Abstract
To study the possible use of probiotics in fish farming, we evaluated the in vitro and in vivo antagonism of antibacterial strain Pseudomonas fluorescens strain AH2 against the fish-pathogenic bacterium Vibrio anguillarum. As iron is important in virulence and bacterial interactions, the effect of P. fluorescens AH2 was studied under iron-rich and iron-limited conditions. Sterile-filtered culture supernatants from iron-limited P. fluorescens AH2 inhibited the growth of V. anguillarum, whereas sterile-filtered supernatants from iron-replete cultures of P. fluorescens AH2 did not. P. fluorescens AH2 inhibited the growth of V. anguillarum during coculture, independently of the iron concentration, when the initial count of the antagonist was 100 to 1,000 times greater that of the fish pathogen. These in vitro results were successfully repeated in vivo. A probiotic effect in vivo was tested by exposing rainbow trout (Oncorynchus mykiss Walbaum) to P. fluorescens AH2 at a density of 105 CFU/ml for 5 days before a challenge with V. anguillarum at 104 to 105 CFU/ml for 1 h. Some fish were also exposed to P. fluorescens AH2 at 107 CFU/ml during the 1-h infection. The combined probiotic treatment resulted in a 46% reduction of calculated accumulated mortality; accumulated mortality was 25% after 7 days at 12°C in the probiotic-treated fish, whereas mortality was 47% in fish not treated with the probiont.
An increasing amount of the world’s fish resources is being supplied by farmed fish. While catches of wild fish have stagnated at approximately 90 million metric tons, the amount of farmed fish has increased from 10 million metric tons in 1984 to more than 20 million metric tons in 1996 (11, 22). Disease is a major problem for the fish farming industry. Although vaccines are being developed and marketed, they generally cannot be used as a universal disease control measure in aquaculture. Juvenile fish are not fully immunocompetent and do not always respond to vaccination. Vaccination by injection, sometimes the only effective route of administration, is impractical when applied to small fish or larger numbers of fish. The addition of substantial amounts of antibiotics and chemotherapeutics remains the method of choice for disease control in many parts of the aquaculture industry. Increased concern about antibiotic-resistant microorganisms (1) has led to several alternative suggestions for disease prevention, including the use of nonpathogenic bacteria as probiotic biocontrol agents (3, 6, 36, 39). Lactic acid bacteria have been tested as probiotics in warm-blooded animals, and attempts have also been made to use lactic acid bacteria as antagonists of fish pathogens (14, 24, 25).
Fluorescent pseudomonads have been used as biocontrol agents in several rhizosphere studies (31), where their inhibitory activity has been attributed to a number of factors, such as the production of antibiotics (29, 34), hydrogen cyanide (38), or iron-chelating siderophores (26, 28). Pseudomonads constitute a large part of the microflora of the gills, skin, and intestinal tracts of live fish (9, 37) and are only rarely reported as pathogens of fish (20). As with their terrestrial counterparts, aquatic pseudomonads are often antagonistic against other microorganisms (15, 27), including fish-pathogenic bacteria (36) and fish-pathogenic fungi (6, 20). One study demonstrated that bathing Atlantic salmon presmolts in a strain of Pseudomonas fluorescens reduced subsequent mortality from stress-induced furunculosis (36).
When tested in vitro, iron limitation has been found to facilitate the antibacterial activity of fluorescent pseudomonads (15, 36). Thus, inhibition may be due to the production of siderophores, which deprive the fish pathogen of iron. Production of siderophores is a virulence factor in many microorganisms, such as members of the family Enterobacteriaceae, Pseudomonas aeruginosa, and Vibrio anguillarum (10), as reviewed by Wooldridge and Williams (40). An efficient salmon furunculosis vaccine elicits antibodies against iron-repressible outer membrane proteins of Aeromonas salmonicida (21). Iron available in the serum of fish, as in mammals (19), is crucial for infection, and fish with iron overload are more prone to attack by V. anguillarum than are fish with low serum iron concentrations (30).
To further study the potential of pseudomonads as biocontrol agents in fish farming, we investigated the inhibitory activity of a P. fluorescens strain of aquatic origin (AH2) against the fish-pathogenic bacterium, V. anguillarum in vitro and in vivo. The influence of iron on the inhibitory activity was assessed because the availability of iron is important in virulence and disease.
(Part of this paper has been presented as posters at the General Meeting of the American Society for Microbiology, 3 to 8 May 1997, and at the Fifth European Marine Microbiology Symposium, 11 to 15 August 1996.)
MATERIALS AND METHODS
Bacterial strains.
A virulent strain of the fish pathogen of V. anguillarum (90-11-287; serotype O1) that carries the pJM1 plasmid was obtained from K. Pedersen, Royal Veterinary and Agricultural University, Copenhagen, Denmark (35). P. fluorescens AH2, which produces several siderophores (2), was isolated from iced freshwater fish (Lates niloticus) (18) and is antagonistic toward several gram-positive and gram-negative bacteria, particularly when iron limited (15, 17).
Media.
M9 salts (32) supplemented with 0.4% glucose and 0.3% Casamino Acids (M9GC) or M9GC plus 3% NaCl (M9GC+NaCl) was used as low-iron culture medium. The total iron concentration, as determined by the phenanthroline method optimized for low concentrations, was estimated to be 0.6 μM (24a). Iron-replete conditions were obtained by adding 100 μM FeCl3. In experiments with pure cultures, bacteria were counted by surface plating on tryptone soy agar plates (Oxoid catalog no. CM131) incubated at 25°C. V. anguillarum was routinely cultured on LB medium (Difco 0446) (5) with a total of 2% NaCl.
Siderophore production.
Siderophore production was assayed on chrome azurol S (CAS) agar (33) based on M9GC (16). In liquid medium, siderophores were detected by the CAS assay (33). Equal volumes of sterile-filtered culture supernatant and CAS assay solution were mixed and left for 30 min at room temperature. The A630 was measured with sterile medium and CAS assay solution (33) as a blank. A negative value indicated the presence of iron-chelating substances such as siderophores (33).
Agar antagonism assay.
Initial screening of antagonism by P. fluorescens AH2 was done in a plate assay. V. anguillarum (100 μl precultured in M9GC+NaCl for 5 days at 15°C) was spread on M9GC+NaCl agar plates with and without additional iron. Wells 3 mm in diameter were punched into the solidified agar, and 10 μl of a 24-h culture of P. fluorescens AH2 was added. The plates were incubated at 15°C, and zones of inhibition around the wells were measured after 3 to 5 days.
Effect of P. fluorescens AH2 supernatants.
P. fluorescens AH2 was precultured in M9GC with and without NaCl and with or without iron (four combinations) and then used to inoculate 50 ml of M9GC in the same four combinations at an initial cell density of 103 to 104 CFU/ml. The flasks were incubated at 12 to 13°C with agitation (150 rpm), and samples were withdrawn daily. One milliliter was used for serial dilutions and estimation of colony counts on tryptone soy agar, and 2 ml was sterile-filtered (0.2-μm pore size; Sartorius no. 16534). The possible inhibitory activity of the sterile-filtered supernatant was tested by adding 100 μl of supernatant to 100 μl of fresh medium in microtiter wells (Nunc microwell 96F) and inoculating it with 10 μl of a dilution of V. anguillarum yielding approximately 104 CFU/ml. Controls were done by inoculating V. anguillarum in 200 μl of M9GC. Each combination was tested in triplicate, and the growth of the fish pathogen was monitored by recording the optical density at 600 nm (OD600) with a Labsystems Multiscan RC microtiter plate reader.
Coculture experiments.
P. fluorescens AH2 and V. anguillarum were precultured separately in M9GC+NaCl at 13°C with aeration for 3 to 5 days. Appropriate dilutions were prepared in physiological saline, and V. anguillarum was inoculated in M9GC+NaCl at an initial cell density of approximately 103 CFU/ml, whereas the initial levels of P. fluorescens AH2 were 104, 105, 106, 107, and 108 CFU/ml. All combinations were done in duplicate. The flasks were incubated at 12 to 13°C with aeration, and samples were withdrawn daily for determination of bacterial cell densities. Numbers of V. anguillarum bacteria were estimated by preparing 10-fold serial dilutions using 1 ml from each dilution to inoculate tubes with 5 ml of H&L medium (23). The tubes were covered with paraffin and incubated at 25°C. The fermentative growth of V. anguillarum caused a change in the pH indicator of the medium. The highest dilution still showing growth was used to calculate the number of V. anguillarum bacteria present. This procedure was chosen because low numbers of V. anguillarum bacteria had to be estimated in a high background of P. fluorescens AH2, which did not grow in the anaerobic H&L tubes. Inoculation of H&L medium with 109 P. fluorescens AH2 bacteria did not affect the color of the medium.
Probiotic treatment and infection of fish.
P. fluorescens AH2 was grown for 6 days at 13°C (150 rpm) in M9GC without NaCl addition, and V. anguillarum 90-11-287 was grown for 16 h in tryptone soy broth. A total of 644 rainbow trout (Oncorhynchus mykiss Walbaum) weighing approximately 40 g were divided into eight 600-liter tanks, and fish from four of the tanks were exposed for 5 days to P. fluorescens AH2 at a level of 105 CFU/ml at 12°C (long-term treatment) by adding the bacteria to the water. All fish were exposed to V. anguillarum at a level of 104 to 105 CFU/ml for 1 h in 50% seawater (i.e., a total of 1.5% NaCl) at 12°C. Half of the probiotically treated fish and half of the untreated fish were also treated with P. fluorescens AH2 (107 CFU/ml) during exposure to V. anguillarum (short-term treatment). The P. fluorescens AH2 bacteria used for this short-term treatment were cultured at 13°C for 7 days in M9GC with NaCl added. All fish, independently of probiotic treatment, were physically handled in the same manner. After infection, the fish were kept at 12°C in fresh water in eight 600-liter tanks with a water flow of 50 liters/h and fed in accordance with the BioMar A/S Ecolife 19 feeding table by automatic feeders during a 10-h daily feeding period. Dead fish were collected and recorded daily. V. anguillarum was isolated from deceased fish by inoculation of head kidney smears on blood agar plates, and its identity was verified by Mono VaR (BioNor Aqua, Skien, Norway) serum agglutination. Data were recorded as accumulated mortality. The calculated accumulated mortality was derived by adding the effect of a particular treatment to the average mortality of all of the eight tanks and subtracting half of the effect of the two individual factors (7) (no significant two-factor interactions between the two treatments were recorded). The statistical significance of the two individual probiotic treatments, as well as that of the combined effect, was calculated by the analysis of variance program in the statistical software package Statgraphics (Statistical Graphics Corporation, Princeton, N.J.).
RESULTS
Agar antagonism assay.
P. fluorescens AH2 caused a clearing zone with a radius of 3 to 5 mm in lawns of V. anguillarum in the absence of iron. No inhibition zones were observed when the medium was supplemented with iron.
Effect of P. fluorescens AH2 supernatants.
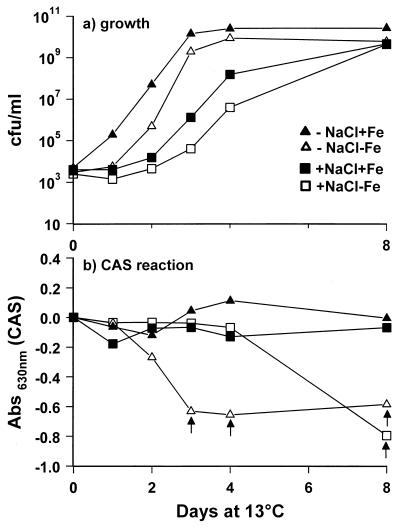
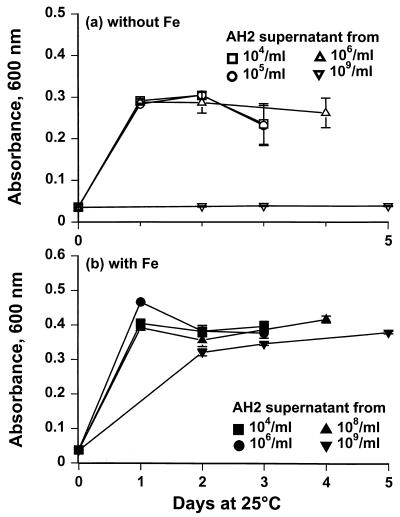
Strain AH2 grew well and produced siderophores in Fe-limited M9GC with and without 3% NaCl (Fig. 1). A weak CAS reaction (A630, −0.25) was measured when counts of strain AH2 reached 106 cells/ml. A significant CAS reaction (A630, ∼−0.6), indicating high levels of siderophores, was not seen until strain AH2 reached 109 CFU/ml (Fig. 1). Addition of iron caused more-rapid growth and a slightly higher maximum cell density, whereas 3% NaCl caused a prolonged lag phase and a slightly lower growth rate (Fig. 1). Sterile-filtered strain AH2 supernatants with a strong CAS reaction were inhibitory to V. anguillarum (Fig. 1 and 2). In contrast, supernatants from iron-replete cultures of strain AH2 or supernatants obtained from iron-limited cultures at low cell density did not inhibit the growth of the fish pathogen (Fig. 2b). The iron-replete cultures showed no sign of iron limitation, as the CAS reaction remained negative. Addition of iron to inhibitory sterile-filtered supernatants with a strong CAS reaction eliminated the inhibitory effect. Growth curves of V. anguillarum in M9GC+NaCl (controls) were identical to curves obtained with supernatants from low counts of strain AH2 that were not CAS positive (data not shown).
FIG. 1.
Growth (a) and CAS reaction (b) of P. fluorescens AH2 grown in M9GC with or without 3% NaCl with or without 0.1 mM FeCl3 at 13°C. The arrows indicate sampling points where sterilely filtered supernatants were inhibitory to V. anguillarum.
FIG. 2.
Growth of V. anguillarum at 25°C in M9GC–3% NaCl with spent supernatants from P. fluorescens AH2 cultured without (a) or with (b) 100 μM FeCl3.
Coculture experiments.
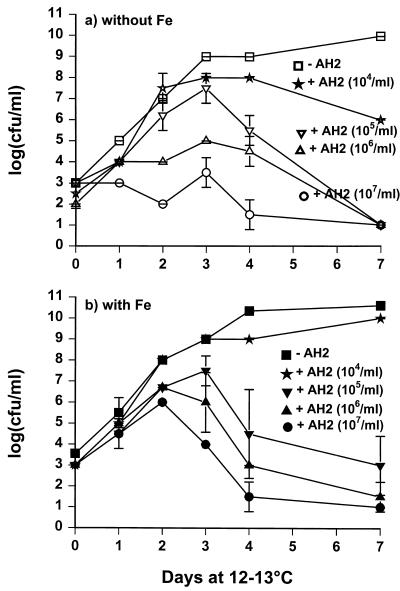
The growth of V. anguillarum was inhibited under iron-limited conditions by strain AH2 inoculated at an initial level of 106 to 107 CFU/ml (Fig. 3a). Lower concentrations of strain AH2 (104 to 105 CFU/ml) allowed initial growth of V. anguillarum, but cell densities never reached the level of the control. High inoculum concentrations (105 to 107 CFU/ml) of strain AH2 under iron-replete conditions allowed an initial increase of V. anguillarum followed by a decrease in the viable count (Fig. 3b). Growth of P. fluorescens AH2 was not affected by coculturing with V. anguillarum (data not shown).
FIG. 3.
Growth of V. anguillarum at 13°C in M9GC–3% NaCl with and without P. fluorescens AH2 at different initial cell densities without (a) or with (b) 100 μM FeCl3.
Probiotic treatment and infection of fish.
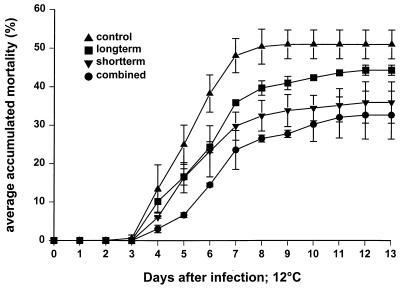
The accumulated mortality of infected fish not treated with strain AH2 reached 50% 9 days after infection with the onset of mortality at day 3. The mortality leveled off after the 7th day, at which the accumulated mortality was 47% (Fig. 4). No dead fish were found in control tanks not exposed to V. anguillarum. Both the long-term and short-term treatments with strain AH2 caused a decrease in accumulated mortality, to 44 and 35%, respectively. Combining the treatments caused a further reduction in accumulated mortality to 32% (Fig. 4). No significant two-factor interactions between the treatments was seen, and the effect of the two treatments combined was therefore regarded as additive (Table 1). The effect of probiotic treatment was most pronounced during the first days of infection, but all probiotic treatments were also significantly different from the infection control when the mortality stabilized (Fig. 4 and Table 1).
FIG. 4.
Accumulated mortality (percentage with two tanks of each treatment) of rainbow trout infected with V. anguillarum with and without probiotic treatment with P. fluorescens AH2. Long-term treatment was 5 days of exposure to P. fluorescens AH2 at 105 CFU/ml. Short-term treatment consisted of addition of P. fluorescens AH2 at 107 CFU/ml during exposure to V. anguillarum.
TABLE 1.
Calculated average accumulated mortality of rainbow trout after infection with V. anguillarum and probiotic treatment with P. fluorescens AH2
| Day after infection | Calculated accumulated avg % mortality | Effect on relative % survivala of:
|
Standard deviation of effect (%) | ||
|---|---|---|---|---|---|
| Long-term AH2 treatment | Short-term AH2 treatment | Combined AH2 treatments | |||
| 7 | 46.6 | 19.7*** | 32.8*** | 46.1*** | 2.7 |
| 10 | 49.8 | 12.9* | 28.7*** | 37.1*** | 2.4 |
| 13 | 50.1 | 10.0 | 26.5** | 33.1*** | 3.1 |
Relative percent survival = [1 − (mortality of treated fish)/(mortality of control)] × 100. ∗∗∗, P < 0.01; ∗∗, P < 0.05; ∗, P < 0.10.
DISCUSSION
The concept of biological disease control, particularly using nonpathogenic bacterial strains for disease prevention, has received widespread attention during the last decade. Fuller (13) defined a probiotic as “a live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance.” However, as the skin and gill microflora of fish must be assumed also to contribute to disease prevention, we have used a broader definition here, namely, a live microbial supplement which beneficially affects the host animal by improving its microbial balance. A strain of P. fluorescens was successfully used to reduce the frequency of stress-induced infections by A. salmonicida (36). Austin et al. (3) reported that exposure to a nonpathogenic Vibrio alginolyticus strain reduced subsequent mortality due to vibriosis. To our knowledge, our data are the first to demonstrate a reduction in vibriosis-caused mortality in trout by the use of a probiotic P. fluorescens strain.
In agreement with other studies (15, 26, 41), we have seen that under iron-limited conditions, P. fluorescens AH2 is inhibitory to V. anguillarum when tested in vitro in a well diffusion assay or when sterile-filtered supernatants were tested. Similar to Smith and Davey (36), we found that addition of iron to sterile-filtered supernatants of strain AH2 eliminated the inhibitory activity. The appearance of inhibitory activity in spent supernatants from iron-limited P. fluorescens AH2 coincided with a strong CAS reaction, indicating the presence of siderophores. However, our data do not allow us to specifically implicate siderophores in the active mechanism.
Despite the importance of iron limitation seen in the deferred end point assays, iron was not as important during coculture, when inhibition was more a function of the density of the antagonist than of the iron concentration (Fig. 3). Due to its fast growth, P. fluorescens AH2 may compete for other nutrients, occupy colonization sites, or excrete antibacterial substances (29, 34). However, such substances, if produced, were not present in sufficient concentrations to allow detection in vitro in supernatants from iron-enriched cultures.
High levels of P. fluorescens AH2 were required before inhibition of V. anguillarum could be detected in coculture assays. In agreement with earlier studies of interactions between fish spoilage bacteria (17), sterile-filtered supernatant from P. fluorescens AH2 did not inhibit the growth of V. anguillarum until an antagonist level of 108 CFU/ml was reached. A number of studies have assessed the numbers of pseudomonads required to protect against various plant diseases. Xu and Gross (41) found that compared to infection with 104 Erwinia carotovora bacteria per potato seed, 106 antagonist bacteria per potato seed increased emergence 32% and 1010 antagonist bacteria per potato seed increased emergence 96%. It has been reported similarly that 106 CFU of a fluorescent pseudomonad per seed was required to protect sunflower seeds against Sclerotinia wilt, and protection increased with increasing amounts of the pseudomonad (12). Our studies also show that the antagonist must be present at significantly higher levels than the pathogen, and the degree of inhibition increases with the level of the antagonist. Thus, during coculture, 107 to 109 CFU/ml was required to inhibit the growth of the pathogen (Fig. 3). Therefore, a potential probiotic culture must either be supplied on a regular basis or be able to colonize and multiply on or in the host.
Rhizosphere studies often have great difficulties moving from in vitro to in vivo situations (8). In preliminary in vivo studies with P. fluorescens AH2 (data not shown), we found that short-term exposure, even to high numbers of the bacterium, had no effect on subsequent fish mortality. However, with more-constant exposure to AH2, a significant reduction in mortality was obtained (Fig. 4 and Table 1). The fact that the reduction in mortality obtained by the two different treatments was additive indicated that further reduction in mortality may be obtained by optimizing the procedure. Probiotic treatment of fish thus offers a very promising alternative to the use of antibiotics and chemotherapy.
ACKNOWLEDGMENTS
This study was partly financed by the Danish Biotechnology Program 1991–1995, the Danish Food Technology Program, the Danish Ministry for Food, Agriculture and Fisheries, and the Academy for the Technical Sciences (T.F.N.).
We thank Søren S. Jørgensen for determination of the iron concentration in the defined medium. Valuable comments from two reviewers and Einar Ringø are appreciated.
REFERENCES
- 1.Amábile-Cuevas C F, Gárdenas-Garciá M, Ludgar M. Antibiotic resistance. Am Sci. 1995;83:320–329. [Google Scholar]
- 2.Anthoni U, Christophersen C, Nielsen P H, Gram L, Petersen B O. Pseudomonine, an isoxazole with siderophoric activity from Pseudomonas fluorescens AH2, isolated from Lake Victorian Nile perch. J Nat Prod Chem. 1995;58:1786–1789. [Google Scholar]
- 3.Austin B, Stuckey L F, Robertson P A W, Effendi I, Griffith D R W. A probiotic strain of Vibrio alginolyticus effective in reducing diseases caused by Aeromonas salmonicida, Vibrio anguillarum and Vibrio ordalii. J Fish Dis. 1995;18:93–96. [Google Scholar]
- 4.Aznar R, Alcaide E. Siderophores and related outer membrane proteins produced by pseudomonads isolated from eels and freshwater. FEMS Microbiol Lett. 1992;98:269–276. doi: 10.1016/0378-1097(92)90168-n. [DOI] [PubMed] [Google Scholar]
- 5.Bertani G. Studies of lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bly J E, Quiniou S M-A, Lawson L A, Clem L W. Inhibition of Saprolegnia pathogenic for fish by Pseudomonas fluorescens. J Fish Dis. 1997;20:35–40. [Google Scholar]
- 7.Box G E P, Hunter W G, Hunter J S. Statistics for experimenters. New York, N.Y: John Wiley & Sons, Inc.; 1978. [Google Scholar]
- 8.Buyer J S. Abstracts of the 94th General Meeting of the American Society for Microbiology 1994. Washington, D.C: American Society for Microbiology; 1994. Siderophore production by Pseudomonas B10 in the rhizosphere, abstr. N188; p. 349. [Google Scholar]
- 9.Cahill M M. Bacterial flora of fishes: a review. Microb Ecol. 1990;19:21–41. doi: 10.1007/BF02015051. [DOI] [PubMed] [Google Scholar]
- 10.Crosa J H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980;283:566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- 11.Csavas I. World aquaculture status and outlook. INFOFISH Int. 1994;5:47–54. [Google Scholar]
- 12.Expert J M, Digat B. Biocontrol of Sclerotinia wilt of sunflower by Pseudomonas fluorescens and Pseudomonas putida strains. Can J Microbiol. 1995;41:685–691. [Google Scholar]
- 13.Fuller R. A review. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 14.Gatesoupe F-J. Lactic acid bacteria increase the resistance of turbot larvae, Scophthalmus maximus, against pathogenic vibrio. Aquat Living Resour. 1994;7:277–282. [Google Scholar]
- 15.Gram L. Inhibitory effect against pathogenic and spoilage bacteria of Pseudomonas strains isolated from spoiled and fresh fish. Appl Environ Microbiol. 1993;59:2197–2203. doi: 10.1128/aem.59.7.2197-2203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gram L. The influence of substrate on siderophore production by fish spoilage bacteria. J Microbiol Methods. 1996;25:199–205. [Google Scholar]
- 17.Gram L, Melchiorsen J. Interaction between Pseudomonas spp. and Shewanella putrefaciens in fish model systems. J Appl Bacteriol. 1996;80:589–595. doi: 10.1111/j.1365-2672.1996.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 18.Gram L, Wedell-Neergaard C, Huss H H. The bacteriology of fresh and spoiling Lake Victorian Nile perch (Lates niloticus) Int J Food Microbiol. 1990;10:303–316. doi: 10.1016/0168-1605(90)90077-i. [DOI] [PubMed] [Google Scholar]
- 19.Griffith E. Iron and biological defence mechanisms. In: Gould G W, Charnley A K, Rhodes-Roberts M E, Cooper R M, Board R G, editors. Natural antimicrobial systems. Part 1. Antimicrobial systems in plants and animals. Bath, United Kingdom: Bath University Press; 1986. pp. 56–71. [Google Scholar]
- 20.Hatai K, Willoughby L G. Saprolegnia parasitica from rainbow trout inhibited by the bacterium Pseudomonas fluorescens. Bull Eur Assn Fish Pathol. 1988;8:27–29. [Google Scholar]
- 21.Hirst I D, Ellis A E. Iron-regulated outer membrane proteins of Aeromonas salmonicida are important protective antigens in Atlantic salmon against furunculosis. Fish Shellfish Immunol. 1994;4:29–45. [Google Scholar]
- 22.Hjul P. The outlook for world aquaculture. INFOFISH Int. 1997;1:27–30. [Google Scholar]
- 23.Hugh R, Leifson E. The taxonomic significance of fermentative versus oxidative gram-negative bacteria. J Bacteriol. 1953;66:24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jöborn A, Olsson C J, Kjelleberg S. Colonization in the fish intestinal tract and production of inhibitory substances in intestinal mucus and faecal extracts by Carnobacterium sp. strain K1. J Fish Dis. 1997;20:383–392. [Google Scholar]
- 24a.Jørgensen, S. S. (Royal Veterinary and Agricultural University, Copenhagen, Denmark). Personal communication.
- 25.Kjelleberg, S., P. Conway, R. Hammond, A. Jöborn, C. Olsson, and A. Westerdahl. 1997. Protection against pathogenic microorganisms. International patent application no. PCT/GB96/01936, international publication no. WO 97/06811.
- 26.Kloepper J W, Leong J, Teintze M, Schroth M N. Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria. Nature. 1980;286:885–886. [Google Scholar]
- 27.Lemos M L, Toranzo A E, Barja J L. Antibiotic activity of epiphytic bacteria isolated from intertidal seaweeds. Microb Ecol. 1985;11:149–163. doi: 10.1007/BF02010487. [DOI] [PubMed] [Google Scholar]
- 28.Loper J E, Buyer J S. Siderophores in microbial interactions on plant surfaces. Mol Plant-Microbe Interact. 1991;4:5–13. [Google Scholar]
- 29.Mazzola M, Cook R J, Thomashow L, Weller D M, Pierson L S., III Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol. 1992;58:2616–2624. doi: 10.1128/aem.58.8.2616-2624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakai T, Kanno T, Cruz E R, Muroga K. The effects of iron compounds on the virulence of Vibrio anguillarum in Japanese eels and ayu. Fish Pathol. 1987;22:185–189. [Google Scholar]
- 31.O’Sullivan D J, O’Gara F. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev. 1992;56:662–676. doi: 10.1128/mr.56.4.662-676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 34.Shanahan P, O’Sullivan D J, Simpson P, Glennon J D, O’Gara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol. 1992;58:353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skov M N, Pedersen K, Larsen J L. Comparison of pulsed-field gel electrophoresis, ribotyping, and plasmid profiling for typing of Vibrio anguillarum serovar O1. Appl Environ Microbiol. 1995;61:1540–1545. doi: 10.1128/aem.61.4.1540-1545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith P, Davey S. Evidence for the competitive exclusion of Aeromonas salmonicida from fish with stress-inducible furunculosis by a fluorescent pseudomonad. J Fish Dis. 1993;16:521–524. [Google Scholar]
- 37.Strøm E, Ringø E. Changes in the bacterial composition of early developing cod, Gadus morhua (L.), larvae following inoculation of Lactobacillus plantarum into the water. In: Walther B T, Pyhn H J, editors. Physiological and biochemical aspects of fish development. Bergen, Norway: University of Bergen; 1993. pp. 226–228. [Google Scholar]
- 38.Voisard C, Keel C, Haas D, Defago G. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J. 1989;8:351–358. doi: 10.1002/j.1460-2075.1989.tb03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerdahl A, Olsson J C, Kjelleberg S, Conway P L. Isolation and characterization of turbot (Scophthalmus maximus)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol. 1991;57:2223–2228. doi: 10.1128/aem.57.8.2223-2228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 41.Xu G-W, Gross D C. Selection of fluorescent pseudomonads antagonistic to Erwinia carotovora and suppressive of potato seed piece decay. Phytopathology. 1986;76:414–422. [Google Scholar]