ABSTRACT
Ceftolozane-tazobactam (C/T), imipenem-relebactam (IMR), and ceftazidime-avibactam (CZA) were tested against 2,531 P. aeruginosa strains isolated from patients in the United States from 2018 to 2020 as part of the SMART (Study for Monitoring Antimicrobial Resistance Trends) surveillance program. MICs were determined by CLSI broth microdilution and interpreted using CLSI M100 (2021) breakpoints. Imipenem-, IMR-, or C/T-nonsusceptible isolates were screened for β-lactamase genes: 96.4% of all isolates and ≥70% of multidrug-resistant (MDR), pan-β-lactam-nonsusceptible, and difficult-to-treat resistance (DTR) isolates were C/T-susceptible; 52.2% of C/T-nonsusceptible isolates remained susceptible to IMR compared to 38.9% for CZA; and 1.7% of isolates tested were nonsusceptible to both C/T and IMR versus 2.2% of isolates with a C/T-nonsusceptible and CZA-resistant phenotype (a difference of 12 isolates). C/T and IMR modal MICs for pan-β-lactam-nonsusceptible isolates remained at or below their respective susceptible MIC breakpoints from 2018 to 2020, while the modal MIC for CZA increased 2-fold from 2018 to 2019 and exceeded the CZA-susceptible MIC breakpoint in both 2019 and 2020. Only six of 802 molecularly characterized isolates carried a metallo-β-lactamase, and two isolates carried a GES carbapenemase. Most P. aeruginosa isolates were C/T-susceptible, including many with MDR, pan-β-lactam-nonsusceptible, DTR, CZA-resistant, and IMR-nonsusceptible phenotypes. While C/T was the most active antipseudomonal agent, IMR demonstrated greater activity than CZA against isolates nonsusceptible to C/T.
KEYWORDS: ceftolozane-tazobactam, imipenem-relebactam, ceftazidime-avibactam, Pseudomonas aeruginosa, United States, SMART, surveillance
INTRODUCTION
Ceftolozane-tazobactam (C/T), imipenem-relebactam (IMR), and ceftazidime-avibactam (CZA) are preferred options in the treatment of patients with suspected or documented serious infections caused by non-carbapenemase-producing multidrug-resistant (MDR) and difficult-to-treat resistance (DTR) P. aeruginosa (1). P. aeruginosa isolates with MDR and DTR phenotypes are common in many countries, including the United States (2–6). Previous studies of clinical isolates of P. aeruginosa from patients in the United States have reported that C/T, IMR, and CZA have sustained their potent in vitro activities against P. aeruginosa over time (2–4, 7–11). Lower C/T, IMR, and CZA susceptibilities have been reported in regions outside North America in association with higher numbers of isolates carrying metallo-β-lactamases (12). The current report provides an update on antimicrobial susceptibility testing results for P. aeruginosa isolates submitted to the SMART (Study for Monitoring Antimicrobial Resistance Trends) surveillance program from 2018 to 2020 by clinical laboratories in the United States. We focused our evaluation on the activity of C/T (FDA approved, 2014) and the newer β-lactam/non-β-lactam β-lactamase inhibitor combinations IMR (2019) and CZA (2015) over a time period (2018 to 2020) when the agents were available clinically for use in the United States.
RESULTS
A proportion of 96.4% of all isolates tested were susceptible to C/T compared to 94.4% CZA susceptible and 91.5% IMR-susceptible (Table 1). A proportion of 81.6% of P. aeruginosa isolates were susceptible to cefepime, while <80% of isolates were susceptible to the other β-lactams tested and to levofloxacin. Higher percentages of MDR, pan- β-lactam-nonsusceptible, and DTR isolates were susceptible to C/T than to CZA (by 13 to 25%) or to IMR (by 17 to 24%). A proportion of 52.2% of C/T-nonsusceptible isolates were susceptible to IMR compared to 38.9% susceptible for CZA (Table 2). The percent susceptible rate for C/T was 16% higher than that for CZA against IMR-nonsusceptible isolates and 16% higher than that for IMR against CZA-resistant isolates. C/T retained activity against 61.0% of CZA-resistant P. aeruginosa, whereas 38.9% of C/T-nonsusceptible isolates were CZA-susceptible. A proportion of 1.7% of isolates tested were nonsusceptible to both C/T and IMR compared to 2.2% of isolates with a C/T-nonsusceptible and CZA-resistant phenotype (a difference of 12 isolates).
TABLE 1.
Percent susceptible to ceftolozane-tazobactam, imipenem-relebactam, ceftazidime-avibactam, and comparators among all P. aeruginosa isolates and subsets of MDR, pan-β-lactam-nonsusceptible, and DTR isolatesa
| Phenotype | No. (% of all isolates) | Antimicrobial agent, % susceptible |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C/T | IMR | CZA | TZP | FEP | CAZ | MEM | IMI | ATM | AMK | LVX | ||
| All | 2,531 (100) | 96.4 | 91.5 | 94.4 | 77.0 | 81.6 | 79.9 | 78.3 | 66.0 | 69.9 | 97.0 | 66.8 |
| MDR | 319 (12.6) | 75.9 | 58.9 | 63.3 | 8.8 | 12.2 | 16.6 | 19.7 | 16.3 | 3.8 | 84.0 | 18.5 |
| Pan-β-lactam-NS | 207 (8.2) | 73.4 | 51.2 | 50.2 | 0 | 0 | 0 | 0 | 0 | 0 | 84.5 | 15.9 |
| DTR | 169 (6.7) | 69.8 | 46.2 | 45.0 | 0 | 0 | 0 | 0 | 0 | 0 | 82.8 | 0 |
C/T, ceftolozane-tazobactam; IMR, imipenem-relebactam; CZA, ceftazidime-avibactam; TZP, piperacillin-tazobactam; FEP, cefepime; CAZ, ceftazidime; MEM, meropenem; IMI, imipenem; ATM, aztreonam; AMK, amikacin; LVX, levofloxacin; NS, nonsusceptible; R, resistant; MDR, multidrug-resistant (resistant to ≥3 sentinel agents [AMK, ATM, FEP, colistin, IMI, LVX, and TZP]); pan-β-lactam-NS, nonsusceptible to all tested β-lactams (excluding C/T, IMR, and CZA); DTR, difficult-to-treat resistance (nonsusceptible to all tested β-lactams, excluding C/T, IMR, and CZA, and fluoroquinolones).
TABLE 2.
Cross-susceptibility to ceftolozane-tazobactam, imipenem-relebactam, and ceftazidime-avibactam among P. aeruginosa isolates with different phenotypesa
| Phenotype | No. (% of all isolates) | Antimicrobial agent, % susceptible |
||
|---|---|---|---|---|
| C/T | IMR | CZA | ||
| C/T-NS | 90 (3.6) | 0 | 52.2 | 38.9 |
| IMR-NS | 214 (8.5) | 79.9 | 0 | 64.0 |
| CZA-R | 141 (5.6) | 61.0 | 45.4 | 0 |
C/T, ceftolozane-tazobactam; IMR, imipenem-relebactam; CZA, ceftazidime-avibactam; NS, nonsusceptible; R, resistant.
Percentages of C/T-susceptible isolates were similar (95.5 to 98.2%) across four United States census regions (see Table S1 and Fig. S1 in the supplemental material). In vitro susceptibility to C/T was higher than that to IMR and CZA among all, MDR, pan-β-lactam-nonsusceptible, and DTR P. aeruginosa isolates collected in each of the four regions (Table S1, Fig. S1).
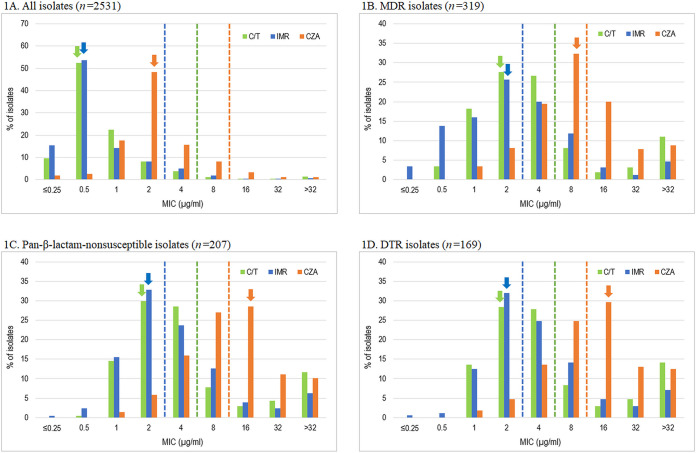
Figure 1A to D provides MIC distributions for C/T, IMR, and CZA for all isolates and isolates with specific antimicrobial-nonsusceptible and -resistant phenotypes. Shift or drift in MIC distributions and modal MICs suggest future changes in antimicrobial susceptibility overall or within specific phenotype subgroups. The C/T, IMR, and CZA modal MICs were 0.5, 0.5, and 2 μg/mL, respectively, for all isolates of P. aeruginosa. For MDR, pan-β-lactam-nonsusceptible, and DTR phenotypes, the modal MIC for C/T of 2 μg/mL was one doubling dilution below the susceptible MIC breakpoint, and the modal MIC for IMR (2 μg/mL) was at the susceptible IMR MIC breakpoint. The modal MIC for CZA was at its susceptible MIC breakpoint (8 μg/mL) for MDR isolates but was greater than its susceptible MIC breakpoint for both pan-β-lactam-nonsusceptible and DTR isolates (16 μg/mL). The CZA modal MIC for C/T-nonsusceptible isolates was >32 μg/mL (31.1% of isolates) compared to a modal MIC of 2 μg/mL (23.3%) for IMR (Fig. S2). Figure S2 also compares the cumulative percentage of C/T-nonsusceptible isolates inhibited by doubling-dilution increases in IMR and CZA. Of the 90 C/T-nonsusceptible isolates identified in the study, 22 (24.4%) were IMR-susceptible and CZA-resistant, while 10 (11.1%) were IMR-nonsusceptible and CZA-susceptible.
FIG 1.
Distribution of ceftolozane-tazobactam, ceftazidime-avibactam, and imipenem-relebactam MIC values against all P. aeruginosa isolates and resistant subsets. C/T, ceftolozane-tazobactam; IMR, imipenem-relebactam; CZA, ceftazidime-avibactam; MDR, multidrug resistant (resistant to ≥3 sentinel agents [amikacin, aztreonam, cefepime, colistin, imipenem, levofloxacin, and piperacillin-tazobactam]); pan-β-lactam-nonsusceptible, nonsusceptible to all tested β-lactams (excluding C/T, IMR, and CZA); DTR, difficult-to-treat resistance (nonsusceptible to all tested β-lactams [excluding C/T, IMR, and CZA] and fluoroquinolones). Dashed lines indicate the respective susceptible breakpoints. Arrows indicate the mode of the respective MIC distributions.
To evaluate in vitro activity over time, MIC distributions of C/T, IMR, and CZA against pan-β-lactam-nonsusceptible isolates were compared over the 3-year study period (2018 to 2020) (Fig. 2). Notably, the modal MIC of CZA increased 2-fold from 2018 to 2019 (and remained 2-fold higher in 2020) and exceeded the CZA-susceptible MIC breakpoint in both 2019 and 2020. In comparison, C/T and IMR modal MICs remained at their respective susceptible MIC breakpoints (or one doubling dilution lower for C/T in 2019) from 2018 to 2020. No statistically significant differences in corresponding percent susceptibilities were noted over this 3-year time period (P > 0.05, Cochran-Armitage test).
FIG 2.
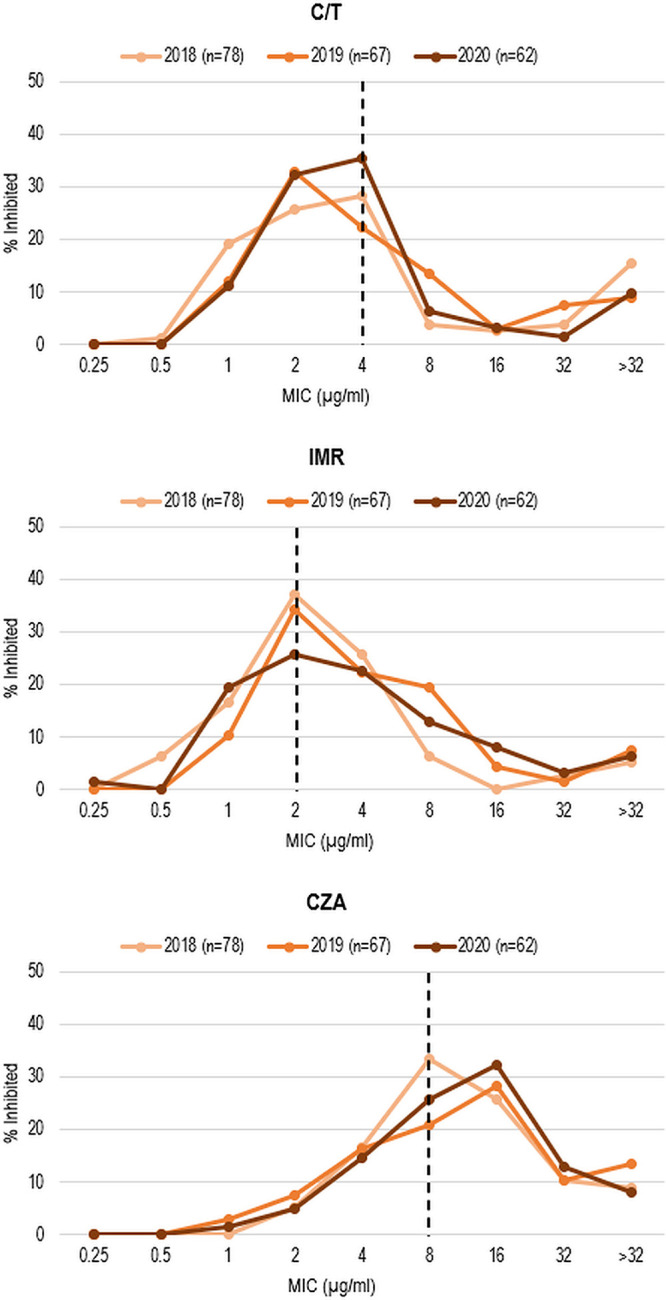
MIC distributions for ceftolozane-tazobactam, imipenem-relebactam, and ceftazidime-avibactam against pan-β-lactam-nonsusceptible P. aeruginosa isolates, by year. C/T, ceftolozane-tazobactam; IMR, imipenem-relebactam; CZA, ceftazidime-avibactam. CLSI susceptibility breakpoints are indicated by dashed lines.
A total of 802 isolates of P. aeruginosa were characterized molecularly based upon the selection criteria. In 792 isolates (98.8%), no acquired β-lactamases were detected; in all but one isolate, PDC (Pseudomonas-derived cephalosporinase) (AmpC) was detected. Carbapenemases were detected in only 9.9%, 4.1%, 5.9%, and 1.0% of molecularly characterized C/T-nonsusceptible (n = 81), IMR-nonsusceptible (n = 196), CZA-resistant (n = 119), and IMI-nonsusceptible (n = 781) isolates, respectively. Five isolates producing IMP-type metallo-β-lactamases (MBLs) were collected in the West (IMP-13, n = 2; IMP-15, n = 1), Midwest (IMP-13, n = 1), and South (IMP-75, n = 1) regions, and one isolate carrying a VIM-2 MBL was collected in the Midwest. All MBL-positive isolates tested as resistant to C/T, CZA, and IMR. Two isolates carrying GES-type carbapenemases were collected in the West (GES-20 [also carrying a GES-19 ESBL], n = 1; tested as resistant to C/T, CZA, and IMR) and in the Northeast (GES-5; tested as resistant to IMR, intermediate to C/T, and susceptible to CZA). Two additional isolates carrying only ESBLs were collected in the Northeast (VEB-14, n = 1; tested as resistant to C/T and CZA and as intermediate to IMR) and in the Midwest (TEM-type, n = 1; tested as resistant to C/T and CZA and as susceptible to IMR) (data not shown).
DISCUSSION
In the current study, 96.4% of P. aeruginosa isolates collected from patients in United States hospitals in 2018 to 2020 were susceptible to C/T; >90% of isolates were also susceptible to amikacin (97.0% susceptible), CZA (94.4%), and IMR (91.5%), while other β-lactam agents and levofloxacin had percent susceptible rates of ≤82% (Table 1). In contrast to many regions in the world where carbapenemase rates among resistant P. aeruginosa isolates are higher (13, 14), almost all (98.8%) carbapenem-, IMR-, or C/T-nonsusceptible isolates of P. aeruginosa did not carry an acquired β-lactamase, including carbapenemases, confirming earlier reports of isolates from United States patients (14, 15).
MDR phenotypes are commonly observed among P. aeruginosa isolates in the United States, particularly among isolates from patients with lower respiratory tract infections (2–5). The MDR rate in the current study, 12.6%, was comparable to rates for P. aeruginosa isolates tested from U.S. patients in the cited studies. Among these MDR P. aeruginosa isolates, 75.9% of isolates were C/T-susceptible compared to 63.3% CZA-susceptible and 58.9% IMR-susceptible. DTR isolates of Gram-negative bacilli are associated with increased therapeutic failure and mortality, especially in severely ill patients (16, 17). In the current study, 6.7% of P. aeruginosa isolates were DTR; percent susceptible rates for DTR isolates exceeded 50% only for amikacin (82.8% susceptible) and C/T (69.8% susceptible).
We found subtle but notable differences in the overlap in nonsusceptibility and resistance to C/T, IMR, and CZA (Table 2). We noted among C/T-nonsusceptible isolates that a greater percentage of isolates were IMR-susceptible (52.2%) than CZA-susceptible (38.9%), among IMR-nonsusceptible isolates that C/T was more active (79.9% susceptible) than CZA (64.0%), among CZA-resistant isolates that C/T was more active (61.0%) than IMR (45.4%), and that the CZA modal MIC for C/T-nonsusceptible isolates was >32 μg/mL compared to 2 μg/mL for IMR. Previously, Fraile-Ribot et al. reported 50% (39/78) of C/T-resistant and 61% (51/84) of CZA-resistant isolates that were not carbapenemase producers to be IMR-susceptible (18), similar to results in the current study (Table 2). We also observed that C/T and IMR modal MICs for pan-β-lactam-nonsusceptible isolates remained at or below their respective susceptible MIC breakpoints from 2018 to 2020, while the modal MIC for CZA increased 2-fold from 2018 to 2019 and exceeded the CZA-susceptible MIC breakpoint in both 2019 and 2020 (Fig. 2), although the corresponding annual percent susceptible values for each agent did not change significantly.
In the current study, 98.3% of all isolates were C/T-susceptible and/or IMR-susceptible, compared to 97.8% of all isolates being C/T-susceptible and/or CZA-susceptible (a difference of 12 isolates). This difference, although slight (0.5% of isolates), suggests a greater propensity for nonoverlapping mechanisms of resistance between C/T and IMR than between C/T and CZA. These observations align with the reports of sporadic isolates of P. aeruginosa that developed cross-resistance to C/T, CZA, and/or cefiderocol during therapy due to amino acid substitutions, insertions, and/or deletions within the Ω-loop of AmpC (PDC) or adjacent AmpR regions (R1 and R2) (sometimes in combination with the ESBLs PER-1, GES-2, and OXA-15) but that maintain susceptibility to or result in lower MICs for IMR and/or piperacillin-tazobactam (18–23).
Ceftolozane is a poor inducer of AmpC (PDC) production (24, 25). In addition, it is only slowly hydrolyzed, if at all, by most AmpC β-lactamases (24, 25). Tazobactam is also a poor inducer of AmpC production; however, tazobactam becomes a poor inhibitor in the presence of high levels of AmpC (24–26). OprD loss, derepression/hyperproduction of AmpC, and MexAB/OprM efflux are not associated with C/T resistance (15). In comparison, CZA resistance mechanisms in P. aeruginosa may also be related to AmpC derepression and increased expression of RND (resistance-nodulation-division) efflux pumps (e.g., MexAB-OprM).
A single study of 46 isolates of CZA-resistant P. aeruginosa collected from United States hospitals in 2015 suggested that a complex combination of MexAB-OprM overexpression and mutations in efflux regulators, penicillin-binding proteins (PBPs), and chaperone proteins was responsible for resistance; AmpC Ω-loop mutations were rarely associated with CZA resistance in this set of isolates (27). However, several other studies have shown that mutations in the Ω-loop broaden the AmpC binding pocket to permit cephalosporins with bulkier R2 side chains (such as the 2-methyl-3-aminopyrazolium of ceftolozane) to enter, resulting in increased catalysis of both ceftazidime and ceftolozane (28, 29). Mutations in the Ω-loop also reduce the affinity of the AmpC binding pocket for avibactam (28). These AmpC structural modifications are also hypothesized to enable carbapenems to rotate their bulky 6α-hydroxyethyl side chain within the AmpC binding pocket to prevent hydrolysis (30). Imipenem is not effluxed by the MexAB/OprM efflux pump or affected significantly by AmpC overexpression but is affected by OprD porin loss with concomitant AmpC hyperexpression (31). These properties suggest that IMR is an option for rescue therapy in cases of pseudomonal infection where C/T and CZA resistance arises during therapy.
Based on in vitro testing of current (2018 to 2020) clinical isolates of P. aeruginosa collected in the United States, C/T remains highly active and provides an important treatment option for patients with infections caused by antimicrobial-nonsusceptible and -resistant P. aeruginosa. C/T was the most active antipseudomonal β-lactam/β-lactamase inhibitor combination tested, and IMR demonstrated greater activity than CZA against C/T-nonsusceptible isolates.
MATERIALS AND METHODS
Bacterial isolates.
Twenty-four medical laboratories in 16 states in the United States collected 14,177 isolates of Gram-negative bacilli from 2018 to 2020 as part of the SMART surveillance program; 2,351 (16.6%) of these isolates were P. aeruginosa. Bloodstream (n = 237, 9.4% of isolates), intra-abdominal (n = 255, 10.1%), lower respiratory tract (n = 1,781, 70.4%), and urinary tract (n = 258, 10.2%) infection specimens accounted for the P. aeruginosa collected. All isolates were sent to IHMA (Schaumburg, IL), where organism identity was confirmed using matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Billerica, MA).
Antimicrobial susceptibility testing.
The CLSI broth microdilution method was used to determine MICs (32, 33) at IHMA on in-house-prepared panels. Avibactam was obtained from Advanced ChemBlocks, Inc. (Burlingame, CA); ceftolozane, imipenem, and relebactam from Merck & Co., Inc. (Kenilworth, NJ); and tazobactam from USP. Other antimicrobial agents were purchased from commercial sources. Cefiderocol is not an agent currently tested by the SMART surveillance program and meropenem-vaborbactam was not tested in 2018, but vaborbactam was not expected to improve the activity of meropenem against P. aeruginosa (34).
MICs were interpreted using 2021 CLSI breakpoints (32). MDR isolates were resistant to three or more of the following seven sentinel antimicrobial agents: amikacin, aztreonam, cefepime, colistin, imipenem, levofloxacin, and piperacillin-tazobactam. Pan-β-lactam-nonsusceptible isolates were nonsusceptible (intermediate or resistant MICs) to all traditional β-lactams tested (aztreonam, ceftazidime, cefepime, piperacillin-tazobactam, imipenem, and meropenem); this definition excluded C/T, IMR, and CZA. DTR isolates were nonsusceptible to all tested traditional β-lactams (excluding C/T, IMR, and CZA) and fluoroquinolones (ciprofloxacin [tested only in 2018] and levofloxacin) (16).
Screening for β-lactamase genes.
Isolates testing as nonsusceptible to imipenem (MIC of ≥4 μg/mL), IMR (MIC of ≥4 μg/mL), or C/T (MIC of ≥8 μg/mL) were screened for the presence of genes encoding ESBLs, acquired AmpC β-lactamases, PDC (Pseudomonas-derived cephalosporinase), serine carbapenemases, and metallo-β-lactamases using published multiplex PCR assays and sequencing (Sanger) as previously described (35, 36). For P. aeruginosa collected in 2020 only, C/T-nonsusceptible, imipenem-nonsusceptible, and IMR-nonsusceptible isolates were characterized by short-read whole-genome sequencing (2 × 150 bp reads; Illumina HiSeq) and analyzed using CLC Genomics Workbench (Qiagen) (37). Of 885 isolates that met the molecular testing criteria, 82 randomly selected isolates collected in 2020 were not characterized. One isolate collected in 2018 that met the molecular testing criteria was also not characterized.
Data availability.
Data are available on request.
ACKNOWLEDGMENTS
We thank all SMART participants for their contributions to the program.
Funding for this research, which included compensation for services related to preparing the manuscript, was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA. S.H.L. and D.F.S. work for IHMA, which receives funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA, for the SMART surveillance program. J.A.K. is a consultant to IHMA and an employee of Shared Health Manitoba and the University of Manitoba. C.A.D., D.W.H., M.T.W., K.Y., F.S., and M.R.M. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA, and own stock in Merck & Co., Inc., Kenilworth, New Jersey, USA. The IHMA authors do not have personal financial interests in the sponsor of the manuscript (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA).
The sponsor participated in the development of the overall study design, but collection and testing of isolates, data analysis, and manuscript preparation were independently performed by IHMA.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2021. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P aeruginosa). Clin Infect Dis 72:e169–e183. doi: 10.1093/cid/ciaa1478. [DOI] [PubMed] [Google Scholar]
- 2.Sader HS, Flamm RK, Carvalhaes CG, Castanheira M. 2020. Comparison of ceftazidime-avibactam and ceftolozane-tazobactam in vitro activities when tested against Gram-negative bacteria isolated from patients hospitalized with pneumonia in United States medical centers (2017–2018). Diagn Microbiol Infect Dis 96:114833. doi: 10.1016/j.diagmicrobio.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Shortridge D, Pfaller MA, Streit JM, Flamm RK. 2020. Antimicrobial activity of ceftolozane/tazobactam tested against contemporary (2015–2017) Pseudomonas aeruginosa isolates from a global surveillance programme. J Glob Antimicrob Resist 21:60–64. doi: 10.1016/j.jgar.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Sader HS, Huband MD, Castanheira M, Flamm RK. 2017. Pseudomonas aeruginosa antimicrobial susceptibility results from four years (2012 to 2015) of the International Network for Optimal Resistance Monitoring Program in the United States. Antimicrob Agents Chemother 61:e02252-16. doi: 10.1128/AAC.02252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lob SH, Hoban DJ, Young K, Motyl MR, Sahm DF. 2018. Activity of imipenem/relebactam against Gram-negative bacilli from global ICU and non-ICU wards: SMART 2015–2016. J Glob Antimicrob Resist 15:12–19. doi: 10.1016/j.jgar.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Karlowsky JA, Lob SH, Young K, Motyl MR, Sahm DF. 2021. Activity of ceftolozane/tazobactam against Gram-negative isolates from patients with lower respiratory tract infections–SMART United States 2018–2019. BMC Microbiol 21:74. doi: 10.1186/s12866-021-02135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lob SH, Hoban DJ, Young K, Motyl MR, Sahm DF. 2020. Activity of ceftolozane-tazobactam and comparators against Pseudomonas aeruginosa from patients in different risk strata–SMART United States 2016–2017. J Glob Antimicrob Resist 20:209–213. doi: 10.1016/j.jgar.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Malan S, Mishra AJ, Mushtaq A, Brinkman CL, Patel R. 2018. In vitro activity of imipenem-relebactam and ceftolozane-tazobactam against resistant Gram-negative bacilli. Antimicrob Agents Chemother 62:e00533-18. doi: 10.1128/AAC.00533-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shortridge D, Castanheira M, Pfaller MA, Flamm RK. 2017. Ceftolozane/tazobactam activity against Pseudomonas aeruginosa clinical isolates from U.S. hospitals: report from the PACTS antimicrobial surveillance program, 2012 to 2015. Antimicrob Agents Chemother 61:e00465-17. doi: 10.1128/AAC.00465-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell DJ, Sader HS, Flamm RK, Jones RN. 2014. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents 43:533–539. doi: 10.1016/j.ijantimicag.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Castanheira M, Duncan LR, Mendes RE, Sader HS, Shortridge D. 2018. Activity of ceftolozane-tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae isolates collected from respiratory tract specimens of hospitalized patients in the United States during 2013 to 2015. Antimicrob Agents Chemother 62:e02125-17. doi: 10.1128/AAC.02125-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlowsky JA, Hackel MA, Bouchillon SK, Sahm DF. 2020. In vitro activity of WCK 5222 (cefepime-zidebactam) against worldwide collected Gram-negative bacilli not susceptible to carbapenems. Antimicrob Agents Chemother 64:e01432-20. doi: 10.1128/AAC.01432-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiratisin P, Kazmierczak K, Stone GG. 2021. In vitro activity of ceftazidime/avibactam and comparators against carbapenemase-producing Enterobacterales and Pseudomonas aeruginosa isolates collected globally between 2016 and 2018. J Glob Antimicrob Resist 27:132–141. doi: 10.1016/j.jgar.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, Sahm DF, Bradford PA. 2016. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:1067–1078. doi: 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castanheira M, Mills JC, Farrell DJ, Jones RN. 2014. Mutation-driven β-lactam resistance mechanisms among contemporary ceftazidime-non-susceptible Pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob Agents Chemother 58:6844–6850. doi: 10.1128/AAC.03681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, Palmore TN, Rhee C, Klompas M, Dekker JP, Powers JH, III, Suffredini AF, Hooper DC, Fridkin S, Danner RL, National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI). 2018. Difficult-to-treat resistance in Gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis 67:1803–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huh K, Chung DR, Ha YE, Ko J-H, Kim S-H, Kim M-J, Huh HJ, Lee NY, Cho SY, Kang C-I, Peck KR, Song J-H, Korean Antimicrobial Resistance Surveillance Network (KARS-Net) Investigators. 2020. Impact of difficult-to-treat resistance in Gram-negative bacteremia on mortality: retrospective analysis of nationwide surveillance data. Clin Infect Dis 71:e487–e496. doi: 10.1093/cid/ciaa084. [DOI] [PubMed] [Google Scholar]
- 18.Fraile-Ribot PA, Zamorano L, Orellana R, del Barrio-Tofiño E, Sánchez-Diener I, Cortes-Lara S, López-Causapé C, Cabot G, Bou G, Martínez-Martínez L, Oliver A, Galán F, Gracia I, Rodríguez MA, Martín L, Sánchez JM, Viñuela L, García MV, Lepe JA, Aznar J, López-Hernández I, Seral C, Castillo-García FJ, López-Calleja AI, Aspiroz C, de la Iglesia P, Ramón S, Riera E, Pérez MC, Gallegos C, Calvo J, Quesada MD, Marco F, Hoyos Y, Horcajada JP, Larrosa N, José González J, Tubau F, Capilla S, Pérez-Moreno MO, Centelles MJ, Padilla E, Rivera A, Mirelis B, Rodríguez-Tarazona RE, Arenal-Andrés N, Ortega MDP, Megías G, García I, Colmenarejo C, on behalf of the GEMARA-SEIMC/REIPI Pseudomonas Study Group, et al. 2020. Activity of imipenem-relebactam against a large collection of Pseudomonas aeruginosa clinical isolates and isogenic β-lactam-resistant mutants. Antimicrob Agents Chemother 64:e02165-19. doi: 10.1128/AAC.02165-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arca-Suárez J, Vázquez-Ucha JC, Fraile-Ribot PA, Lence E, Cabot G, Martínez-Guitián M, Lasarte-Monterrubio C, Rodríguez-Iglesias M, Beceiro A, González-Bello C, Galán-Sánchez F, Oliver A, Bou G. 2020. Molecular and biochemical insights into the in vivo evolution of AmpC-mediated resistance to ceftolozane/tazobactam during treatment of an MDR Pseudomonas aeruginosa infection. J Antimicrob Chemother 75:3209–3217. doi: 10.1093/jac/dkaa291. [DOI] [PubMed] [Google Scholar]
- 20.Arca-Suárez J, Lasarte-Monterrubio C, Rodino-Janeiro B-K, Cabot G, Vázquez-Ucha JC, Rodríguez-Iglesias M, Galán-Sánchez F, Beceiro A, González-Bello C, Oliver A, Bou G. 2021. Molecular mechanisms driving in vivo development of OXA-10-mediated resistance to ceftolozane/tazobactam and ceftazidime/avibactam during treatment of XDR Pseudomonas aeruginosa infections. J Antimicrob Chemother 76:91–100. doi: 10.1093/jac/dkaa396. [DOI] [PubMed] [Google Scholar]
- 21.Fraile-Ribot PA, Cabot G, Mulet X, Periañez L, Martín-Pena ML, Juan C, Pérez JL, Oliver A. 2018. Mechanisms leading to in vivo ceftolozane/tazobactam resistance development during the treatment of infections caused by MDR Pseudomonas aeruginosa. J Antimicrob Chemother 73:658–663. doi: 10.1093/jac/dkx424. [DOI] [PubMed] [Google Scholar]
- 22.Rubio AM, Kline EG, Jones CE, Chen L, Kreiswirth BN, Nguyen MH, Clancy CJ, Cooper VS, Haider G, Van Tyne D, Shields RK. 2021. In vitro susceptibility of multidrug-resistant Pseudomonas aeruginosa following treatment-emergent resistance to ceftolozane-tazobactam. Antimicrob Agent Chemother 65:e00084-21. doi: 10.1128/AAC.00084-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simner PJ, Beisken S, Bergman Y, Posch AE, Cosgrove SE, Tamma PD. 2021. Cefiderocol activity against clinical Pseudomonas aeruginosa isolates exhibiting ceftolozane-tazobactam resistance. Open Forum Infect Dis 8:ofab311. doi: 10.1093/ofid/ofab311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, Lagacé-Wiens PRS, Rubinstein E, Gin AS, Walkty A, Hoban DJ, Lynch JP, III, Karlowsky JA. 2014. Ceftolozane/tazobactam: a novel cephalosporin/beta-lactamase inhibitor combination with activity against multidrug-resistant Gram-negative bacilli. Drugs 74:31–51. doi: 10.1007/s40265-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 25.Tamma PD, Doi Y, Bonomo RA, Johnson JK, Simner PJ, Antibacterial Resistance Leadership Group. 2019. A primer on AmpC β-lactamases: necessary knowledge for an increasingly multidrug-resistant world. Clin Infect Dis 69:1446–1455. doi: 10.1093/cid/ciz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacoby GA. 2009. AmpC β-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castanheira M, Doyle TB, Smith CJ, Mendes RE, Sader HS. 2019. Combination of MexAB-OprM overexpression and mutations in efflux regulators, PBPs and chaperone proteins is responsible for ceftazidime/avibactam resistance in Pseudomonas aeruginosa clinical isolates from US hospitals. J Antimicrob Chemother 74:2588–2595. doi: 10.1093/jac/dkz243. [DOI] [PubMed] [Google Scholar]
- 28.Slater CL, Winogrodzki J, Fraile-Ribot PA, Oliver A, Khajehpour M, Mark BL. 2020. Adding insult to injury: mechanistic basis of how AmpC mutations allow Pseudomonas aeruginosa to accelerate cephalosporin hydrolysis and evade avibactam. Antimicrob Agents Chemother 64:e00894-20. doi: 10.1128/AAC.00894-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berrazeg M, Jeannot K, Enguéné VYN, Broutin I, Loeffert S, Fournier D, Plésiat P. 2015. Mutations in β-lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrob Agents Chemother 59:6248–6255. doi: 10.1128/AAC.00825-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lahiri SD, Walkup GK, Whiteaker JD, Palmer T, McCormack K, Tanudra MA, Nash TJ, Thresher J, Johnstone MR, Hajec L, Livchak S, McLaughlin RE, Alm RA. 2015. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J Antimicrob Chemother 70:1650–1658. doi: 10.1093/jac/dkv004. [DOI] [PubMed] [Google Scholar]
- 31.Young K, Painter RE, Raghoobar SL, Hairston NN, Racine F, Wisniewski D, Balibar CJ, Villafania A, Zhang R, Sahm DF, Blizzard T, Murgolo N, Hammond ML, Motyl MR. 2019. In vitro studies evaluating the activity of imipenem in combination with relebactam against Pseudomonas aeruginosa. BMC Microbiol 19:150. doi: 10.1186/s12866-019-1522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute. 2021. M100 performance standards for antimicrobial susceptibility testing, 31st ed. Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed, M07-A11. Approved standard. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 34.Melinta Therapeutics. 2021. Vabomere (meropenem and vaborbactam) for injection, for intravenous use, prescribing information. http://www.vabomere.com/media/pdf/vabomere-us-prescribing-information.pdf.
- 35.Lob SH, Biedenbach DJ, Badal RE, Kazmierczak KM, Sahm DF. 2015. Antimicrobial resistance and resistance mechanisms of Enterobacteriaceae in ICU and non-ICU wards in Europe and North America: SMART 2011–2013. J Glob Antimicrob Resist 3:190–197. doi: 10.1016/j.jgar.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Nichols WW, de Jonge BLM, Kazmierczak KM, Karlowsky JA, Sahm DF. 2016. In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to ceftazidime-avibactam (INFORM 2012 to 2014). Antimicrob Agents Chemother 60:4743–4749. doi: 10.1128/AAC.00220-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estabrook M, Kazmierczak KM, Wise M, Arhin FF, Stone GG, Sahm DF. 2021. Molecular characterization of clinical isolates of Enterobacterales with elevated MIC values for aztreonam-avibactam from the INFORM global surveillance study, 2012–2017. J Glob Antimicrob Resist 24:316–320. doi: 10.1016/j.jgar.2021.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 and Fig. S1 and S2. Download aac.00189-22-s0001.pdf, PDF file, 0.2 MB (195.5KB, pdf)
Data Availability Statement
Data are available on request.