Abstract
Study Objectives
Obstructive sleep apnea is associated with neurobehavioral dysfunction, but the relationship between disease severity as measured by the apnea-hypopnea index and neurobehavioral morbidity is unclear. The objective of our study is to compare the neurobehavioral morbidity of mild sleep-disordered breathing versus obstructive sleep apnea.
Methods
Children 3–12 years old recruited for mild sleep-disordered breathing (snoring with obstructive apnea-hypopnea index < 3) into the Pediatric Adenotonsillectomy Trial for Snoring were compared to children 5–9 years old recruited for obstructive sleep apnea (obstructive apnea-hypopnea 2–30) into the Childhood Adenotonsillectomy Trial. Baseline demographic, polysomnographic, and neurobehavioral outcomes were compared using univariable and multivariable analysis.
Results
The sample included 453 participants with obstructive sleep apnea (median obstructive apnea-hypopnea index 5.7) and 459 participants with mild sleep-disordered breathing (median obstructive apnea-hypopnea index 0.5). By polysomnography, participants with obstructive sleep apnea had poorer sleep efficiency and more arousals. Children with mild sleep-disordered breathing had more abnormal executive function scores (adjusted odds ratio 1.96, 95% CI 1.30–2.94) compared to children with obstructive sleep apnea. There were also elevated Conners scores for inattention (adjusted odds ratio 3.16, CI 1.98–5.02) and hyperactivity (adjusted odds ratio 2.82, CI 1.83–4.34) in children recruited for mild sleep-disordered breathing.
Conclusions
Abnormal executive function, inattention, and hyperactivity were more common in symptomatic children recruited into a trial for mild sleep-disordered breathing compared to children recruited into a trial for obstructive sleep apnea. Young, snoring children with only minimally elevated apnea-hypopnea levels may still be at risk for deficits in executive function and attention.
Trial Registration
Pediatric Adenotonsillectomy for Snoring (PATS), NCT02562040; Childhood Adenotonsillectomy Trial (CHAT), NCT00560859
Keywords: pediatric, sleep-disordered breathing, obstructive sleep apnea, behavior, neurocognition
Statement of Significance.
Obstructive sleep apnea is associated with neurobehavioral dysfunction, but the relationship between disease severity as measured by the apnea-hypopnea index and neurobehavioral morbidity is unclear. We compared baseline characteristics of 861 children recruited into two randomized controlled trials, the Pediatric Adenotonsillectomy Trial for Snoring (apnea-hypopnea index < 3) and the Childhood Adenotonsillectomy Trial (apnea-hypopnea index 2–30). Among symptomatic children who were adenotonsillectomy candidates, there were more abnormalities in executive function, inattention, and hyperactivity in the cohort with lower apnea-hypopnea levels. Snoring children without frequent apneas may still be at risk for deficits in executive function and attention, which suggests that snoring is a behavioral risk factor for neurobehavioral morbidity.
Introduction
Pediatric sleep-disordered breathing (SDB) is a common condition that encompasses a spectrum of disorders ranging from mild sleep-disordered breathing (mSDB), defined as primary snoring without frequent respiratory events, to severe obstructive sleep apnea (OSA), characterized by frequent apneas and/or hypopneas and oxyhemoglobin desaturation. Both mSDB and OSA are associated with neurobehavioral morbidity, but the relationship between disease severity, as measured by the apnea-hypopnea index (AHI), and neurocognitive morbidity is unclear [1–6]. A recent large cohort study showed that parent-reported snoring was associated with behavioral impairments and gray matter cortical thinning. However, the lack of polysomnography measures precluded assessment of whether the findings were associated with OSA severity [7]. While more severe neurocognitive impairment might be expected to correlate with more frequent respiratory disturbances, several studies have shown equivalent outcomes when comparing children with mSDB to those with OSA [1, 2, 8, 9]. One hypothesis is that the increased work of breathing and sympathetic activation during snoring can cause neurobehavioral deficits in children with mSDB even without apneas and gas exchange abnormalities [10, 11].
The objective of this study is to compare the neurobehavioral morbidity of pediatric OSA with mSDB by comparing the baseline characteristics of participants recruited into two trials, the Childhood Adenotonsillectomy Trial (CHAT) [6] and the Pediatric Adenotonsillectomy Trial for Snoring (PATS) [12]. Both are multicenter randomized controlled trials that compare neurobehavioral outcomes for children randomized to adenotonsillectomy versus watchful waiting with supportive care, but CHAT included children with habitual snoring and an obstructive apnea-hypopnea index (oAHI) between 2 and 30, whereas PATS included children with habitual snoring and an oAHI under 3 [6, 12]. The study similarities provide an opportunity to compare the role of AHI entry criteria on the neurobehavioral morbidity of participants, and to consider other factors that may influence the characteristics of children participating in two different trials. We hypothesized that neurobehavioral morbidity would be worse in the cohort recruited on the basis of OSA compared to the cohort recruited on the basis of mSDB.
Methods
Study design
CHAT and PATS are both multicenter, single-blind randomized controlled trials. Children meeting inclusion criteria were randomly assigned to adenotonsillectomy or watchful waiting. Children underwent baseline assessments that included clinical evaluation, polysomnographic testing with scoring at a centralized sleep reading center with the same scoring staff, and parent behavior ratings. The study protocols were similarly developed, largely by the same group of investigators, and data similarly monitored and processed.
Written informed consent was obtained from caregivers and assent obtained for children over 7 years old at the start of the study. Independent data and safety monitoring boards reviewed interim data on study safety and quality. The studies were approved by the institutional review boards at each participating site (Supplementary Table 1). CHAT was conducted between 2007 and 2012, and PATS is an ongoing study that enrolled participants between 2016 and 2020.
Inclusion criteria
In CHAT, inclusion criteria included: 1) age 5 to 9 years old; 2) tonsillar hypertrophy (> Brodsky 1+, or obstructing at least 25% of the airway); 3) caregiver-reported snoring at least 3 nights per week; 4) surgical candidacy; 5) English proficiency; 6) polysomnography showing oAHI between 2 and 30 events per hour or obstructive apnea index between 1 and 20 events per hour. Children were excluded from the study if they had recurrent tonsillitis, a body mass index (BMI) z-score > 3, arterial oxyhemoglobin under 90% for over 2% of total sleep time, or use of attention-deficit/hyperactivity disorder (ADHD) medication.
In PATS, inclusion criteria included: 1) age 3 to 12 years old; 2) tonsillar hypertrophy (Brodsky > 2+, or obstructing at least 50% of the airway); 3) caregiver-reported snoring at least 3 nights per week; 4) surgical candidacy; 5) polysomnography showing oAHI under 3 events per hour and obstructive apnea index under 1 event per hour. They were excluded from the study if they had recurrent tonsillitis, a BMI z-score > 3, or any arterial oxyhemoglobin desaturations under 90% in conjunction with obstructive events. Use of ADHD medication was not an exclusion criterion.
Both studies enrolled only families willing to accept surgery or watchful waiting as possible treatment options for the duration of the trial (7 months for CHAT and 12 months for PATS), and excluded children with severe comorbidities (eg, cardiopulmonary diseases, bleeding disorders, epilepsy, severe diabetes, poorly-controlled asthma), Down syndrome or developmental delay.
Outcome assessments
We analyzed the neurobehavioral measures that were common to both trials, which are the Behavior Rating Inventory of Executive Function (BRIEF), the Child Behavior Checklist (CBCL), and the Conners 3rd edition. The BRIEF (PAR Inc, Lutz, Florida) is a caregiver-completed assessment of executive function [13]. The Global Executive Composite (GEC) T-score comprises age- and gender-adjusted summary measures of behavioral regulation and metacognition. A higher score indicates worse functioning, and a T-score above 65 is considered abnormally elevated. The BRIEF 2nd edition was administered for children 6 years and older or 5 years old and in kindergarten, while the BRIEF Preschool version (BRIEF-P) was administered for children 3-4 years old or 5 years old and in preschool.
The CBCL and Conners are caregiver-completed assessments of child behavior [14, 15]. The CBCL/1.5–5 (ASEBA, Burlington, VT) was administered for children 5 years and younger in age, while the CBCL/6–18 was administered for children 6 years and older. The total score combines the internalizing and externalizing scores. Higher T-scores indicate worse function, and a T-score of 64 or higher is considered clinically abnormal. The Conners Pearson Assessments, San Antonio, TX) long form was administered in CHAT, while the short form was administered to children 6 years and older in PATS. The subscales with the highest correlations were compared across the two studies (Supplementary Table 2) [15]. Higher T-scores on the Conners indicate worse functioning, and a T-score of 65 or higher is considered elevated.
Analysis
The mSDB group was comprised of PATS trial participants (oAHI < 3), while the OSA group was comprised of CHAT trial participants (oAHI 2–30). Baseline demographic characteristics, BMI percentile, ADHD history, OSA-18 scores (a validated disease-specific quality of life instrument [16]), behavior ratings, and polysomnography features were compared between the groups using t-tests for continuous parametric data, Wilcoxon tests for nonparametric continuous data, and chi-squared tests for categorical data. The oAHI was defined as all obstructive apneas plus hypopneas with 3% desaturation or arousal divided by total sleep time. Spearman correlation was used to assess the correlation with behavioral measures. To assess differences in neurobehavioral morbidity, univariable and multivariable logistic regression was performed to estimate the odds ratio of abnormal BRIEF, CBCL, and Conners scores given group (PATS, CHAT) using the cutoffs described above after adjusting for age, gender, race, low maternal education (defined as high school or less), BMI percentile, ADHD (defined as reported history of known ADHD), and recruitment source (otolaryngology, sleep clinic, or other). All statistical analysis was performed with SAS (SAS, Cary, NC). Alpha < 0.05 was considered significant.
Results
Sample characteristics
Sample characteristics of the 453 CHAT participants and 459 PATS participants are described in Table 1. Participants in CHAT had a higher mean age and BMI percentile compared to those in PATS, and were more likely to be Black, come from a low-income family, and have a mother with a high school education or lower. These characteristics are consistent with findings from the screening data from the CHAT study that demonstrated that Black children were more likely to be eligible based on an elevated AHI compared to those screened for the study and found to have a lower AHI [17]. Consistent with the study designs, CHAT participants also were less likely to have a history of ADHD. The disease-specific quality of life measured by the OSA-18 survey was similar with an average score of 51.9 (95% CI 50.4-53.4) in PATS compared to 53.6 (95% CI 51.9-55.3; p = 0.142) in CHAT.
Table 1.
Study characteristics
| Characteristic | Overall (N = 912) |
Study | P-value | |
|---|---|---|---|---|
| PATS (N = 459, 50.3%) | CHAT (N = 453, 49.7%) |
|||
| Age (years), mean ± SD | 6.3 ± 1.9 | 6.1 ± 2.3 | 6.6 ± 1.4 | 0.0005 |
| Male, n (%) | 448 (49.1%) | 229 (49.9%) | 219 (48.3%) | 0.640 |
| Race White non-Hispanic, n (%) Black, n (%) Hispanic, n (%) Other, n (%) |
377 (41.4%) 367 (40.3%) 112 (12.3%) 55 (6.0%) |
236 (51.5%) 121 (26.4%) 75 (16.4%) 26 (5.7%) |
141 (31.1%) 246 (54.3%) 37 (8.2%) 29 (6.4%) |
<0.0001 |
| Mother with high school diploma or lower, n (%) | 226 (25.0%) | 86 (18.8%) | 140 (31.3%) | <0.0001 |
| Annual household income <$60,000 | 512 (63.0%) | 232 (54.7%) | 280 (72.0%) | <0.0001 |
| BMI Percentile, mean % ± SD | 66.8 ± 31.1 | 63.6 ± 31.1% | 70.2 ± 31.8% | 0.0013 |
| ADHD History, n (%) | 58 (6.4%) | 45 (9.8%) | 13 (2.9%) | <0.0001 |
| OSA-18 Score, mean ± SD | 52.8 ± 17.6 | 51.9 ± 16.6 | 53.6 ± 18.6 | 0.142 |
| BRIEF GEC T-score, mean ± SD | 52.8 ± 12.1 | 55.6 ± 12.2 | 49.9 ± 11.3 | <0.0001 |
| CBCL T-score, mean ± SD Internalizing Externalizing Total |
51.8 ± 11.2 51.4 ± 11.1 53.1 ± 11.0 |
52.0 ± 11.2 51.1 ± 11.3 53.2 ± 11.1 |
51.5 ± 11.1 51.6 ± 10.9 52.8 ± 10.8 |
0.508 0.510 0.672 |
| Conners T-score, mean ± SD Defiance Executive Functioning Peer Relations Hyperactivity/Impulsivity Inattention |
51.0 ± 11.5 54.5 ± 11.8 51.1 ± 10.6 57.6 ± 13.2 55.0 ± 13.1 |
52.1 ± 11.2 57.0 ± 12.4 52.2 ± 11.3 62.4 ± 15.1 60.9 ± 14.8 |
51.5 ± 11.7 53.0 ± 11.1 50.4 ± 10.1 55.0 ± 11.2 51.7 ± 10.8 |
0.072 <0.0001 0.045 <0.0001 <0.0001 |
| Polysomnogram Characteristics, mean ± SD Obstructive apnea-hypopnea index Percentage of sleep time with end-tidal CO2 > 50 mmHg Arousal index Central apnea index Percentage of sleep time with SpO2s < 90% Average SpO2 Sleep efficiency Wake after sleep onset (min) N1 sleep percent N2 sleep percent N3 sleep percent N REM percent Average heart rate |
4.3 ± 5.5 6.3% ± 16.0 7.1 ± 3.0 0.8 ± 1.1 0.1% ± 0.2 97.4% ± 1.1 87.2% ± 8.4 41.2 ± 39.2 8.0% ± 3.9 31.1% ± 7.6 31.1% ± 7.6 17.8% ± 4.5 82.6 ± 9.3 |
0.8 ± 0.7 2.3% ± 8.8 5.7 ± 1.9 0.5 ± 0.7 0.0% ± 0.0 97.5% ± 1.1 88.4% ± 7.9 34.5 ± 35.4 7.4% ± 3.5 44.6% ± 7.8 30.7% ± 7.8 17.3% ± 4.7 81.0 ± 9.3 |
7.8 ± 5.9 10.8% ± 20.5 8.5 ± 3.2 1.0 ± 1.3 0.1% ± 0.3 97.4% ± 1.0 86.0% ± 8.6 47.9 ± 41.8 8.6% ± 4.1 41.4% ± 7.7 31.6% ± 7.4 18.4% ± 4.3 84.2 ± 9.1 |
<0.0001 <0.0001 <0.0001 <0.0001 <0.0001 0.079 <0.0001 <0.0001 <0.0001 <0.0001 0.081 0.0002 <0.0001 |
Race missing for 1 participant. Education missing for 8 participants. Income missing for 99 participants. CBCL missing for 23 participants. Conners limited to n = 704 children > age 5; with 4 participants > age 5 with missing data.
Polysomnography characteristics
As expected, children in CHAT had evidence of more severe SDB compared to the PATS sample, including higher percentage of sleep time with oxyhemoglobin saturation < 90% and sleep time with end-tidal carbon dioxide > 50 mmHg; higher arousal index; and worse sleep efficiency (Table 1). Children in PATS had lower percentage of rapid eye movement (REM) sleep at 17.3% (95% CI 16.9-17.8%) compared to children in CHAT at 18.4% (95% CI 85.2–86.7%; p = 0.0002).
Neurobehavioral outcomes
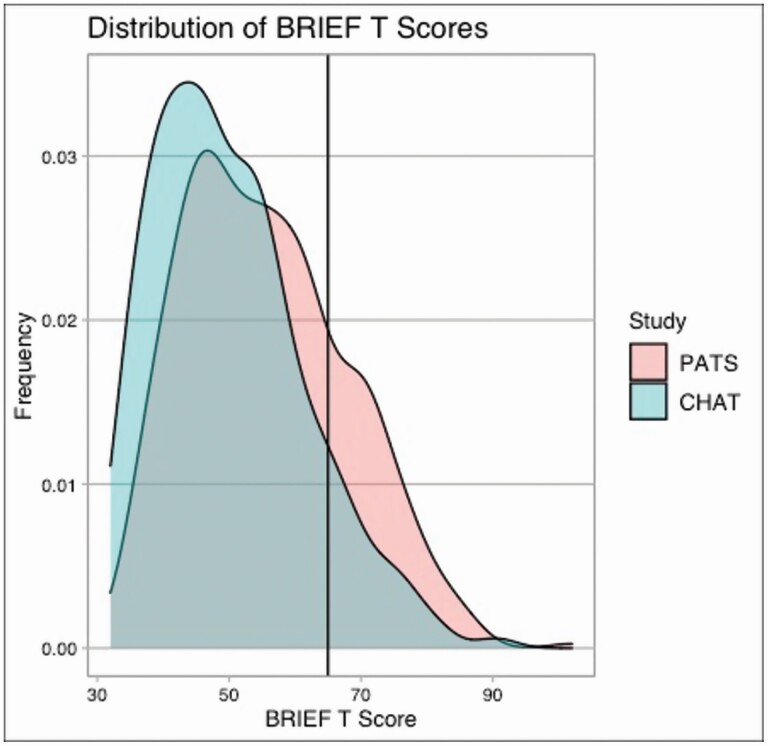
The mean baseline BRIEF score for the children in PATS was higher at 55.6 (95% CI 54.5–56.7), compared to 49.9 (95% CI 48.9–51.0; p < 0.0001) for the children in CHAT. There was a higher frequency of abnormal BRIEF scores for the children in PATS at 23.7% (95% CI 19.8–27.7%) compared to 11.7% (95% CI 8.7–14.7%; p < 0.0001) for the children in CHAT, as seen in Figure 1.
Figure 1.
Distribution of BRIEF scores in PATS (mSDB cohort) compared to CHAT (OSA cohort).
The results of multivariable logistic regression for elevated neurobehavioral measures are seen in Table 2. Adjusted for age, gender, race, low maternal education, BMI percentile, history of ADHD, and recruitment source, the odds of an abnormal BRIEF score was 1.96 (95% CI 1.30–2.94) in the children in PATS compared to the children in CHAT. The adjusted odds of an elevated Conners hyperactivity/impulsivity score was 2.82 (95% CI 1.83–4.34) and an elevated Conners inattention score was 3.16 (95% CI 1.98–5.02) in PATS compared to CHAT. CBCL scores did not significantly differ by group.
Table 2.
Frequencies and odds ratios for abnormal neurocognitive scores for PATS (mSDB cohort) compared to CHAT (OSA cohort)
| Neurocognitive Measure | Frequency in mSDB (95% CI) | Frequency in OSA (95% CI) | Unadjusted odds of abnormal score (95% CI) | P-value | Adjusted odds of abnormal score (95% CI)b | P-value |
|---|---|---|---|---|---|---|
| Abnormal BRIEF (>65), n = 903b | 23.7%(19.8–27.7%) | 11.7% (8.7–14.7%) | 2.35 (1.64–3.36) | <0.0001 | 1.96 (1.30–2.94) | 0.0012 |
| Clinically Abnormal CBCL (>64), n = 889c Internalizing Externalizing Total |
15.4% (12.0–18.7%) 13.4% (10.2–16.5%) 17.6% (14.1–21.1%) |
15.5% (12.1–18.9%) 14.1% (10.8–17.4%) 16.8% (13.3–20.3%) |
0.99 (0.66–1.43) 0.94 (0.64–1.38) 1.06 (0.75–1.50) |
0.971 0.753 0.759 |
0.80 (0.53–1.21) 0.85 (0.54–1.33) 0.95 (0.64–1.41) |
0.292 0.480 0.947 |
| Elevated Conners (>65), n = 698d Defiance Executive Functioning Peer Relations Hyperactivity/ Impulsivity Inattention |
14.3% (10.0–18.6%) 26.6% (21.1–32.1%) 13.9% (9.6–18.2%) 39.8% (33.7–45.9%) 36.1% (30.1–42.1%) |
12.8% (9.7–15.9%) 16.3% (12.9–19.8%) 8.8% (6.2–11.5%) 20.3% (16.6–24.0%) 13.7% (10.5–16.9%) |
1.14 (0.73–1.78) 1.86 (1.28–2.70) 1.67 (1.03–2.70) 2.60 (1.85–3.65) 3.57 (2.46–5.17) |
0.579 0.001 0.038 <0.0001 <0.0001 |
0.97 (0.55–1.68) 1.50 (0.94–2.40) 1.53 (0.85–2.75) 2.82 (1.83–4.34) 3.16 (1.98–5.02) |
0.904 0.087 0.153 <0.0001 <0.0001 |
aAdjusted for age, gender, race, low maternal education, BMI percentile, ADHD, recruitment source.
bCovariates missing for 9 patients.
cCBCL missing for 23 participants.
dConners limited to n = 704 children > age 5; with 6 participants > age 5 with missing data.
Sensitivity analyses
Sensitivity analyses restricted to non-overlapping oAHI, overlapping ages, shared sites, adjusted for income in participants with known income, or excluding children with ADHD history or ADHD medications were all consistent with worse BRIEF, inattention, and hyperactivity scores in the PATS cohort compared to the CHAT cohort (Supplementary Table 3).
The correlation between percentage of REM sleep and BRIEF scores was –0.042 (p = 0.201).
Discussion
We compared the baseline neurobehavioral and polysomnographic data from two cohorts of children, selected using AHI for participation in randomized controlled trials targeting children with different SDB severity levels. We found an increased frequency of abnormal executive function in the snoring children recruited on the basis of snoring (mSDB) compared to children recruited on the basis of OSA. There were also more abnormal inattention and hyperactivity scores in the children with little or no sleep apnea.
Differences in behavioral measures between the two cohorts may be due to participant demographics, polysomnogram findings, study design, or disease type. With regard to participant demographics, the two cohorts differed in age, race, parental education, and household income. However, BRIEF T-scores, which are already adjusted externally for gender and age, were additionally adjusted for these characteristics in our statistical models. The addition of these covariates to the models did not substantively change the odds of an abnormal BRIEF score in the PATS as compared to the CHAT sample. In fact, CHAT participants were more likely to be from lower socio-economic households, which would tend to bias the findings toward greater impairment in that sample [18]. Therefore, it is unlikely that participant demographics account for the higher neurobehavioral morbidity in the sample selected on the basis of mSDB.
With regard to polysomnography parameters, lower levels of oxyhemoglobin saturation, higher end-tidal carbon dioxide values, and greater sleep fragmentation were observed in CHAT versus PATS. In contrast, REM sleep was modestly lower in PATS than CHAT. REM sleep has been implicated in memory consolidation and emotional regulation [19–21] and was reported to be inversely correlated with Conners score [22]. While intriguing, this finding does not readily explain the group differences since we did not find a significant correlation between percentage of REM sleep and BRIEF scores, and the magnitude of difference was small.
With regard to study design, parents and clinicians may differ in their comfort in participation in trials that challenge the current dogma of treating OSA with adenotonsillectomy, and there may also be secular differences resulting from the timing of the two studies. Differential referral patterns could also contribute as all children had to have some symptoms to present to a clinician, and they also had to be adenotonsillectomy candidates and be willing to participate in a trial in order to be included. A key factor in surgical trials is that both the clinician and the family have equipoise—i.e., feel that the child would do equally well with either surgery or watchful waiting. We cannot exclude the possibility that PATS clinicians had equipoise for randomizing more symptomatic children, while CHAT clinicians had equipoise for randomizing less symptomatic children. This is challenging to investigate because equipoise is difficult to measure and can change over time [23]. However, the children had comparable quality of life scores at baseline and did not significantly differ in withdrawal or screen failure rates. Yet unmeasured selection bias may still have contributed to families being more willing to participate in PATS if their children were more symptomatic.
An important difference between the eligibility criteria of the two studies is the exclusion from the CHAT study of participants taking ADHD medication. The association between ADHD history and an elevated BRIEF score is well-established [24–26]. Neurobehavioral differences were found in our primary analyses adjusting for ADHD history, as well as in secondary analyses excluding those children from analysis.
We consider the effect of disease type on neurobehavior. Both mSDB and OSA are associated with neurobehavioral morbidity [2, 3, 8, 9], as well as with functional MRI brain changes [27–31]. The mechanisms mediating the neurobehavioral effects of OSA are considered, most likely, to be through effects of nocturnal hypoxemia and arousal-related sleep disruption. While hypoxemia is exclusive to children with OSA and arousals are more severe in those children, some studies have shown equivalent morbidity and neurobehavioral deficits in children with habitual snoring compared to OSA [1, 2, 8, 9]. A few studies have similarly found poorer behavioral functioning in mSDB compared to more severe disease [8, 32, 33]. Different hypotheses exist for the behavioral sequelae associated with snoring, including both mechanistic pathways as well as environmental factors [34]. First, even without apneas, snoring may cause increased work of breathing and sympathetic activation [10, 11]. There is also a complex relationship between oxygenation and cognition; the changes in cerebral oxygenation during obstructive and central events are similar [35], and mild intermittent hypoxia may increase cerebral circulation and possibly be neuroprotective [36–39]. Finally, some studies have found elevated proinflammatory markers like interleukins and urinary leukotrienes in children with mild OSA compared to children with more severe disease [40, 41], which may also be relevant to neurocognition.
Importantly, the traditional AHI may not be a good predictor of behavioral and cardiovascular outcomes. For instance, the AHI does not correlate with quality of life or parent-reported symptomatology in children [42–44]. Our results suggest that risk stratification based on AHI does not identify children with higher neurobehavioral morbidity. The identification of metrics that better predict neurobehavior remains a research need [45]. For instance, cyclic alternating pattern was shown in CHAT to be associated with BRIEF and Conners scores [46]. Respiratory cycle-related EEG changes may also be useful, for example, in the identification of sleepiness [47–49]. Future investigation of more detailed indices of sleep macro- and microarchitecture may further elucidate whether biomarkers of cognition associate with SDB.
In both cohorts, we found elevated frequency of abnormal BRIEF scores, consistent with the neurobehavioral effects of both mSDB and OSA. Based on the T-score distribution, 6.7% of children overall would be expected to have an elevated BRIEF score, although this varies slightly based on age and gender. We found 23.7% of children in the mSDB cohort and 11.7% of the children in the OSA cohort had an elevated BRIEF score. The mean BRIEF score in the mSDB cohort was 55.6 and in the OSA cohort 49.9. By comparison, other studies report a mean BRIEF score of 48-61.4 for mSDB, with 3 of 4 studies reporting elevated BRIEF scores [32, 50–52]. For OSA, the mean BRIEF score reported in the literature ranges from 51-62, with all 3 studies reporting elevated BRIEF scores [1, 32, 51]. Two studies present BRIEF scores by disease severity, neither of which found significant differences across the range of AHI [32, 51]. However, power in these studies was limited by the relatively small sample sizes in the different disease severity groups.
We also found differences in the Conners subscales of executive functioning, hyperactivity/impulsivity, and inattention, but no differences in the CBCL scores between the two groups. Findings indicating study differences on the BRIEF but not on the CBCL or OSA-18 may reflect differences in item content. The BRIEF assesses a broader range of behaviors related to difficulties in behavioral, emotional, and cognitive self-regulation than the other two scales; thus, it is more likely to tap into the wide range of weaknesses exhibited by children with mSDB [34]. Inattention and executive functioning may be more sensitive than other emotional and behavioral characteristics to sleep disruption. Clinical, epidemiological, and imaging studies have shown that attention and executive function are particularly sensitive to SDB and its treatment in young children [4, 5, 53]. These are clinically important domains that affect behavior and are of importance to families and teachers.
These results must be interpreted in the context of study design. As discussed previously, differential referral patterns and the exclusion of children with ADHD in CHAT could have contributed to differences in group composition between the two studies. One limitation is that the Conners long form was administered in CHAT, while the short form was administered in PATS, though subscales with high correlation were chosen as comparators across studies. Additionally, little data were available for children who were screened but not randomized or who were found to be ineligible, which limited our analysis of possible selection biases. The study strengths include the relatively large sample size with inclusion of children from geographically diverse sites. The prospectively collected data with consistency in study investigators, study outcomes, and polysomnogram scoring facilitates comparison across studies.
In summary, this analysis of two cohorts of children representing a wide spectrum of SDB with similar sites, sleep study scoring, and outcome measurement found a higher frequency of abnormal scores for executive function, attention, and hyperactivity in the children recruited on the basis of mSDB compared to those enrolled in a trial that targeted OSA. The differences were partially explained by differences in trial exclusion criteria (i.e., ADHD history). Our findings suggest that traditional polysomnography metrics used to measure disease severity in SDB do not align with or predict neurobehavioral morbidity. Children with mild disease (eg, oAHI < 3 in the mSDB trial) may have significant neurobehavioral problems that are under-recognized. Our results emphasize the need for measures of SDB that are more sensitive to neurobehavioral outcomes and that can better identify the children who are most at risk.
Supplementary Material
Disclosure statement
Financial disclosures: Supported by NIH NHLBI 1U01HL125307, 1U01HL125295, American Thoracic Society ASPIRE Fellowship. Dr. Ishman was a consultant for Inspire Medical in 2019.
Non-financial disclosures: None.
Author contributions
Dr. Yu contributed to study conception and design, data analysis and interpretation, and manuscript drafting. Drs. Radcliffe, Taylor contributed to study conception and design, data interpretation, and critical revisions for important intellectual content. Drs. Amin, Baldassari, Boswick, Chervin, Elden, Furth, Garetz, George, Ishman, Kirkham, Liu, Mitchell, Naqvi, Rosen, Ross, Shah, Tapia, Young, and Zopf contributed to data acquisition, data interpretation, and critical revisions for important intellectual content. Dr. Wang contributed to study conception and design, data analysis and interpretation, and critical revisions for important intellectual content.
Dr. Redline contributed to study conception and design, data interpretation, and critical revisions for important intellectual content. All authors contributed to final approval of the manuscript and agree to be accountable for all aspects of the work.
Subject: Epidemiology (Pediatric): Outcomes & Management
References
- 1. Biggs SN, et al. Long-term changes in neurocognition and behavior following treatment of sleep disordered breathing in school-aged children. Sleep 2014;37(1):77–84. doi: 10.5665/sleep.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chervin RD, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics 2006;117(4):e769–e778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emancipator JL, et al. Variation of cognition and achievement with sleep-disordered breathing in full-term and preterm children. Arch Pediatr Adolesc Med. 2006;160(2):203–210. [DOI] [PubMed] [Google Scholar]
- 4. Gottlieb DJ, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145(4):458–464. [DOI] [PubMed] [Google Scholar]
- 5. Landau YE, et al. Impaired behavioral and neurocognitive function in preschool children with obstructive sleep apnea. Pediatr Pulmonol. 2012;47(2):180–188. [DOI] [PubMed] [Google Scholar]
- 6. Marcus CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Isaiah A, et al. Associations between frontal lobe structure, parent-reported obstructive sleep disordered breathing and childhood behavior in the ABCD dataset. Nat Commun. 2021;12(1):2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackman AR, et al. Sleep-disordered breathing in preschool children is associated with behavioral, but not cognitive, impairments. Sleep Med. 2012;13(6):621–631. [DOI] [PubMed] [Google Scholar]
- 9. Melendres MC, et al. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics 2004;114(3):768–775. [DOI] [PubMed] [Google Scholar]
- 10. Nisbet LC, et al. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep Med Rev. 2014;18(2):179–189. [DOI] [PubMed] [Google Scholar]
- 11. Kwok KL, et al. BP and arterial distensibility in children with primary snoring. Chest 2003;123(5):1561–1566. [DOI] [PubMed] [Google Scholar]
- 12. Wang R, et al. Pediatric Adenotonsillectomy Trial for Snoring (PATS): protocol for a randomised controlled trial to evaluate the effect of adenotonsillectomy in treating mild obstructive sleep-disordered breathing. BMJ Open 2020;10(3):e033889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gioia GAI PK, et al. Behavior Rating Inventory of Executive Function. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 14. Achenbach TM, et al. Behavioral problems and competencies reported by parents of normal and disturbed children aged four through sixteen. Monogr Soc Res Child Dev. 1981;46(1):1–82. [PubMed] [Google Scholar]
- 15. Conners CK. Conners 3rd Edition Manual. Tonawanda, NY: Multi-Health Systems; 2008. [Google Scholar]
- 16. Franco RA, Jr., et al. ; First place--resident clinical science award. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 19992000;123(1 Pt 1):9–16. [DOI] [PubMed] [Google Scholar]
- 17. Weinstock TG, et al. Predictors of obstructive sleep apnea severity in adenotonsillectomy candidates. Sleep 2014;37(2):261–269. doi: 10.5665/sleep.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Isaiah A, et al. Association between habitual snoring and cognitive performance among a large sample of preadolescent children. JAMA Otolaryngol Head Neck Surg 2021;147(5):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hou J, et al. REM sleep deprivation-induced circadian clock gene abnormalities participate in hippocampal-dependent memory impairment by enhancing inflammation in rats undergoing sevoflurane inhalation. Behav Brain Res. 2019;364:167–176. [DOI] [PubMed] [Google Scholar]
- 20. Marks GA, et al. A functional role for REM sleep in brain maturation. Behav Brain Res. 1995;69(1-2):1–11. [DOI] [PubMed] [Google Scholar]
- 21. Pesonen AK, et al. REM sleep fragmentation associated with depressive symptoms and genetic risk for depression in a community-based sample of adolescents. J Affect Disord. 2019;245:757–763. [DOI] [PubMed] [Google Scholar]
- 22. Um YH, et al. Association between sleep parameters and cognitive function in drug-naive children with attention-deficit hyperactivity disorder: a polysomnographic study. Sleep Med. 2016;21:165–170. [DOI] [PubMed] [Google Scholar]
- 23. Joffe S, et al. Equipoise: asking the right questions for clinical trial design. Nat Rev Clin Oncol. 2012;9(4):230–235. [DOI] [PubMed] [Google Scholar]
- 24. Alloway TP, et al. The diagnostic utility of behavioral checklists in identifying children with ADHD and children with working memory deficits. Child Psychiatry Hum Dev. 2009;40(3):353–366. [DOI] [PubMed] [Google Scholar]
- 25. Mahone EM, et al. Validity of the behavior rating inventory of executive function in children with ADHD and/or Tourette syndrome. Arch Clin Neuropsychol. 2002;17(7):643–662. [PubMed] [Google Scholar]
- 26. Qian Y, et al. Do executive function deficits differentiate between children with attention deficit hyperactivity disorder (ADHD) and ADHD comorbid with oppositional defiant disorder? A cross-cultural study using performance-based tests and the behavior rating inventory of executive function. Clin Neuropsychol. 2010;24(5):793–810. [DOI] [PubMed] [Google Scholar]
- 27. Philby MF, et al. Reduced regional grey matter volumes in pediatric obstructive sleep apnea. Sci Rep. 2017;7:44566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kheirandish-Gozal L, et al. Preliminary functional MRI neural correlates of executive functioning and empathy in children with obstructive sleep apnea. Sleep 2014;37(3):587–592. doi: 10.5665/sleep.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horne RSC, et al. Regional brain tissue changes and associations with disease severity in children with sleep-disordered breathing. Sleep 2018;41(2). doi: 10.1093/sleep/zsx203. [DOI] [PubMed] [Google Scholar]
- 30. Halbower AC, et al. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med. 2006;3(8):e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan KC, et al. Neurocognitive dysfunction and grey matter density deficit in children with obstructive sleep apnoea. Sleep Med. 2014;15(9):1055–1061. [DOI] [PubMed] [Google Scholar]
- 32. Bourke R, et al. Cognitive and academic functions are impaired in children with all severities of sleep-disordered breathing. Sleep Med. 2011;12(5):489–496. [DOI] [PubMed] [Google Scholar]
- 33. Bourke RS, et al. Neurobehavioral function is impaired in children with all severities of sleep disordered breathing. Sleep Med. 2011;12(3):222–229. [DOI] [PubMed] [Google Scholar]
- 34. Biggs SN, et al. The conundrum of primary snoring in children: what are we missing in regards to cognitive and behavioural morbidity? Sleep Med Rev. 2014;18(6):463–475. [DOI] [PubMed] [Google Scholar]
- 35. Tamanyan K, et al. The impact of central and obstructive respiratory events on cerebral oxygenation in children with sleep disordered breathing. Sleep 2019;42(5). doi: 10.1093/sleep/zsz044. [DOI] [PubMed] [Google Scholar]
- 36. Almendros I, et al. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. 2014;307(2):L129–L140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beaudin AE, et al. Impact of obstructive sleep apnoea and intermittent hypoxia on cardiovascular and cerebrovascular regulation. Exp Physiol. 2017;102(7):743–763. [DOI] [PubMed] [Google Scholar]
- 38. Bouslama M, et al. Protective effects of intermittent hypoxia on brain and memory in a mouse model of apnea of prematurity. Front Physiol. 2015;6:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yaffe K, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306(6):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Biyani S, et al. Urinary Leukotriene E4 Levels in Children with Sleep-Disordered Breathing. Otolaryngol Head Neck Surg. 2018;158(5):947–951. [DOI] [PubMed] [Google Scholar]
- 41. Smith DF, et al. Inflammatory milieu and cardiovascular homeostasis in children with obstructive sleep apnea. Sleep 2017;40(4). doi: 10.1093/sleep/zsx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baldassari CM, et al. Correlation between REM AHI and quality-of-life scores in children with sleep-disordered breathing. Otolaryngol Head Neck Surg. 2014;151(4):687–691. [DOI] [PubMed] [Google Scholar]
- 43. Isaiah A, et al. Predictors of behavioral changes after adenotonsillectomy in pediatric obstructive sleep apnea: a secondary analysis of a randomized clinical trial. JAMA Otolaryngol Head Neck Surg 2020;146(10):900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paruthi S, et al. Effect of adenotonsillectomy on parent-reported sleepiness in children with obstructive sleep apnea. Sleep 2016;39(11):2005–2012. doi: 10.5665/sleep.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Giordani B, et al. Neuropsychological and behavioral functioning in children with and without obstructive sleep apnea referred for tonsillectomy. J Int Neuropsychol Soc. 2008;14(4):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hartmann S, et al. Cyclic alternating pattern in children with obstructive sleep apnea and its relationship with adenotonsillectomy, behavior, cognition, and quality of life. Sleep. 2021;44(1). doi: 10.1093/sleep/zsaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chervin RD, et al. Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea. Am J Respir Crit Care Med. 2005;171(6):652–658. [DOI] [PubMed] [Google Scholar]
- 48. Chervin RD, et al. Do respiratory cycle-related EEG changes or arousals from sleep predict neurobehavioral deficits and response to adenotonsillectomy in children? J Clin Sleep Med. 2014;10(8):903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chervin RD, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs other surgical care. Sleep 2006;29(4):495–503. PMID: 16676783. [PMC free article] [PubMed] [Google Scholar]
- 50. Hill CM, et al. Increased cerebral blood flow velocity in children with mild sleep-disordered breathing: a possible association with abnormal neuropsychological function. Pediatrics 2006;118(4):e1100–e1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tamanyan K, et al. Age effects on cerebral oxygenation and behavior in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2018;197(11):1468–1477. [DOI] [PubMed] [Google Scholar]
- 52. Waters KA, et al. Cognition After Early Tonsillectomy for Mild OSA. Pediatrics 2020;145(2) [DOI] [PubMed] [Google Scholar]
- 53. Karpinski AC, et al. Risk for sleep-disordered breathing and executive function in preschoolers. Sleep Med. 2008;9(4):418–424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.