ABSTRACT
GST-HG131, a novel dihydroquinolizinone (DHQ) compound, has been shown to reduce circulating levels of HBsAg in animals. This first-in-human trial evaluated the safety, tolerability, and pharmacokinetic profile of GST-HG131 in healthy Chinese subjects. This was a double-blind, randomized, placebo-controlled phase Ia clinical trial that was conducted in two parts. Part A was a single-ascending-dose (SAD; GST-HG131 10 30, 60, 100, 150, 200, 250 or 300 mg or placebo) study, which also assessed the food effect of GST-HG131 100 mg. Part B was a multiple-ascending-dose (MAD; GST-HG131 30, 60 or 100 mg or placebo BID) study. Tolerability assessments included adverse events, vital signs, 12-lead electrocardiogram, physical examination, and clinical laboratory tests. PK analyses were conducted in blood, urine, and fecal samples. Single doses of GST-HG131 ≤ 300 mg and multiple doses of GST-HG131 ≤ 60 mg were generally safe and well tolerated; however, multiple dosing was stopped at GST-HG131 100 mg, as pre-defined stopping rules specified in the protocol were met (Grade II drug related AEs of nausea and dizziness in >50% of subjects). In the SAD study, median tmax of GST-HG131 was 1-6 h, and t1/2 ranged from 3.88 h to 14.3 h. PK parameters were proportional to dose. Exposure was reduced after food intake. In the MAD study, steady-state was attained on day 4, and there was no apparent plasma accumulation of GST-HG131 on day 7 (Racc < 1.5). In conclusion, GST-HG131 exhibited an acceptable safety profile in healthy subjects at single doses ranging from 10-300 mg and multiple doses (BID) ranging from 30-60 mg, and the MAD doses (30 mg and 60 mg BID) that potentially meet the therapeutic AUC requirements. These findings imply GST-HG131 has potential as a therapeutic option for CHB infection. (This study has been registered at ClinicalTrials.gov under identifier NCT04499443.).
KEYWORDS: GST-HG131, pharmacokinetics, safety, hepatitis B virus expression inhibitor, food effect
INTRODUCTION
Globally, an estimated 250 million individuals are living with chronic hepatitis B virus (HBV) infection, a major cause of liver cirrhosis and hepatocellular carcinoma (HCC) (1–3). HBV infection is characterized by high HBV surface antigen (HBsAg) levels in the circulation, while functional cure is defined as an off-therapy loss of serum HBsAg (4–7). Current antiviral agents, such as interferon and necleos(t)ide analogues (NA), have low functional cure rates (8, 9), and there remains an unmet clinical need for novel therapies with improved anti-HBV potency and good tolerability.
RG7834 is a dihydroquinolizinone (DHQ)-based small-molecule HBV expression inhibitor that significantly reduces HBsAg levels in vitro and in vivo (1, 8–12). Although the development of RG7834 was stopped due to toxicity issues, especially the acute neurotoxicity (13), medicinal chemistry optimization of this compound generated GST-HG131 (Fujian Cosunter Pharmaceutical Co., Ltd), a novel inhibitor of HBsAg production with a promising preclinical safety profile that can significantly reduce serum HBsAg levels in models of chronic HBV infection.
In preclinical pharmacology studies, GST-HG131 and RG7834 had similar HBsAg inhibitory activities (IC50 < 10nM) (9). GST-HG131 inhibited HBsAg production in HepG2.2.15 cells at 50% and 90% maximal effective concentration (EC50 and EC90) values of 4.53 and 48.7 nM, respectively. In an adeno-associated virus/HBV (AAV-HBV) model, GST-HG131 (3–30 mg/kg) demonstrated a robust dose-dependent reduction of HBsAg, and GST-HG131 30 mg/kg and RG7834 10 mg/kg had the same rate of HBsAg reduction (unpublished data). In preclinical safety studies, GST-HG131 had a low risk of off-target effects. In rats and beagles, there were no safety signals in a 28-day toxicology study. In rats, a functional observational battery showed a low risk of neurotoxicity.
In rats and beagles, terminal half-life (t1/2) after intravenous administration of GST-HG131 was 2.11–3.24 h, and bioavailability after oral administration of GST-HG131 was 65% or 76.5%, respectively. In mice, rats, dogs and humans, GST-HG131 had a plasma protein binding rate of 59.3%, 57.1%, 61.1% and 57.2%, respectively. In rats, mean total recovery of radioactivity in urine and fecal samples after a single oral administration of 15 mg/100 μCi/kg [14C] GST-HG131 was 95.23%, with urine and feces accounting for 3.01% and 91.48%, respectively.
The objective of this first-in-human trial was to evaluate the safety, tolerability, and pharmacokinetic (PK) profile of GST-HG131 in healthy Chinese subjects. Findings will inform dose optimization for subsequent Phase Ib and Phase II clinical trials in patients with chronic HBV infection.
RESULTS
Baseline characteristics.
A total of 847 subjects were screened, and 126 subjects were enrolled in this study. One subject withdrew from the SAD GST-HG131 10 mg cohort and one subject withdrew from the MAD GST-HG131 60 mg cohort before the first dose of study drug due to abnormal blood pressure. Eight subjects withdrew from the MAD GST-HG131 100 mg cohort due to AEs. Finally, 116 subjects completed the study and were included in the safety, tolerability, and PK analyses. Subjects baseline demographic characteristics (age, weight, and BMI) were well balanced across treatment groups (Table 1).
TABLE 1.
Baseline demographic characteristics of study subjects
| SAD |
MAD |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 mg | 30 mg | 60 mg | 100 mg/group a | 100 mg/group B | 150 mg | 200 mg | 250 mg | 300 mg | Placebo | 30 mg BID | 60 mg BID | 100 mg BID | Placebo | |
| Characteristics | (N = 8) | (N = 8) | (N = 8) | (N = 8) | (N = 8) | (N = 8) | (N = 8) | (N = 8) | (N = 8) | (N = 16) | (N = 10) | (N = 10) | (N = 10) | (N = 6) |
| Age, y | 36.8 ± 8.48 | 35 ± 10.3 | 36.4 ± 11.8 | 37.6 ± 11.3 | 37.4 ± 6.72 | 34.5 ± 9.58 | 37.5 ± 10.0 | 44.4 ± 9.07 | 41.9 ± 9.98 | 36.6 ± 9.84 | 38.5 ± 7.86 | 37.2 ± 7.66 | 42.5 ± 6.87 | 37.8 ± 3.87 |
| Gender, n (%) | ||||||||||||||
| Male | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 8 (50.0) | 5 (50.0) | 5 (50.0) | 5 (50.0) | 3 (50.0) |
| Female | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 4 (50.0) | 8 (50.0) | 5 (50.0) | 5 (50.0) | 5 (50.0) | 3 (50.0) |
| ht, cm | 164.0 ± 7.67 | 166.7 ± 13.0 | 161.2 ± 8.46 | 161.7 ± 7.76 | 162.8 ± 7.03 | 162.4 ± 9.77 | 162.6 ± 10.3 | 162.6 ± 7.80 | 161.9 ± 8.92 | 162.7 ± 8.95 | 160.2 ± 5.94 | 161.4 ± 7.98 | 164.6 ± 8.63 | 158.6 ± 9.26 |
| wt, kg | 67.0 ± 9.60 | 66.7 ± 13.4 | 61.2 ± 7.18 | 59.0 ± 6.46 | 57.0 ± 7.18 | 63.3 ± 9.70 | 63.0 ± 5.36 | 63.5 ± 10.1 | 60.1 ± 8.70 | 64.0 ± 10.7 | 60.0 ± 7.79 | 60.5 ± 5.97 | 62.7 ± 9.52 | 60.7 ± 9.54 |
| BMI, kg/m2 | 24.9 ± 2.70 | 23.8 ± 2.05 | 23.6 ± 2.20 | 22.8 ± 2.38 | 21.5 ± 1.51 | 23.9 ± 2.23 | 24.1 ± 2.53 | 23.9 ± 2.59 | 23.1 ± 2.30 | 24.0 ± 2.25 | 23.4 ± 2.12 | 23.1 ± 1.73 | 23.2 ± 2.20 | 23.8 ± 1.94 |
Safety and tolerability.
In the SAD study, 18.8-62.5% of subjects treated with GST-HG131 and 31.3% of subjects treated with placebo reported treatment-emergent AEs (TEAEs). 12.5-50.0% of subjects treated with GST-HG131 and 31.3% of subjects treated with placebo reported drug-related TEAEs.
Drug-related TEAEs most frequently (≥25%) reported by subjects administered GST-HG131 included serum creatinine increased (GST-HG131 30 mg and 200 mg: 25% of subjects each), neutrophil count decreased (GST-HG131 300 mg: 25% of subjects), and WBC count decreased (GST-HG131 300 mg: 25% of subjects). All AEs were mild to moderate (assessed by the investigator as CTCAE grade I and II). No dose effect was observed for any TEAE in SAD study (Table 2).
TABLE 2.
Treatment-related adverse events
| Preferred term | Single ascending dose |
Multiple ascending dose |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 mg | 30 mg | 60 mg | 100 mg fasted | 100 mg fed | 150 mg | 200 mg | 250 mg | 300 mg | Placebo | 30 mg BID | 60 mg BID | 100 mg BID | Placebo | |
| (N = 8) | (N = 8) | (N = 8) | (N = 16) | (N = 16) | (N = 8) | (N = 8) | (N = 8) | (N = 8) | (N = 16) | (N = 10) | (N = 10) | (N = 10) | (N = 6) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| All AEsa | 2 (25.0%) | 3 (37.5%) | 2 (25.0%) | 5 (31.3%) | 3 (18.8%) | 1 (12.5%) | 4 (50.0%) | 2 (25.0%) | 5 (62.5%) | 5 (31.3%) | 5 (50.0%) | 4 (40.0%) | 10 (100%) | 2 (33.3%) |
| Drug related AEs | 2 (25.0%) | 2 (25.0%) | 1 (12.5%) | 4 (25.0%) | 2 (12.5%) | 1 (12.5%) | 4 (50.0%) | 2 (25.0%) | 4 (50.0%) | 5 (31.3%) | 4 (40.0%) | 2 (20.0%) | 10 (100%) | 2 (33.3%) |
| Serum creatinine increased | 0 | 2 (25.0%) | 0 | 0 | 0 | 0 | 2 (25.0%) | 0 | 1 (12.5%) | 0 | 2 (20.0%) | 0 | 3 (30.0%) | 1 (16.7%) |
| Neutrophil count decreased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (25.0%) | 0 | 0 | 0 | 0 | 1 (16.7%) |
| WBC count decreased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (25.0%) | 0 | 0 | 0 | 0 | 0 |
| Blood bilirubin increased | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 1 (12.5%) | 0 | 0 | 1 (6.3%) | 0 | 1 (10.0%) | 0 | 0 |
| Neutrophil count increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5%) | 0 | 0 | 0 | 1 (10.0%) | 0 |
| Positive antinuclear antibodies | 0 | 0 | 1 (12.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| WBC count increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5%) | 0 | 0 | 0 | 1 (10.0%) | 0 |
| Reduced sperm forward movement | 1 (12.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 0 | 0 |
| Blood creatine phosphokinase increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5%) | 0 | 0 | 0 | 0 | 0 |
| Alanine aminotransferase increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 0 | 0 |
| Aspartate aminotransferase increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 0 | 0 |
| Sperm count decreased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 0 | 0 |
| Cacospermia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (30.0%) | 1 (10.0%) | 0 | 1 (16.7%) |
| Abnormal sperm morphology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 | 0 | 0 |
| Lipase increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 0 | 0 |
| Hyperuricemia | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 0 | 1 (12.5%) | 0 | 1 (6.3%) | 0 | 0 | 0 | 0 |
| Hypoalbuminemia | 0 | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypoglycemia | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyponatremia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5%) | 0 | 0 | 0 | 0 | 0 | 3 (30.0%) | 0 |
| Hypokalemia | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 | 1 (10.0%) | 0 |
| Hypophosphatemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 |
| Hypocalcemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 | 0 |
| Hypertriglyceridemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5%) | 0 | 0 | 1 (10.0%) | 0 | 1 (16.7%) |
| Urinary tract infection | 0 | 0 | 0 | 2 (12.5%) | 1 (6.3%) | 1 (12.5%) | 0 | 0 | 1 (12.5%) | 1 (6.3%) | 1 (10.0%) | 0 | 0 | 0 |
| Upper respiratory infection | 0 | 1 (12.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 0 | 0 | 0 | 1 (6.3%) | 1 (6.3%) | 0 | 0 | 1 (12.5%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 1 (12.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dizziness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 (90.0%) | 0 |
| Asthenospermia | 0 | 0 | 1 (12.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (20.0%) | 0 | 0 | 0 |
| Haematuria | 0 | 1 (12.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Supraventricular extrasystole | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (6.3%) | 0 | 0 | 0 | 0 |
| Nausea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 (90.0%) | 0 |
| Vomit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 (60.0%) | 0 |
| Gastroesophageal reflux disease | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (30.0%) | 0 |
| Abdominal discomfort | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (20.0%) | 0 |
| Upper abdominal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 | 0 |
| Retching | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 |
| Cheat discomfort | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 |
| Pharyngeal erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 |
| Tonsil hypertrophy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 |
| Tinnitus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 |
AE, adverse event; data are n (%).
In the MAD study, TEAEs were reported in 50.0%, 40.0%, 100%, and 33.3% of subjects, and drug-related TEAEs were reported in 40.0%, 20.0%, 100%, and 33.3% of subjects administered 30, 60, 100 mg GST-HG131 or placebo, respectively. Multiple doses of GST-HG131 30 mg or 60 mg were generally safe and well tolerated, however, 100% of subjects administered multiple doses of GST-HG131 100 mg reported drug-related TEAEs, including dizziness (reported by 90% of subjects), nausea (reported by 90% of subjects), and vomiting (reported by 60% of subjects). Subjects treated with other doses of GST-HG131 or placebo did not report these TEAEs (Table 3).
TABLE 3.
Drug-related adverse events in subjects treated with multiple doses of GST-HG131 100 mg
| 100 mg BID | 100 mg BID | 100 mg BID | Placebo | Placebo | Placebo | |
|---|---|---|---|---|---|---|
| Preferred term | (N = 10) | (N = 10) | (N = 10) | (N = 2) | (N = 2) | (N =2) |
| Total | CTCAE grade I | CTCAE grade II | Total | CTCAE grade I | CTCAE grade II | |
| Drug related AEsa | 10 (100%) | 3 (30.0%) | 7 (70.0%) | 1 (50.0%) | 1 (50.0%) | 0 |
| Dizziness | 9 (90.0%) | 2 (20.0%) | 7 (70.0%) | 0 | 0 | 0 |
| Nausea | 9 (90.0%) | 3 (30.0%) | 6 (60.0%) | 0 | 0 | 0 |
| Vomit | 6 (60.0%) | 4 (40.0%) | 2 (20.0%) | 0 | 0 | 0 |
| Gastroesophageal reflux disease | 3 (30.0%) | 3 (30.0%) | 0 | 0 | 0 | 0 |
| Abdominal discomfort | 2 (20.0%) | 2 (20.0%) | 0 | 0 | 0 | 0 |
| Retching | 1 (10.0%) | 1 (10.0%) | 0 | 0 | 0 | 0 |
| Serum creatinine increased | 3 (30.0%) | 3 (30.0%) | 0 | 1 (50.0%) | 1 (50.0%) | 0 |
| Neutrophil count decreased | 0 | 0 | 0 | 1 (50.0%) | 1 (50.0%) | 0 |
| Hyponatremia | 2 (20.0%) | 2 (20.0%) | 0 | 0 | 0 | 0 |
| Cheat discomfort | 1 (10.0%) | 1 (10.0%) | 0 | 0 | 0 | 0 |
| Tinnitus | 1 (10.0%) | 1 (10.0%) | 0 | 0 | 0 | 0 |
AE, adverse event; data are n (%).
In the MAD study, > 50% of subjects treated with GST-HG131 100 mg reported grade ≥2 drug-related TEAEs (Table 3). Multiple dosing was stopped at GST-HG131 100 mg, as pre-defined stopping rules specified in the protocol were met.
Pharmacokinetics.
GST-HG131 plasma concentration-time profiles and PK parameters after a single dose of GST-HG131 10–300 mg are shown in Fig. 1 and Table 4. GST-HG131 was absorbed rapidly with a median Tmax between 1.0 h and 3.0 h. Mean t1/2 ranged from 3.88 h to 14.3 h, which tended to increase with the dose escalation. Mean Cmax increased from 223 ng/mL to 5375 ng/mL. Cmax and AUC values increased in a dose-dependent manner. GST-HG131 PK were linear with respect to Cmax, AUC0-t, and AUC0-∞. Dose proportionality was concluded since the 90% CI for the slope ln(Cmax) (β = 0.97 [0.86-0.99]), ln(AUC0-t) (β = 1.07 [1.00-1.15]), and ln(AUC0-∞) (β = 1.07 [1.00-1.15]) on ln(dose) were completely contained within the pre-specified equivalence interval of 0.796–1.204.
FIG 1.
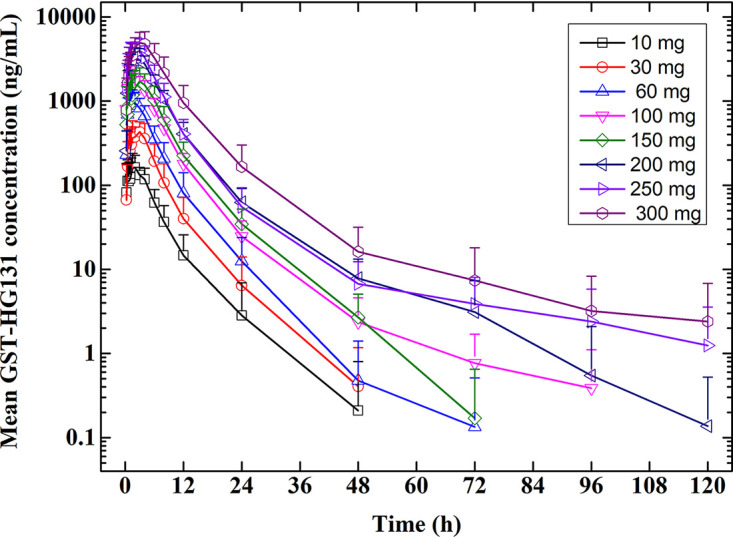
SAD study: GST-HG131 plasma concentration-time profiles in healthy subjects (Semi-log profiles).
TABLE 4.
SAD study: Plasma pharmacokinetic parameters of GST-HG131 in healthy subjectsa
| PK parametes | 10 mg | 30 mg | 60 mg | 100 mg (fasted) | 100 mg (fed) | 150 mg | 200 mg | 250 mg | 300 mg |
|---|---|---|---|---|---|---|---|---|---|
| (N = 8) | (N = 8) | (N = 8) | (N = 16) | (N = 16) | (N = 8) | (N = 8) | (N = 8) | (N = 8) | |
| Tmax (h)b | 1.375 (0.25, 4.00) | 1.500 (0.75, 4.00) | 1.000 (0.50, 3.00) | 1.750 (0.25, 4.00) | 2.500 (1.25, 4.00) | 3.000 (1.00, 6.00) | 2.005 (1.00, 4.00) | 2.000 (0.50, 3.02) | 2.500 (1.25, 4.02) |
| Cmax (n*g/mL) | 223.3 ± 63.1 | 552.9 ± 121.3 | 1314.7 ± 352.2 | 2132.3 ± 595.2 | 1582.3 ± 291.3 | 2035.2 ± 881.7 | 3451.7 ± 820.3 | 4724.1 ± 1243.7 | 5375.7 ± 1682.9 |
| AUC0-t (h*ng/mL) | 1015.6 ± 419.0 | 2762.5 ± 1149.3 | 5908.8 ± 2212.9 | 11568.1 ± 2765.9 | 9791.9 ± 2002.6 | 12983.8 ± 4800.5 | 23737.5 ± 6020.9 | 27962.5 ± 9921.3 | 43337.5 ± 18315.3 |
| AUC0-∞ (h*ng/mL) | 1028.0 ± 422.3 | 2777.5 ± 1150.7 | 5945.0 ± 2209.5 | 11606.9 ± 2769.3 | 9845.6 ± 2004.0 | 13013.8 ± 4819.6 | 23762.5 ± 6036.5 | 28012.5 ± 9968.3 | 43525.0 ± 18383.2 |
| t1/2 (h) | 4.12 ± 1.65 | 4.06 ± 1.02 | 3.88 ± 1.22 | 7.71 ± 5.27 | 9.18 ± 10.9 | 5.63 ± 2.21 | 8.58 ± 4.80 | 11.6 ± 13.6 | 14.3 ± 22.3 |
| CL/F (mL/h) | 10842.5 ± 3316.3 | 12171.3 ± 3981.7 | 11753.8 ± 5777.5 | 9080.0 ± 2188.7 | 10529.4 ± 1989.5 | 14792.5 ± 11166.6 | 8960.0 ± 2503.4 | 9770.0 ± 2857.4 | 7996.3 ± 3274.3 |
| Vz/F (mL) | 58800 ± 10708 | 67300 ± 14679 | 60088 ± 17208 | 94131 ± 49143 | 142881 ± 178253 | 102463 ± 37626 | 107713 ± 59224 | 142925 ± 140744 | 128363 ± 158248 |
Data are expressed as mean ±SD unless otherwise specified. Tmax is expressed as median (range).
Tmax, time to peak plasma concentration; Cmax, peak plasma concentration; AUC0-t, area under the plasma concentration-time curve from time zero to time t; AUC0-∞, area under the plasma concentration-time curve from time zero to infinity; t1/2, terminal elimination half-life; CL/F, apparent clearance; Vz/F, apparent volume of distribution.
GST-HG131 plasma concentration-time profiles in the food-effect study are shown in Fig. 2. In the fed state, Tmax was delayed, and Cmax and AUC were decreased. The Cmax, AUC0-t, and AUC0-∞ geometric least-squares mean ratios (90% CI) were 0.76 (0.65, 0.89), 0.85 (0.81, 0.89), and 0.86 (0.82, 0.90), respectively. Mean recovery of GST-HG131 in urine and fecal samples were 2.368% and 2.797%, respectively, over 120 h post-dose.
FIG 2.
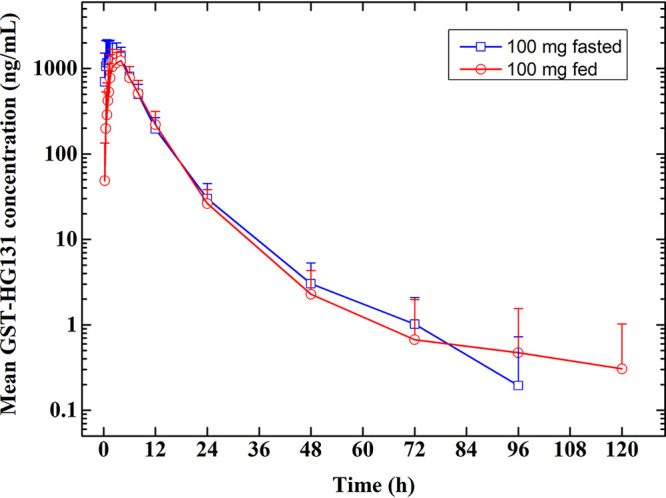
Food-effect study: GST-HG131 100 mg (single dose) plasma concentration-time profiles in healthy subjects (Semi-log profiles).
GST-HG131 plasma concentration-time profiles and PK parameters (days 1 and 7) after multiple doses of GST-HG131 30–100 mg are shown in Fig. 3 and Table 5. Median Tmax was 1.5–2.0 h and 1.385–4.5 h on days 1 and 7, respectively. Mean t1/2,ss ranged from 7.20 h to 10.0 h, and Racc was <1.5, implying no plasma accumulation of GST-HG131 on day 7. Steady-state conditions were reached on day 4. Trough levels prior to first administration of GST-HG131 30 mg, 60 mg, or 100 mg on days 4–7 were 82.6–90.9, 213.8−263.8, 395.5−426.5 ng/mL, respectively. Trough levels (12h) prior to second administration of GST-HG131 30 mg, 60 mg, or 100 mg on day 7 were 59.3, 182.3 and 460.4 ng/mL.
FIG 3.
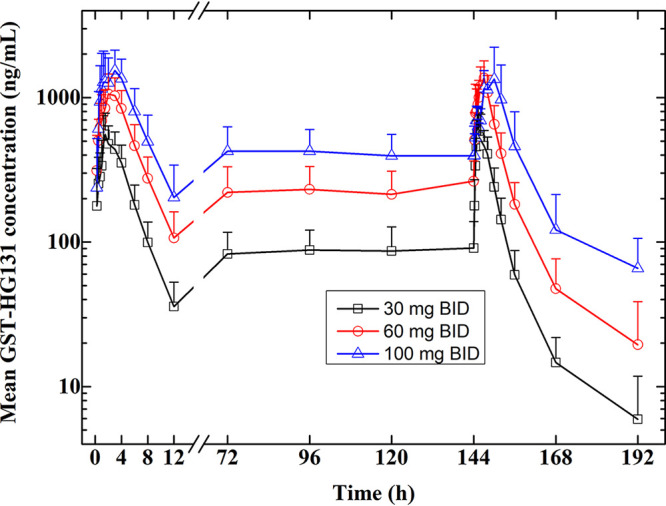
MAD study: GST-HG131 plasma concentration-time profiles in healthy subjects (Semi-log profiles).
TABLE 5.
MAD study: Plasma pharmacokinetic parameters of GST-HG131 in healthy subjectsa
| PK parametes | 30 mg BID | 60 mg BID | 100 mg BID |
|---|---|---|---|
| D1 | (N = 10) | (N = 10) | (N = 10) |
| Tmax (h)b | 1.500 (0.75,3.00) | 2.000 (0.75,3.00) | 1.750 (1.00,4.00) |
| Cmax (n*g/mL) | 645.9 ± 145.4 | 1250.9 ± 276.7 | 1993.3 ± 548.5 |
| AUC0-t (h*ng/mL) | 2656.0 ± 698.8 | 6122.0 ± 1637.9 | 9574.0 ± 2873.1 |
| AUC0-∞ (h*ng/mL) | 2792.0 ± 764.4 | 6588.0 ± 1845.1 | 10532.0 ± 3475.7 |
| t1/2 (h) | 2.52 ± 0.343 | 2.86 ± 0.526 | 2.91 ± 0.586 |
| CL/F (mL/h) | 11504.0 ± 3165.8 | 9674.0 ± 2365.1 | 10587.0 ± 3836.6 |
| Vz/F (mL) | 40770 ± 8325.5 | 39400 ± 9664.8 | 42500 ± 11362 |
| D7 | (N = 10) | (N = 10) | (N = 2) |
| Tmax,ss (h) | 1.385 (0.75,4.00) | 2.500 (0.50,3.00) | 4.500 (3.00,6.00) |
| Cmax,ss (n*g/mL) | 698.4 ± 232.4 | 1587.2 ± 337.5 | 1698.1 ± 382.7 |
| Cmin,ss (n*g/mL) | 57.9 ± 29.3 | 180.6 ± 72.5 | 368.4 ± 206.6 |
| AUC0-t,ss (h*ng/mL) | 3910.0 ± 1322.6 | 10320.0 ± 3123.6 | 16650.0 ± 8273.2 |
| AUC0-∞,ss (h*ng/mL) | 3983.0 ± 1335.5 | 10589.0 ± 3363.0 | 17600.0 ± 8909.6 |
| AUC0-12h,ss (h*ng/mL) | 3220.0 ± 1040.2 | 8146.0 ± 2072.2 | 10920.0 ± 4072.9 |
| t1/2,ss (h) | 7.20 ± 1.80 | 7.92 ± 1.78 | 10.0 ± 1.10 |
| CL/F,ss (mL/h) | 10291.0 ± 3629.8 | 7784.0 ± 1893.0 | 9830.0 ± 3634.5 |
| DF (%) | 241.3 ± 54.2 | 211.3 ± 30.7 | 153.2 ± 37.5 |
| RacAUC0-12h (%) | 121.5 ± 24.4 | 135.0 ± 23.4 | 132.3 ± 23.1 |
Data are expressed as mean ±SD unless otherwise specified. Tmax is expressed as median (range).
Tmax, time to peak plasma concentration; Cmax, peak plasma concentration; AUC0-t, area under the plasma concentration-time curve from time zero to time t; AUC0-∞, area under the plasma concentration-time curve from time zero to infinity; t1/2, terminal elimination half-life; CL/F, apparent clearance; Vz/F, apparent volume of distribution, Tmax,ss, time to peak plasma concentration at steady-state; Cmax,ss, peak plasma concentration at steady-state; AUC0-t,ss, area under the plasma concentration-time curve from time zero to time t at steady-state; AUC0-∞,ss, area under the plasma concentration-time curve from time zero to infinity at steady-state; AUC0-12ht,ss, area under the plasma concentration-time curve from time zero to 12 h at steady-state; t1/2,ss, terminal elimination half-life at steady-state; CL/F,ss, apparent clearance at steady-state; Vz/F, apparent volume of distribution; DF, fluctuation percentage; Rac, accumulation factor.
DISCUSSION
GST-HG131 is a novel inhibitor of HBsAg production which optimized from the first oral HBV expression inhibitor RG7834. The anti-HBV potency of GST-HG131 is well-demonstrated in both in vitro HepG2.2.15 cells and PHH infection assays. GST-HG131 had similar HBsAg inhibitory activities with RG7834 (IC50 < 10nM) in preclinical pharmacology studies (9). Efficacy of GST-HG131 against HBV was shown in AAV-HBV mice model by demonstrating a significant reduction of HBsAg. The safety was well in rats and beagles in a 28-day toxicology study and showed a low risk of neurotoxicity, which was observed in RG7834 (13). This Phase I clinical trial was designed evaluate the safety, tolerability, and PK profile of GST-HG131 in healthy Chinese subjects.
In this study healthy subjects were randomized to receive a single oral dose of GST-HG131 10, 30, 60, 100, 150, 200, 250, or 300 mg, multiple oral doses of GST-HG131 30, 60 or 100 mg (BID), or matching placebo. The maximum recommended starting dose (MSRD) in humans was calculated as 8.72–20.2 mg based on the NOAEL in rats (15 mg/kg) and beagles (100 mg/kg), considering a safety factor of 60. The maximal tolerated dose (MTD) in humans was calculated as 363.6 mg and 697.7–3488.4 mg based on MTDs in rats (300 mg/kg) and beagles (200−1000 mg/kg), considering a safety factor of 10. Although GST-HG131was safe and well-tolerated in preclinical animal studies, and single doses of GST-HG131 ≤ 300 mg and multiple doses of GST-HG131 ≤ 60 mg were generally safe and well tolerated in this first-in-human study, multiple dosing was stopped at GST-HG131 100 mg, as pre-defined stopping rules specified in the protocol were met (Grade II drug related AEs of nausea and dizziness in >50% of subjects).
There were no serious AEs or deaths in the SAD or MAD studies. No dose effect was observed for any TEAE. However, the MAD study was stopped at the GST-HG131 100 mg cohort as >50% of the subjects treated with multiple doses of GST-HG131 100 mg reported grade 2 drug-related AEs, including dizziness (reported by 90% of subjects), nausea (reported by 90% of subjects), and vomiting (reported by 60% of subjects), which were not reported by subjects in all SAD doses and MAD doses ≤60 mg BID treated with GST-HG131 or placebo. In preclinical toxicology studies, vomiting, salivation and opisthotonos were observed in beagles treated with GST-HG131 1000 mg/kg. Except the neurotoxicity of RG7834, no other adverse reaction was mentioned about RG7834 and other DHQ compounds (13).
In the MAD study, the half-life of GST-HG131 ranged from 7.20 h to 10.0 h, implying GST-HG131 is suitable for twice daily dosing. In the SAD study, Cmax and AUC values increased in a dose-dependent manner. In previous human, monkey, dog, rat and mouse liver microsomes and liver cells incubation tests, the prototype drug GST-HG131 was the main component (>75%) after incubation. In vivo metabolic tests, the relative abundance of GST-HG131 (percentage of GST-HG131 in plasma to total plasma radioactivity) were 69.2–75.4% and 86.4–90.2% in SD rats and beagle dog. Prototype drugs GST-HG131 was the main existing form in previous studies. In rats, mean total recovery of radioactivity in fecal samples after a single oral administration of 15 mg/100 μCi/kg [14C] GST-HG131 was 91.5%. In humans, only 2.797% of GST-HG131 was excreted through the feces, the phenomenon that requires further investigation in a radio-labeling study.
The efficacy of GST-HG131 in patients with CHB infection remains to be elucidated. GST-HG131 inhibited HBsAg production in HepG2.2.15 cells at an EC90 value of 48.7 nM (21 ng/mL). The initial effective dose in humans was calculated as 160.8 mg, corresponding to an effective dose of 30 mg/kg in mice. The mean steady state trough concentration for the MAD GST-HG131 30 mg cohort (free drug: 24.8 ng/mL) reached the target concentration, and the mean AUC of the plasma concentration of GST-HG131 was greater than the effective dose extrapolated from studies in mice (160.8 mg) (Table 6). The safety, tolerability, and PK profile of GST-HG131 in the healthy Chinese subjects included in this first-in-human study suggests postprandial doses of GST-HG131 30 mg BID and GST-HG131 60 mg BID could be used in a Phase Ib study in patients with HBV infection.
TABLE 6.
Pharmacokinetic profiles of GST-HG131
| Species | Dose | AUCss (h*ng/mL, D7) | Ctrough (ng/mL,D7) |
|---|---|---|---|
| HepG2.2.15 cell | NA | NA | EC90: 48.71 nM (21 ng/mL) |
| Mice | Effective dose (single dose) | AUC0-t: 5660 (free: 2304) | NA |
| Healthy subjects | 30 mg (BID) | AUC0-12h,ss: 3220 (free: 1378) | C12h: 57.9 (free: 24.8) |
| 60 mg (BID) | AUC0-12h,ss: 8146 (free: 3486) | C12h: 180.6 (free: 77.3) |
In conclusion, GST-HG131 exhibited an acceptable safety, tolerability and PK profile in healthy subjects at single doses ranging from 10–300 mg and multiple doses (BID) ranging from 30–60 mg, and the MAD doses (30 mg and 60 mg BID) that potentially meet the therapeutic AUC requirements. These findings and preclinical efficacy data imply GST-HG131 has potential as a therapeutic option for CHB infection. GST-HG131 30 mg BID and 60 mg BID may be used in future clinical trials.
MATERIALS AND METHODS
This was a single-center clinical trial conducted at the Jilin University First Affiliated Hospital-Phase I Clinical Research Center, Changchun City, China between August 18, 2020 and July 6, 2021 (NCT04499443 [ClinicalTrials.gov]). This first-in-human study was approved by the Ethics Committee at the Jilin University First Affiliated Hospital-Clinical Research Institute and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All study subjects gave written informed consent.
Study subjects.
This study was conducted in healthy Chinese volunteers. Key inclusion criteria were: 1) males or females (not pregnant or lactating) aged 18–55 years; 2) body mass index (BMI) 18-28 kg/m2 (body weight: males, ≥ 50 kg, females, ≥ 45 kg); and 3) no clinically significant abnormal findings at screening on physical examination, medical history, and/or clinical laboratory tests. Key exclusion criteria were: 1) chronic smoking or alcohol and/or drug abuse; 2) use of any medication within the 14 days prior to initiation of study drug (defined as GST-HG131 or placebo) or during the study; 3) blood donation within the 3 months prior to study initiation; 4) exposure to any CYP3A4, P-gp, or BCRP inducers or inhibitors within the 3 months prior to initiation of study drug; or 5) participation in a clinical trial of another investigational drug within the 3 months prior to study initiation.
Study design.
This was a double-blind, randomized, placebo-controlled Phase Ia clinical trial that was conducted in two parts. Part A was a single-ascending-dose (SAD) study comprising eight cohorts (n = 10 per cohort). Subjects in each cohort were randomly assigned 4:1 to receive a single dose of GST-HG131 (10, 30, 60, 100, 150, 200, 250, or 300 mg) or placebo in the fasting state. An additional eight subjects were enrolled in the 100 mg cohort to assess the food effect of GST-HG131 in a two-period crossover study with a 7-day washout period. During the fed condition, after an overnight fast of approximately 10 h, high-fat (approximately 50% of total caloric content of the meal), high calorie (approximately 800–1000 kcal) breakfast will be consumed starting 30 min prior to dosing. The breakfast (two boiled eggs, 20 g butter, 20 g bacon, one slice of toast 50 g, 115 g fries and 240 mL whole milk) should be completed prior to dosing.
The initial GST-HG131 dose (10 mg) was selected based on preclinical studies. MRSD were calculated at 20.2 and 8.72 mg using a 60-fold safety margin to the no-observed-adverse-effect-level (NOAEL) in rats and beagles, respectively. The minimal anticipated biological effect level (MABEL) was 160.8 mg, calculated from an efficacy study in mice. Dose-escalation occurred after a satisfactory medical review of safety data at days 2, 4, and 6 post-dose. The Principal Investigator and study Sponsor determined each subsequent dose level.
Part B was a multiple-ascending-dose (MAD) study comprising three cohorts (n = 12 per cohort). Subjects in each cohort were randomly assigned 5:1 to receive GST-HG131 (30, 60, or 100 mg) or placebo in the fasting state twice-daily for 7 days. Doses administered in the MAD study were determined after a review of the safety and PK data from the SAD study. Dose-escalation occurred after a satisfactory medical review of safety data at days 3, 6, and 9 after treatment initiation. The Principal Investigator and study Sponsor approved each subsequent dose level.
Each SAD and MAD cohort included two sentinel subjects, safety results for the sentinel subjects were reviewed at 48 h (SAD) or 72 h (MAD) post-dose, prior to enrolling additional subjects.GST-HG131 and placebo were provided by the sponsor (Fujian Cosunter Pharmaceutical Co., Ltd.), and had identical packaging, labeling, appearance, and administration schedules.
Study termination criteria was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0 and defined as the emergence of the following drug-related adverse events (AEs): in a dose group, more than half of participants suffered Grade 2 drug-related AEs, or more than quarter of participants suffered Grade 3–4 treatment-related AEs or one subject experienced a drug-related serious adverse event (SAE).
PK analysis.
Blood samples (4 mL each) for PK analysis were collected via an indwelling intravenous angiocatheter into K2EDTA containing tubes at pre-specified time points. Plasma was isolated after the first 0.5–1 mL of blood had been discarded.
In the SAD study, blood samples were collected at 0 h (pre-dose) and 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, 96, and 120 h post-dose. For the food-effect cohort, blood samples were collected at the same time points on days 1 and 8. In the MAD study, blood samples were collected on day 1 (0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 10, and 12 h) and day 7 (0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, and 48 h). Samples for the determination of GST-HG131 trough concentration were collected on day 4 to day 6 at 0 h (pre-dose).
In period-2 (fasting state) of the food-effect study, urine samples were collected at 0 h, 0–6 h, 6–12 h, 12–24 h, 24–48 h, 48–72 h, 72–96 h, and 96–120 h post-dose. Fecal samples were collected at 0–120 h post-dose.
All samples were stored at −80°C until analysis.
The concentrations of GST-HG131 in plasma, urine, and fecal samples were determined with a validated liquid chromatography-tandem mass spectrometry method using an LC-30AD HPLC system (Shimadzu, Tokyo, Japan) equipped with a Triple Quad 6500 + (AB Sciex, Toronto, ON, Canada). Calibration ranges for assays in plasma, urine, and feces were 1.00∼2000 ng/mL. Accuracies were -6.1%∼4.9%, -7.3%∼14.9% and -4.7%∼ 9.6%, and precision was within 6.2% CV, 10.1% CV and 8.1% CV in plasma, urine, and feces, respectively.
Safety.
Adverse events (AEs), vital signs (body temperature, blood pressure at rest, heart rate, respiratory rate), and findings on electrocardiograms, physical examination, and clinical laboratory tests (biochemistry, hematology, urinalysis, reticulocyte, thyroid function, antinuclear antibody [ANA] series, and immune globulin) were evaluated according to the NCI-CTCAE v5.0. Thyroid ultrasound, semen analysis and sperm DNA fragmentation testing was performed, and sex hormone levels were measured. The incidence and severity of AEs and their relationship to study drug were recorded.
Statistical analysis.
Plasma PK data were analyzed using standard non-compartmental methods with WinNonlin v8.0 (Certara USA Inc.). PK parameters included peak plasma concentration (Cmax), time to peak plasma concentration (Tmax), area under the plasma concentration–time curve from time zero to the last time point with a quantifiable concentration (AUC0-t), AUC from time zero to infinity (AUC0-∞), t1/2, clearance (CL/F), and apparent volume of distribution (Vz/F). The amount (Ae) and apparent fraction (Fe) of GST-HG131 recovered in the urine and fecal samples over 120 h post-dose were calculated.
Statistical analysis was performed with SAS software, v 9.4 (SAS Institute Inc., United States). Continuous variables are provided as number, mean ± standard deviation, median, maximum, and minimum. Categorical variables are provided as frequency and percentage. Dose proportionality analysis on Cmax and AUC was assessed using log-transformed data regressions.
In the food-effect study, mixed-effects modeling with period and treatment as fixed effects and subjects specified as nested random effects was used to compare log-transformed Cmax, AUC0-t, and AUC0-∞ values between the fasted and fed states. Geometric mean ratios and corresponding 90% confidence intervals (CIs) for Cmax, AUC0-t, and AUC0-∞ were calculated.
ACKNOWLEDGMENTS
This work was financially supported by the capital construction funds within the provincial budget in 2020 (innovation capacity construction, project: 2020C038-1) and the National Natural Science Foundation of China (Project: 82104314).
The authors have no conflicts of interest.
Contributor Information
Junqi Niu, Email: junqiniu@jlu.edu.cn.
Yanhua Ding, Email: dingyanh@jlu.edu.cn.
REFERENCES
- 1.Han X, Zhou C, Jiang M, Wang Y, Wang J, Cheng Z, Wang M, Liu Y, Liang C, Wang J, Wang Z, Weikert R, Lv W, Xie J, Yu X, Zhou X, Luangsay S, Shen HC, Mayweg AV, Javanbakht H, Yang S. 2018. Discovery of RG7834: the first-in-class selective and orally available small molecule hepatitis B virus expression inhibitor with novel mechanism of action. J Med Chem 61:10619–10634. 10.1021/acs.jmedchem.8b01245. [DOI] [PubMed] [Google Scholar]
- 2.Roca Suarez AA, Testoni B, Zoulim F. 2021. HBV 2021: new therapeutic strategies against an old foe. Liver Int 41(Suppl 1):15–23. 10.1111/liv.14851. [DOI] [PubMed] [Google Scholar]
- 3.Schinazi RF, Ehteshami M, Bassit L, Asselah T. 2018. Towards HBV curative therapies. Liver Int 38(Suppl 1):102–114. 10.1111/liv.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dusheiko G. 2021. Hepatitis B cure: how and when. Liver Int 41(Suppl 1):24–29. 10.1111/liv.14837. [DOI] [PubMed] [Google Scholar]
- 5.Fanning GC, Zoulim F, Hou J, Bertoletti A. 2019. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov 18:827–844. 10.1038/s41573-019-0037-0. [DOI] [PubMed] [Google Scholar]
- 6.Lee HW, Lee JS, Ahn SH. 2020. Hepatitis B virus cure: targets and future therapies. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsounis EP, Tourkochristou E, Mouzaki A, Triantos C. 2021. Toward a new era of hepatitis B virus therapeutics: the pursuit of a functional cure. World J Gastroenterol 27:2727–2757. 10.3748/wjg.v27.i21.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menne S, Wildum S, Steiner G, Suresh M, Korolowicz K, Balarezo M, Yon C, Murreddu M, Hong X, Kallakury BV, Tucker R, Yang S, Young JAT, Javanbakht H. 2020. Efficacy of an inhibitor of hepatitis B virus expression in combination with entecavir and interferon-α in woodchucks chronically infected with woodchuck hepatitis virus. Hepatol Commun 4:916–931. 10.1002/hep4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller H, Wildum S, Luangsay S, Walther J, Lopez A, Tropberger P, Ottaviani G, Lu W, Parrott NJ, Zhang JD, Schmucki R, Racek T, Hoflack JC, Kueng E, Point F, Zhou X, Steiner G, Lütgehetmann M, Rapp G, Volz T, Dandri M, Yang S, Young JAT, Javanbakht H. 2018. A novel orally available small molecule that inhibits hepatitis B virus expression. J Hepatol 68:412–420. 10.1016/j.jhep.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Mueller H, Lopez A, Tropberger P, Wildum S, Schmaler J, Pedersen L, Han X, Wang Y, Ottosen S, Yang S, Young JAT, Javanbakht H. 2019. PAPD5/7 are host factors that are required for hepatitis B virus RNA stabilization. Hepatology 69:1398–1411. 10.1002/hep.30329. [DOI] [PubMed] [Google Scholar]
- 11.Nagpal N, Wang J, Zeng J, Lo E, Moon DH, Luk K, Braun RO, Burroughs LM, Keel SB, Reilly C, Lindsley RC, Wolfe SA, Tai AK, Cahan P, Bauer DE, Fong YW, Agarwal S. 2020. Small-molecule PAPD5 inhibitors restore telomerase activity in patient stem cells. Cell Stem Cell 26:896–909.e8. 10.1016/j.stem.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun L, Zhang F, Guo F, Liu F, Kulsuptrakul J, Puschnik A, Gao M, Rijnbrand R, Sofia M, Block T, Zhou T. 2020. The dihydroquinolizinone compound RG7834 inhibits the polyadenylase function of PAPD5 and PAPD7 and accelerates the degradation of matured hepatitis B virus surface protein mRNA. Antimicrob Agents Chemother 65. 10.1128/AAC.00640-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang N, Sun L, Noe D, Lam PYS, Zhou T, Block TM, Du Y. 2021. Hepatoselective dihydroquinolizinone bis-acids for HBsAg mRNA degradation. ACS Med Chem Lett 12:1130–1136. 10.1021/acsmedchemlett.1c00228. [DOI] [PMC free article] [PubMed] [Google Scholar]


