leprosy, mainly prospective studies capable of analyzing risk associations and reporting other types of mutations.
In conclusion, this study revealed a very low percentage of AMR in leprosy in Brazil and, consequently, stresses that the standard WHO-MDT regimen is effective in preventing cases of resistant leprosy. Moreover, the results support that ofloxacin is the best choice to replace rifampin in cases of intolerance and resistance in Brazil.
MATERIALS AND METHODS
Study design, population, and data source.
This was a nationwide cross-sectional study of all leprosy cases that included the collection of biological materials for the investigation of AMR in Brazil. Patients were classified mainly as new leprosy cases and those who needed to be retreated. We also recorded and classified the main situations that require retreatment as follows. (i) Suspected therapeutic failure: patients who, at the end of the first adequate leprosy treatment still present signs of disease activity, such as untreatable leprosy reactions and anergic forms of leprosy. (ii) Relapse: patients who developed disease activity long after the first treatment. The inclusion criteria for AMR investigation in Brazil comprised patients referred to sentinel centers classified as new multibacillary cases with a bacilloscopic index (BI) greater than or equal to 2+. Additionally, cases of relapsed leprosy and cases classified as suspected therapeutic failure were investigated without any restriction related to the BI (22).
The demographic, clinical, and laboratory data of leprosy cases investigated regarding AMR were obtained from the databases of the Electronic Forms System (FORMSUS) from 1 October 2018 to 30 September 2020, which is the system of the surveillance plan. Additional information from previous and current treatment of patients classified as relapse and suspected therapeutic failure were also collected from the Information System of Notifiable Diseases (SINAN) from 2001 to 2020. Both are systems of the MH. Patients without laboratory results from the molecular investigation were excluded. The study was approved by the Research Ethics Committee of the Faculdade de Saúde da Universidade de Brasília (no. 4.391.397). Patient informed consent was waived after ethical approval, as relevant data were included in the national surveillance system.
AMR detection.
The detection of AMR was performed in three reference laboratories (Fundação Alfredo da Matta, Instituto Lauro Souza Lima, and Fundação Oswaldo Cruz) that standardized the molecular technique according to WHO guidelines (29, 30). The technique used is a DNA-based direct-sequencing PCR molecular test to detect mutations or single-nucleotide polymorphisms (SNPs) that have been associated with M. leprae resistance to drugs (31). The laboratories conducted the PCR for amplification of M. leprae DNA fragments isolated from a patient’s skin biopsy. Then, genetic sequencing (PCR-DNA sequencing) was used to identify mutations or SNPs present in the drug resistance determinant regions (DRDRs) of the folP1 gene for dapsone, rpoB gene for rifampin, and gyrA gene for ofloxacin (30).
Skin biopsy specimens were obtained from the border of a skin lesion. The collection of biological material from new multibacillary and relapse patients was performed at the time of diagnosis (22). The target regions of the folP1 (gene ID: 908646), rpoB (gene ID: 910599), and gyrA (gene ID: 908154) genes, available under GenBank accession no. NC002677, were used as standards. The sequences were aligned using MEGA7, Molecular Evolutionary Genetics Analysis version 7.0, for larger data sets (32).
Data analysis.
The proportion of cases of resistant leprosy among the leprosy cases investigated was calculated as recommended by WHO (33). To identify factors associated with the presence of AMR, a bivariate analysis was conducted using Pearson’s chi-square test or Fischer’s exact test for categorical variables (sex, region, case, clinical form, and reaction). Numerical variables (age and BI) were compared using the Mann–Whitney U test. Variables that could significantly influence the occurrence of AMR were selected by a leprosy specialist based on clinical criteria and were inserted into a multivariate logistic regression model. A cutoff point of 50 years was considered for age based on the mean value found for leprosy patients. Subgroup analyses were also used to explore the characteristics of new leprosy cases, relapsed leprosy cases, and suspected therapeutic failure cases. Associations are expressed as odds ratios (ORs) with 95% confidence intervals (CIs) in the bivariate analysis and adjusted ORs (aORs) in the multivariate analysis to reduce confounding and corresponding P values. Losses were ignored, and missing values resulted in the exclusion of the case in the multivariate analysis.
Rstudio software version 1.2.1335 (Rstudio Team, Boston, USA) was used for statistical analysis, and the QGIS Geographic Information System (Open-Source Geospatial Foundation Project version 2.18; http://qgis.osgeo.org) was used for map preparation.
ACKNOWLEDGMENTS
We thank all the staff members of the Brazilian Ministry of Health for their support in the acquisition of relevant data for the performance of the present study. We thank the Federal District Research Foundation (FAP-DF) that supported this publication (Edital no. 11/2022). There was no funding source for this study. E.S.N.A: conceptualization, investigation, data curation, writing – original draft, writing – review & editing, visualization; J.G.B: investigation, data curation, writing – original draft; J.S.S: data curation, writing – original draft; C.R.F.C: data curation, writing – review & editing; P.S.R: data curation, writing – review & editing; M.O.M: data curation, writing – review & editing; C.O.F: data curation, writing – review & editing; C.M.G: conceptualization, writing – review & editing, visualization; W.N.A: conceptualization, investigation, formal analysis, data curation, writing – original draft, writing – editing, visualization. We declare no competing interests.
REFERENCES
- 1.Han XY, Sizer KC, Velarde-Félix JS, Frias-Castro LO, Vargas-Ocampo F. 2012. The leprosy agents Mycobacterium lepromatosis and Mycobacterium leprae in Mexico. Int J Dermatol 51:952–959. 10.1111/j.1365-4632.2011.05414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2020. Global leprosy (Hansen disease) update, 2019: time to step-up prevention initiatives. Weekly Epidemiological Record 95:417–440. http://www.who.int/wer. [Google Scholar]
- 3.World Health Organization. 2009. Guidelines for global surveillance of drug resistance in Leprosy. WHO Regional Office for South-East Asia, New Delhi. https://apps.who.int/iris/handle/10665/205158. [Google Scholar]
- 4.Matsuoka M. 2010. Drug resistance in leprosy. Jpn J Infect Dis 63:1–7. 10.7883/yoken.63.1. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, He J, Liu J, You Y, Yuan L, Wen Y. 2019. Nested PCR and the TaqMan SNP genotyping assay enhanced the sensitivity of drug resistance testing of Mycobacterium leprae using clinical specimens of leprosy patients. PLoS Negl Trop Dis 13:e0007946. 10.1371/journal.pntd.0007946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauffour A, Lecorche E, Reibel F, Mougari F, Raskine L, Aubry A, Jarlier V, Cambau E, CNR-MyRMA. 2018. Prospective study on antimicrobial resistance in leprosy cases diagnosed in France from 2001 to 2015. Clin Microbiol Infect 24:1213.e5–1213.e8. 10.1016/j.cmi.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Lavania M, Singh I, Turankar R, Ahuja M, Pathak V, Sengupta U, Das L, Kumar A, Darlong J, Nathan R, Maseey A. 2018. Molecular detection of multidrug-resistant Mycobacterium leprae from Indian leprosy patients. J Glob Antimicrob Resist. 12:214–9. 10.1016/j.jgar.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Nery JAC, Sales AM, Hacker MAVB, Moraes MO, Maia RC, Sarno EN, Illarramendi X. 2021. Low rate of relapse after twelve-dose multidrug therapy for hansen’s disease: a 20-year cohort study in a brazilian reference center. PLoS Negl Trop Dis 15:e0009382. 10.1371/journal.pntd.0009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opromolla DVA. 1997. Leprosy therapy. Medicina, Ribeirão Preto 30:345–350. 10.11606/issn.2176-7262.v30i3p345-350. (In Portuguese). [DOI] [Google Scholar]
- 10.Cambau E, Saunderson P, Matsuoka M, Cole ST, Kai M, Suffys P, Rosa PS, Williams D, Gupta UD, Lavania M, Cardona-Castro N, Miyamoto Y, Hagge D, Srikantam A, Hongseng W, Indropo A, Vissa V, Johnson RC, Cauchoix B, Pannikar VK, Cooreman EAWD, Pemmaraju VRR, Gillini L, WHO surveillance network of antimicrobial resistance in leprosy. 2018. Antimicrobial resistance in leprosy: results of the first prospective open survey conducted by a WHO surveillance network for the period 2009–15. Clin Microbiol Infect 24:1305–1310. 10.1016/j.cmi.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silva Rocha A, Cunha M.dG, Diniz LM, Salgado C, Aires MAP, Nery JA, Gallo EN, Miranda A, Magnanini MMF, Matsuoka M, Sarno EN, Suffys PN, de Oliveira MLW. 2012. Drug and multidrug resistance among Mycobacterium leprae isolates from Brazilian relapsed leprosy patients. J Clin Microbiol 50:1912–1917. 10.1128/JCM.06561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contreras Mejía MDC, Porto Dos Santos M, Villarouco da Silva GA, da Motta Passos I, Naveca FG, Souza Cunha M.dG, Moraes MO, de Paula L. 2014. Identification of primary drug resistance to rifampin in mycobacterium leprae strains from leprosy patients in Amazonas State. J Clin Microbiol 52:4359–4360. 10.1128/JCM.01688-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damasco M, Talhari S, Viana SM, Signorelli M, Saad MH, Andrade LM. 1986. Secondary dapsone-resistant leprosy in Brazil: a preliminary report. Lepr Rev 57:5–8. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. 2016. Global Leprosy Strategy 2016–2020: accelerating towards a leprosy-free world. WHO Regional Office for South-East Asia, New Delhi, India. https://apps.who.int/iris/handle/10665/208824. [Google Scholar]
- 15.Beltrán-Alzate C, López Díaz F, Romero-Montoya M, Sakamuri R, Li W, Kimura M, Brennan P, Cardona-Castro N. 2016. Leprosy drug resistance surveillance in Colombia: the experience of a sentinel country. PLoS Negl Trop Dis 10:e0005041. 10.1371/journal.pntd.0005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh SK, Kumar A, Nath G, Singh TB, Mishra MN. 2018. Resistance to anti leprosy drugs in multi-bacillary leprosy: a cross sectional study from a tertiary care centre in eastern Uttar Pradesh, India. Indian J Dermatol Venereol Leprol 84:275–279. 10.4103/ijdvl.IJDVL_34_16. [DOI] [PubMed] [Google Scholar]
- 17.Wahyuni R, Adriaty D, Iswahyudi I, Rosita C, Agusni I, Izumi S. 2012. Profile of mutation on drug resistance Mycobacterium leprae isolates in Indonesia collected during 2003–2011. Microbiol Indones 6:135–138. 10.5454/mi.6.3.7. [DOI] [Google Scholar]
- 18.Dalawi I, Tang MM, Osman AS, Ismail M, Bakar RSA, Dony JF, Zainol J, Johar A. 2017. Drug resistance pattern of Mycobacterium leprae from mouse footpad cultivation between 1997 to 2013 in Malaysia. Leprosy Rev 88:463–477. 10.47276/lr.88.4.463. [DOI] [Google Scholar]
- 19.Coulibaly N’Golo D, Roger D, Kakou NG, Solange, Henri K, Christiane AA, Aboubacar S, Philippe BAD, Vagamon B, Mireille D. 2020. Drugs susceptibility testing in leprosy patients from Côte d’Ivoire reveals multidrugs resistance combination cases to dapsone, rifampicin and ofloxacin. Am J Microbiol Res 8:160–163. http://www.sciepub.com/ajmr/abstract/12657. [Google Scholar]
- 20.Liu D, Zhang Q, Sun Y, Wang C, Zhang Y, Fu X, Chen M, Zhou G, Yu X, Wang J, Liu H, Zhang F. 2015. Drug resistance in Mycobacterium leprae from patients with leprosy in China. Clin Exp Dermatol 40:908–911. 10.1111/ced.12665. [DOI] [PubMed] [Google Scholar]
- 21.Rosa PS, D’Espindula HRS, Melo ACL, Fontes ANB, Finardi AJ, Belone AFF, Sartori BGC, Pires CAA, Soares CT, Marques FB, Branco FJD, Baptista IMFD, Trino LM, Fachin LRV, Xavier MB, Floriano MC, Ura S, Diório SM, Delanina WFB, Moraes MO, Virmond MCL, Suffys PN, Mira MT. 2020. Emergence and transmission of drug-multidrug-resistant Mycobacterium leprae in a former leprosy colony in the Brazilian Amazon. Clin Infect Dis 70:2054–2061. 10.1093/cid/ciz570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brazil Ministry of Health, Secretary of Health Surveillance. 2020. Technical note no. 8/2020-CGDE/DCCI/SVS/MS: surveillance of antimicrobial resistance in leprosy. Ministry of Health, Brazil. http://www.aids.gov.br/pt-br/legislacao/nota-tecnica-no-82020-cgdedccisvsms. (In Portuguese). [Google Scholar]
- 23.Brazil Ministry of Health, Secretary of Health Surveillance. 2016. Guidelines for surveillance, care and elimination of leprosy as a public health problem. Ministry of Health, Brazil. https://www.saude.gov.br/images/pdf/2016/fevereiro/04/diretrizes-eliminacao-hanseniase-367. (In Portuguese). [Google Scholar]
- 24.Guerrero MI, Colorado CL, Torres JF, León CI. 2013. Is drug-resistant Mycobacterium leprae a real cause for concern? First approach to molecular monitoring of multibacillary Colombian patients with and without previous leprosy treatment. biomedica 34:137–147. 10.7705/biomedica.v34i0.1686. [DOI] [PubMed] [Google Scholar]
- 25.Chokkakula S, Chen Z, Wang L, Jiang H, Chen Y, Shi Y, Zhang W, Gao W, Yang J, Li J, Li X, Shui T, He J, Shen L, Liu J, Wang D, Wang H, Chen H, Kuang Y, Li B, Chen Z, Wu A, Yu M, Yan L, Suryadevara NC, Vissa V, Liu W, Wang H. 2019. Molecular surveillance of antimicrobial resistance and transmission pattern of Mycobacterium leprae in Chinese leprosy patients. Emerg Microbes Infect 8:1479–1489. 10.1080/22221751.2019.1677177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahajan NP, Lavania M, Singh I, Nashi S, Preethish-Kumar V, Vengalil S, Polavarapu K, Pradeep-Chandra-Reddy C, Keerthipriya M, Mahadevan A, Yasha TC, Nandeesh BN, Gnanakumar K, Parry GJ, Sengupta U, Nalini A. 2020. Evidence for Mycobacterium leprae drug resistance in a large cohort of leprous neuropathy patients from India. Am J Trop Med 102:547–552. 10.4269/ajtmh.19-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avanzi C, Maia RC, Benjak A, Nery JA, Sales AM, Miranda A, Duppre NC, Nogueira Brum Fontes A, Pereira da Silva T, Olmo Pinheiro R, Neves-Manta F, Moreira SJM, Busso P, Sarno EN, Suffys PN, Cole ST, Moraes MO. 2020. Emergence of Mycobacterium leprae rifampin resistance evaluated by whole-genome sequencing after 48 years of irregular treatment. Antimicrob Agents Chemother 64. 10.1128/AAC.00330-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrade ESN, Brandão JG, da Silva JS, Kurizky PS, Rosa PS, de Araújo WN, Gomes CM. 2021. A systematic review and meta-analysis of studies on the diagnostic accuracy and screening of tests to detect antimicrobial resistance in leprosy. Diagn Microbiol Infect Dis 100:115325. 10.1016/j.diagmicrobio.2021.115325. [DOI] [PubMed] [Google Scholar]
- 29.Brazil Ministry of Health, Secretary of Health Surveillance. 2018. Technical note no. 31 CGHDE/CGLAB/DEVIT/SVS/MS: implementation of the protocol for the investigation of drug resistance in leprosy and establishment of the sample shipment flow. Ministry of Health, Brazil. [Google Scholar]
- 30.World Health Organization. 2017. A guide for surveillance of antimicrobial resistance in leprosy. World Health Organization, Regional Office for South-East Asia, New Delhi, India. https://apps.who.int/iris/rest/bitstreams/1137285/retrieve. [Google Scholar]
- 31.Sekar B, Arunagiri K, Kumar BN, Narayanan S, Menaka K, Oommen PK. 2011. Detection of mutations in folP1, rpoB and gyrA genes of M. leprae by PCR- direct sequencing - a rapid tool for screening drug resistance in leprosy. Lepr Rev 82:36–45. [PubMed] [Google Scholar]
- 32.Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. 2017. Global leprosy strategy 2016–2020. Accelerating towards a leprosy-free world. Monitoring and Evaluation Guide. WHO Regional Office for South-East Asia, New Delhi, India. https://apps.who.int/iris/handle/10665/254907. [Google Scholar]