ABSTRACT
Bedaquiline and clofazimine are increasingly used to treat infections with Mycobacterium abscessus. We determined distributions of MICs by broth microdilution for bedaquiline and clofazimine for 61 M. abscessus clinical isolates using different media and incubation times. We show that incubation time and growth media critically influence the MIC. Our data will aid in defining future clinical breakpoints for in vitro susceptibility testing for bedaquiline and clofazimine in M. abscessus.
KEYWORDS: ECOFF, MIC, Mycobacterium abscessus, antibiotic susceptibility testing, bedaquiline, clofazimine, epidemiological cutoff
TEXT
Increasing numbers of infections due to Mycobacterium abscessus are being reported, presenting mostly as pulmonary disease in patients with cystic fibrosis (CF), bronchiectasis, or chronic obstructive pulmonary disease (COPD) and as skin and soft tissue infections following trauma or surgery (1, 2). M. abscessus belongs to the rapidly growing mycobacteria and consists of the three subspecies M. abscessus subsp. abscessus, M. abscessus subsp. massiliense, and M. abscessus subsp. bolletii (3). M. abscessus naturally exhibits extensive drug resistance (4), and treatment requires individual multidrug regimens that are based on in vitro susceptibility testing and clinical expertise (5, 6). Treatment options are limited especially for macrolide-resistant M. abscessus, which is common due to expression of the inducible 23S rRNA methyltransferase Erm(41) (7). Recently approved drugs for combination therapy of multidrug-resistant tuberculosis, such as bedaquiline, a diarylquinoline antibiotic, and clofazimine, a key drug in therapy for leprosy, have been increasingly used for the treatment of infections with nontuberculous mycobacteria, particularly for M. abscessus (8–10). Procedures for in vitro susceptibility testing of these two drugs in M. abscessus have not yet been standardized. Bedaquiline and clofazimine were not included in commercial microdilution panels until recently, when Thermo Fisher Scientific (Waltham, MA) released the RAPMYCO2 Sensititre plate with clofazimine. Accordingly, no clinical breakpoints have been defined by the Clinical and Laboratory Standards Institute (CLSI) or by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) to separate bedaquiline and clofazimine susceptible from resistant M. abscessus strains. The frequency of M. abscessus strains categorized as clofazimine resistant in different studies varies between 0% and 95%, with a pooled rate of in vitro resistance of 16% (95% confidence interval, 4.0% to 16%) (11). Determination of MIC distributions and epidemiological cutoff values (ECOFF) is a prerequisite for assignment of clinical breakpoints (12).
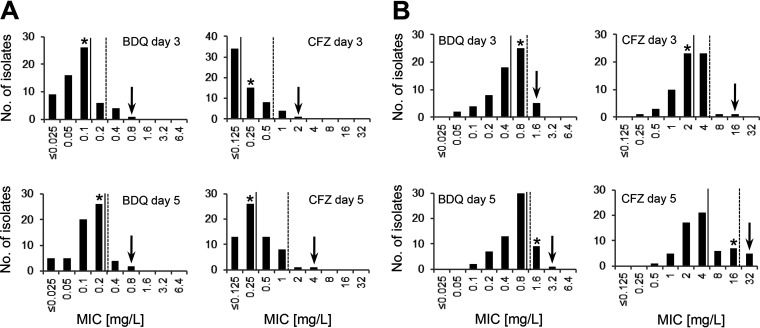
In this study, we determined the MICs of bedaquiline and clofazimine for the type strain M. abscessus ATCC 19977 and 61 M. abscessus nonduplicate (one isolate per patient) clinical isolates from the years 2008 to 2013 that were isolated at or submitted to our laboratory and for which antibiotic susceptibility testing was requested. Fifty-one (84%) M. abscessus isolates were of respiratory origin, and six isolates (10%) were of nonrespiratory origin. For four isolates (6%), the clinical origin was unknown. Thirty-one (51%) M. abscessus isolates were recovered from patients with CF, 19 (31%) M. abscessus isolates were recovered from non-CF patients, and for 11 (18%) isolates, the CF status of the patient was not known. The isolates were selected to comprise the three M. abscessus subspecies and include M. abscessus subsp. abscessus (n = 32), M. abscessus subsp. bolletii (n = 17), and M. abscessus subsp. massiliense (n = 12). Subspecies assignment was done by combined 16S rRNA gene, rpoB, and erm(41) sequence analysis (13). MIC determination was conducted by in-house broth microdilution according to CLSI guidelines, except for the incubation temperature, which was set at 37°C (14). Bedaquiline (Adooq, Irvine, CA) and clofazimine (Sigma-Aldrich, St. Louis, MO) were dissolved in 100% dimethyl sulfoxide (DMSO) and diluted in cation-adjusted Mueller-Hinton broth (CAMHB) (Merck, Darmstadt, Germany) to final concentrations of 0.025 mg/L to 6.4 mg/L for bedaquiline and 0.125 mg/L to 32 mg/L for clofazimine (2-fold serial dilutions with a maximum final DMSO concentration of 6.25% [vol/vol]). MIC50 and MIC90 values of 0.1 mg/L and 0.2 mg/L, respectively, for bedaquiline and of ≤0.125 mg/L and 0.5 mg/L, respectively, for clofazimine were calculated when growth was judged after 3 days of incubation in CAMHB in polystyrene plates (Greiner Bio-One, Monroe, NC) (Fig. 1A). The exact definition of the MIC50 but not of the MIC90 value of clofazimine was hampered by the limits of the test range. Tentative ECOFFs of 0.8 mg/L for bedaquiline and of 2 mg/L for clofazimine were assigned by visual inspection of the histograms (eyeball method) (15, 16). We did not observe significant differences between the three M. abscessus subspecies. For M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense, the MIC50 values for bedaquiline were 0.05 mg/L, 0.1 mg/L, and 0.1 mg/L, respectively, and the MIC90 values were 0.1 mg/L, 0.2 mg/L, and 0.4 mg/L. Clofazimine MIC50 values of ≤0.125 mg/L, ≤0.125 mg/L, and 0.25 mg/L were found for M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense. The clofazimine MIC90 value was 0.5 mg/L for all three subspecies. The MIC of the type strain M. abscessus ATCC 19977 was determined at 0.1 mg/L for bedaquiline and at 0.25 mg/L for clofazimine. For a subset of 12 isolates (four each of M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense), the MIC of bedaquiline and clofazimine was also determined at 30°C. For bedaquiline, an MIC50 of 0.025 mg/L and an MIC90 of 0.05 mg/L was observed; for clofazimine, the MIC50 and MIC90 values were ≤0.125 mg/L and 0.5 mg/L, respectively. For the CLSI quality control strain Mycobacterium peregrinum ATCC 700686, we determined the MIC of bedaquiline to be 0.0031 to 0.0125 mg/L (median, 0.0063 mg/L) and the MIC of clofazimine to be 0.0625 to 0.25 mg/L (median, 0.125 mg/L) for 10 replicates (incubation at 30°C). Incubation of M. peregrinum ATCC 700686 at 37°C resulted in comparable MIC ranges (median MIC for bedaquiline, 0.0125 mg/L, and for clofazimine, 0.125 mg/L). CLSI has not yet published quality control concentration ranges for M. peregrinum ATCC 700686 for bedaquiline and clofazimine (17).
FIG 1.
MIC distributions of bedaquiline (BDQ) and clofazimine (CFZ) for M. abscessus complex isolates (n = 61) determined in CAMHB without (A) and with (B) 10% OADC and read after 3 and 5 days of incubation at 37°C. MIC50 (continuous line), MIC90 (broken line), and tentative ECOFF values (arrow) are indicated as well as MIC values of the type strain M. abscessus ATCC 19977 (*).
Reported bedaquiline and clofazimine MIC distributions for M. abscessus vary between laboratories and definition of uniform ECOFF values has not been possible so far (Table 1). In particular, reported MIC50 and MIC90 values for clofazimine vary from 0.12 mg/L to 4 mg/L and from 0.25 mg/L to 32 mg/L, respectively. A recent study of M. abscessus pulmonary disease demonstrated that clofazimine MICs of ≤1 mg/L were most often associated with sputum conversion to negative in patients who were being treated with a clofazimine-containing drug regimen. The effect was more pronounced when MIC values were ≤0.25 mg/L, while no culture conversion was observed for MIC values exceeding 2 mg/L (18). Of note, MIC determination in this study by Kwak et al. was conducted in 7H9 broth supplemented with oleic acid, albumin, dextrose, and catalase (OADC), which is not recommended by CLSI, and showed a bimodal distribution of MICs (18). Most likely, for M. abscessus as for Mycobacterium tuberculosis, the bedaquiline and clofazimine MIC values are affected by in vitro culture conditions, which may account for the diverging MIC results obtained in different studies (19, 20).
TABLE 1.
In vitro MIC studies for bedaquiline and clofazimine for M. abscessus complex
| Studya | No. of isolates | Method | Mediumb | MIC range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) |
|---|---|---|---|---|---|---|
| Bedaquiline | ||||||
| This study | 61 | Broth microdilution | CAMHB | ≤0.025 to 0.8 | 0.1 | 0.2 |
| This study | 61 | Broth microdilution | CAMHB OADC | 0.05 to 1.6 | 0.4 | 0.8 |
| Asami et al., 2021 (24) | 70 | Broth microdilution | CAMHB | 0.06 to 0.25 | 0.13 | 0.25 |
| Chew et al., 2021 (25) | 211 | Broth microdilution | CAMHB | 0.008 to 0.25 | 0.06 | 0.12 |
| Gumbo et al., 2020 (26) | 20 | Broth microdilution | NA | 0.25 to 1 | 1 | 1 |
| Sarathy et al., 2020 (27) | 12 | Broth microdilution | 7H9 ADC | 0.08 to 0.42c | 0.28c | 0.36c |
| Sorayah et al., 2019 (28) | 17 | Broth microdilution | 7H9 ADC | 0.02 to 0.38c | 0.06c | 0.21c |
| Viljoen et al., 2019 (29) | 18 | Broth microdilution | CAMHB | 0.032 to 0.128 | 0.064 | 0.128 |
| Kim et al., 2019 (30) | 132 | Broth microdilution | CAMHB | ≤0.016 to 0.5 | 0.062 | 0.125 |
| Brown-Elliott and Wallace, 2019 (31) | 104 | Broth microdilution | CAMHB | 0.003 to 0.5 | 0.06 | 0.12 |
| Li et al., 2018 (32) | 197 | Broth microdilution | CAMHB | 0.007 to 1 | 0.062 | 0.125 |
| Dupont et al., 2018 (33) | 30 | Broth microdilution | CAMHB | 0.031 to 0.125 | 0.062 | 0.125 |
| Vesenbeckh et al., 2017 (21) | 20 | Agar dilution | 7H10 OADC | 0.12 to 1 | 0.5 | 1 |
| Pang et al., 2017 (34) | 381 | Broth microdilution | CAMHB | 0.016 to >16 | 0.13 | >16 |
| Clofazimine | ||||||
| This study | 61 | Broth microdilution | CAMHB | ≤0.125 to 2 | ≤0.125 | 0.5 |
| This study | 61 | Broth microdilution | CAMHB OADC | 0.25 to 16 | 2 | 4 |
| Asami et al., 2021 (24) | 70 | Broth microdilution | CAMHB | 0.25 to 1 | 0.5 | 1 |
| Chew et al., 2021 (25) | 211 | Broth microdilution | CAMHB | 0.008 to 1 | 0.12 | 0.25 |
| Kwak et al., 2021 (18) | 40 | Broth microdilution | 7H9 OADC | 0.031 to 16 | 4 | 8 |
| Luo et al., 2018 (35) | 40 | Broth microdilution | CAMHB | 0.031 to >8 | 4 | >8 |
| Shen et al., 2018 (36) | 20 | Broth microdilution | CAMHB | 0.25 to 128 | NA | 32 |
| Schwartz et al., 2018 (37) | 17 | Broth microdilution | CAMHB | 0.38 to 3 | 1.5 | 3 |
| Kim et al., 2015 (38) | 57 | Broth microdilution | CAMHB | ≤1 to ≥4 | NA | ≤1 |
| Singh et al., 2014 (39) | 67 | Broth microdilution | CAMHB | 2 to 8 | 2 | 8 |
| van Ingen et al., 2012 (40) | 390 | Broth microdilution | CAMHB | NA | ≤0.5 | 1 |
| Shen et al., 2012 (41) | 117 | Broth microdilution | CAMHB | 0.03125 to 2 | 0.25 | 0.5 |
Selected studies based on PubMed search including the keywords “Mycobacterium,” “abscessus” and “bedaquiline” or “clofazimine.” Studies that analyzed >10 M. abscessus complex isolates are shown.
7H9 ADC, Middlebrook 7H9 broth 0.2% glycerol, 0.05% Tween 80, 10% ADC (albumin-dextrose-catalase); 7H9/7H10 OADC, Middlebrook 7H9 broth/7H10 agar 10% OADC (oleic acid albumin dextrose catalase); CAMHB, cation-adjusted Mueller-Hinton broth without/with 10% OADC; NA, not available.
MIC calculated in milligrams per liter from original data in μM (molecular weight of bedaquiline, 555.5 g/mol).
We observed an influence of incubation time on MIC. Prolonged incubation of 5 days resulted in slightly increased MIC50 values of 0.2 mg/L for bedaquiline and of 0.25 mg/L for clofazimine (Wilcoxon signed-rank test, bedaquiline [Z = −5.1594, P = <0.00001] and clofazimine [Z = −3.2911, P = 0.001]) (Fig. 1A). All isolates showed sufficient growth to be read at days 3 and 5. We next investigated a putative influence of media composition by including 10% OADC (Becton, Dickinson, Franklin Lakes, NJ). OADC is a growth supplement that contains oleic acid, albumin, dextrose, and catalase and is used in combination with Middlebrook 7H9 broth or CAMHB for standard drug susceptibility testing of M. tuberculosis and slowly growing nontuberculous mycobacteria (14). Media containing OADC were also used for resistance testing of rapidly growing nontuberculous mycobacteria (18, 21), although this is not recommended by CLSI (17). Addition of 10% OADC to CAMHB increased the MIC50 of bedaquiline from 0.1 mg/L to 0.4 mg/L (Z = −6.6573, P = <0.00001) and the MIC50 of clofazimine from ≤0.125 mg/L to 2 mg/L (Z = −6.8463, P = <0.00001) when the MIC results were determined at day three of incubation (Fig. 1B). M. abscessus ECOFFs shifted from 0.8 mg/L to 1.6 mg/L for bedaquiline and from 2 mg/L to 16 mg/L for clofazimine in the presence of OADC. These findings suggest that including OADC to the growth medium increases the MICs for bedaquiline and clofazimine in isolates of M. abscessus. Bedaquiline and clofazimine show high plasma protein binding capacities of >99%, and consequently, the protein content of the culture medium is likely to affect the MIC results as has been previously discussed for M. tuberculosis and MICs to bedaquiline (20). Drug instability during prolonged incubation has been shown to affect MIC values for mycobacteria, particularly the MIC of beta-lactam antibiotics (22). Bedaquiline and clofazimine are, however, stable over time as has been demonstrated for in vitro susceptibility testing of M. tuberculosis (20, 23). For M. abscessus, the increased MIC values observed at 5 days versus 3 days of incubation are most probably the result of increased visible growth due to bacteriostatic rather than bactericidal effects around MIC.
In summary, we show that incubation time and composition of the growth medium critically influence the MICs of bedaquiline and clofazimine in M. abscessus while temperature (37°C versus 30°C) has little effect. With the increased use of bedaquiline and clofazimine for treatment of M. abscessus infections, there is a need for standardized MIC testing and interpretation guidelines. In the meantime, it is important that laboratory testing conditions of bedaquiline and clofazimine are precisely documented. Uniform testing conditions are a prerequisite for data comparison and the definition of ECOFF and clinical breakpoints. Beyond that, clinical trials are needed to establish the correlation between in vitro MIC results and clinical response.
ACKNOWLEDGMENTS
We thank the technicians of the National Reference Center for Mycobacteria, Institute of Medical Microbiology, University of Zurich for their dedicated help, and E. C. Böttger for critical reading of the manuscript and continuous support.
This study was supported by the University of Zurich. We acknowledge financial support from Schweizerische Gesellschaft für Cystische Fibrose (CFCH), Swiss National Science Foundation (310030_197699), and the Federal Office of Public Health (FOPH). F. N. Akdoğan Kittana was supported by TUBITAK Turkey.
B. Schulthess, conceptualization, project administration, formal analysis, visualization, writing – original draft, writing – review & editing; F. N. Akdoğan Kittana, investigation, data curation, writing – review & editing; R. Hömke, resources, methodology, writing – review & editing; P. Sander, supervision, funding acquisition, writing – review & editing.
REFERENCES
- 1.Cowman S, van Ingen J, Griffith DE, Loebinger MR. 2019. Non-tuberculous mycobacterial pulmonary disease. Eur Respir J 54:1900250. doi: 10.1183/13993003.00250-2019. [DOI] [PubMed] [Google Scholar]
- 2.Johansen MD, Herrmann JL, Kremer L. 2020. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol 18:392–407. doi: 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- 3.Tortoli E, Kohl TA, Brown-Elliott BA, Trovato A, Leao SC, Garcia MJ, Vasireddy S, Turenne CY, Griffith DE, Philley JV, Baldan R, Campana S, Cariani L, Colombo C, Taccetti G, Teri A, Niemann S, Wallace RJ, Jr, Cirillo DM. 2016. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. nov. Int J Syst Evol Microbiol 66:4471–4479. doi: 10.1099/ijsem.0.001376. [DOI] [PubMed] [Google Scholar]
- 4.Luthra S, Rominski A, Sander P. 2018. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front Microbiol 9:2179. doi: 10.3389/fmicb.2018.02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. 2020. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis 71:905–913. doi: 10.1093/cid/ciaa1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 71(Suppl 1):i1–i22. doi: 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nash KA, Brown-Elliott BA, Wallace RJ, Jr.. 2009. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philley JV, Wallace RJ, Jr, Benwill JL, Taskar V, Brown-Elliott BA, Thakkar F, Aksamit TR, Griffith DE. 2015. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 148:499–506. doi: 10.1378/chest.14-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B, Jhun BW, Moon SM, Lee H, Park HY, Jeon K, Kim DH, Kim SY, Shin SJ, Daley CL, Koh WJ. 2017. Clofazimine-containing regimen for the treatment of Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 61:e02052-16. doi: 10.1128/AAC.02052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey GB, Tebes P, Vinnard C, Kim D, Hadjiliadis D, Hansen-Flaschen J, Dorgan D, Glaser L, Barton G, Hamilton KW. 2019. Clinical outcomes of clofazimine use for rapidly growing mycobacteria infections. Open Forum Infectious Dis 6:ofz456. [Google Scholar]
- 11.Hajikhani B, Nasiri MJ, Hosseini SS, Khalili F, Karimi-Yazdi M, Hematian A, Nojookambari NY, Goudarzi M, Dadashi M, Mirsaeidi M. 2021. Clofazimine susceptibility testing of Mycobacterium avium complex and Mycobacterium abscessus: a meta-analysis study. J Glob Antimicrob Resist 26:188–193. doi: 10.1016/j.jgar.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 12.European Committee for Antimicrobial Susceptibility Testing. 2017. MIC distributions and epidemiological cut-off value (ECOFF) setting, EUCAST SOP 10.00. European Committee on Antimicrobial Susceptibility Testing. http://www.eucast.org. [Google Scholar]
- 13.Griffith DE, Brown-Elliott BA, Benwill JL, Wallace RJ, Jr.. 2015. Mycobacterium abscessus. “Pleased to meet you, hope you guess my name…” Ann Am Thorac Soc 12:436–439. doi: 10.1513/AnnalsATS.201501-015OI. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes, 2nd ed. CLSI document M24A. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 15.Hombach M, Courvalin P, Böttger EC. 2014. Validation of antibiotic susceptibility testing guidelines in a routine clinical microbiology laboratory exemplifies general key challenges in setting clinical breakpoints. Antimicrob Agents Chemother 58:3921–3926. doi: 10.1128/AAC.02489-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2018. Performance standards for susceptibility testing of mycobacteria, Nocardia spp., and other aerobic Actinomyces, 1st ed. CLSI supplement M62. Clinical and Laboratory Standards Institute, Wayne. PA. [Google Scholar]
- 18.Kwak N, Whang J, Yang JS, Kim TS, Kim SA, Yim JJ. 2021. Minimal inhibitory concentration of clofazimine among clinical isolates of nontuberculous mycobacteria and its impact on treatment outcome. Chest 159:517–523. doi: 10.1016/j.chest.2020.07.040. [DOI] [PubMed] [Google Scholar]
- 19.Lounis N, Vranckx L, Gevers T, Kaniga K, Andries K. 2016. In vitro culture conditions affecting minimal inhibitory concentration of bedaquiline against M. tuberculosis. Med Mal Infect 46:220–225. doi: 10.1016/j.medmal.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Keller PM, Hömke R, Ritter C, Valsesia G, Bloemberg GV, Böttger EC. 2015. Determination of MIC distribution and epidemiological cutoff values for bedaquiline and delamanid in Mycobacterium tuberculosis using the MGIT 960 system equipped with TB eXiST. Antimicrob Agents Chemother 59:4352–4355. doi: 10.1128/AAC.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vesenbeckh S, Schönfeld N, Roth A, Bettermann G, Krieger D, Bauer TT, Rüssmann H, Mauch H. 2017. Bedaquiline as a potential agent in the treatment of Mycobacterium abscessus infections. Eur Respir J 49:1700083. doi: 10.1183/13993003.00083-2017. [DOI] [PubMed] [Google Scholar]
- 22.Rominski A, Schulthess B, Müller DM, Keller PM, Sander P. 2017. Effect of beta-lactamase production and beta-lactam instability on MIC testing results for Mycobacterium abscessus. J Antimicrob Chemother 72:3070–3078. doi: 10.1093/jac/dkx284. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. 2018. Technical manual for drug susceptibility testing of medicines used in the treatment of tuberculosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 24.Asami T, Aono A, Chikamatsu K, Igarashi Y, Morishige Y, Murase Y, Yamada H, Takaki A, Mitarai S. 2021. Efficacy estimation of a combination of triple antimicrobial agents against clinical isolates of Mycobacterium abscessus subsp. abscessus in vitro. JAC Antimicrob Resist 3:dlab004. doi: 10.1093/jacamr/dlab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew KL, Octavia S, Go J, Ng S, Tang YE, Soh P, Yong J, Jureen R, Lin RTP, Yeoh SF, Teo J. 2021. In vitro susceptibility of Mycobacterium abscessus complex and feasibility of standardizing treatment regimens. J Antimicrob Chemother 76:973–978. doi: 10.1093/jac/dkaa520. [DOI] [PubMed] [Google Scholar]
- 26.Gumbo T, Cirrincione K, Srivastava S. 2020. Repurposing drugs for treatment of mycobacterium abscessus: a view to a kill. J Antimicrob Chemother 75:1212–1217. doi: 10.1093/jac/dkz523. [DOI] [PubMed] [Google Scholar]
- 27.Sarathy JP, Ganapathy US, Zimmerman MD, Dartois V, Gengenbacher M, Dick T. 2020. TBAJ-876, a 3,5-dialkoxypyridine analogue of bedaquiline, is active against Mycobacterium abscessus. Antimicrob Agents Chemother 64:e02404-19. doi: 10.1128/AAC.02404-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorayah R, Manimekalai MSS, Shin SJ, Koh WJ, Gruber G, Pethe K. 2019. Naturally-occurring polymorphisms in QcrB are responsible for resistance to telacebec in Mycobacterium abscessus. ACS Infect Dis 5:2055–2060. doi: 10.1021/acsinfecdis.9b00322. [DOI] [PubMed] [Google Scholar]
- 29.Viljoen A, Raynaud C, Johansen MD, Roquet-Banères F, Herrmann JL, Daher W, Kremer L. 2019. Verapamil improves the activity of bedaquiline against Mycobacterium abscessus in vitro and in macrophages. Antimicrob Agents Chemother 63:e00705-19. doi: 10.1128/AAC.00705-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DH, Jhun BW, Moon SM, Kim SY, Jeon K, Kwon OJ, Huh HJ, Lee NY, Shin SJ, Daley CL, Koh WJ. 2019. In vitro activity of bedaquiline and delamanid against nontuberculous mycobacteria, including macrolide-resistant clinical isolates. Antimicrob Agents Chemother 63:e00665-19. doi: 10.1128/AAC.00665-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown-Elliott BA, Wallace RJ, Jr.. 2019. In vitro susceptibility testing of bedaquiline against Mycobacterium abscessus complex. Antimicrob Agents Chemother 63:e01919-18. doi: 10.1128/AAC.01919-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Ye M, Guo Q, Zhang Z, Yang S, Ma W, Yu F, Chu H. 2018. Determination of MIC distribution and mechanisms of decreased susceptibility to bedaquiline among clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother 62:e00175-18. doi: 10.1128/AAC.00175-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dupont C, Viljoen A, Thomas S, Roquet-Banères F, Herrmann JL, Pethe K, Kremer L. 2017. Bedaquiline inhibits the ATP synthase in Mycobacterium abscessus and is effective in infected zebrafish. Antimicrob Agents Chemother 61:e01225-17. doi: 10.1128/AAC.01225-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pang Y, Zheng H, Tan Y, Song Y, Zhao Y. 2017. In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob Agents Chemother 61:e02627-16. doi: 10.1128/AAC.02627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo J, Yu X, Jiang G, Fu Y, Huo F, Ma Y, Wang F, Shang Y, Liang Q, Xue Y, Huang H. 2018. In vitro activity of clofazimine against nontuberculous mycobacteria isolated in Beijing, China. Antimicrob Agents Chemother 62:e00072-18. doi: 10.1128/AAC.00072-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Y, Wang X, Jin J, Wu J, Zhang X, Chen J, Zhang W. 2018. In vitro susceptibility of Mycobacterium abscessus and Mycobacterium fortuitum isolates to 30 antibiotics. Biomed Res Int 2018:4902941. doi: 10.1155/2018/4902941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz M, Fisher S, Story-Roller E, Lamichhane G, Parrish N. 2018. Activities of dual combinations of antibiotics against multidrug-resistant nontuberculous mycobacteria recovered from patients with cystic fibrosis. Microb Drug Resist 24:1191–1197. doi: 10.1089/mdr.2017.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SY, Kim CK, Bae IK, Jeong SH, Yim JJ, Jung JY, Park MS, Kim YS, Kim SK, Chang J, Kang YA. 2015. The drug susceptibility profile and inducible resistance to macrolides of Mycobacterium abscessus and Mycobacterium massiliense in Korea. Diagn Microbiol Infect Dis 81:107–111. doi: 10.1016/j.diagmicrobio.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Singh S, Bouzinbi N, Chaturvedi V, Godreuil S, Kremer L. 2014. In vitro evaluation of a new drug combination against clinical isolates belonging to the Mycobacterium abscessus complex. Clin Microbiol Infect 20:1124–1127. doi: 10.1111/1469-0691.12780. [DOI] [PubMed] [Google Scholar]
- 40.van Ingen J, Totten SE, Helstrom NK, Heifets LB, Boeree MJ, Daley CL. 2012. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob Agents Chemother 56:6324–6327. doi: 10.1128/AAC.01505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen GH, Wu BD, Hu ST, Lin CF, Wu KM, Chen JH. 2010. High efficacy of clofazimine and its synergistic effect with amikacin against rapidly growing mycobacteria. Int J Antimicrob Agents 35:400–404. doi: 10.1016/j.ijantimicag.2009.12.008. [DOI] [PubMed] [Google Scholar]