Abstract
Tumor-derived exosomes (TEX), a subset of small extracellular vesicles (EVs) which originate from the endocytic compartment of tumor cells, are emerging as key players in cancer progression. TEX circulate freely in patients’ body fluids and transfer bioactive cargos from tumor to various recipient cells. The molecular cargo of melanoma cell-derived exosomes (MTEX) mimics that of the tumor, and MTEX serve as a liquid biopsy that provides potentially useful information for cancer diagnosis, prognosis or responses to therapy. Plasma of melanoma patients contains a mix of MTEX and exosomes produced by non-malignant cells (NMTEX). Isolation of these exosome subtypes from the bulk of plasma exosomes is necessary to evaluate contributions of each as potential biomarkers of melanoma progression and outcome. Here, methods for separation of MTEX from T cell-derived exosomes from a single small volume of plasma and for their subsequent molecular and functional characterization are described. Following size exclusion chromatography (SEC) to isolate total plasma exosomes, immune affinity-based capture of MTEX with anti-CSPG4 antibody and then of exosomes produced by T cells with anti-CD3 antibody is used to sequentially isolate the two subsets. This immune capture method enables the recovery of MTEX and CD3+ exosomes in quantities sufficient for molecular profiling by flow cytometry or western blotting and for functional analyses.
Keywords: melanoma cell-derived exosomes (MTEX), non-malignant cell-derived exosomes (NMTEX), CD3+ exosomes, immunoaffinity-based capture, plasma-derived exosomes
Introduction:
Extracellular vesicles (EVs) are a heterogeneous population of nanosize lipid membrane vesicles shed by all cell types into the extracellular space. Exosomes are a small subset (30–150 nm in diameter) of EVs originating from the endocytic compartment and assembled in the multivesicular bodies (MVBs). Exosomes are released from a producer cell upon fusion of MVB with the cell membrane [1,2]. The exosome cell membrane and lumen carry a wide range of biologically active proteins, lipids, and nucleic acids [3–5]. The content and molecular topography of exosomes resemble those of parent cells [6]. These similarities with parent cells, e.g., tumor cells, make exosomes a potential candidate for the tumor liquid biopsy. Exosomes mediate intracellular communications and regulate many physiological and pathophysiological processes, including cancer progression [7–9]. Exosomes can be found in all body fluids, and their frequency as well as content reflect general health and may change depending on pathophysiological conditions [10–12]. Plasma or other body fluid-derived exosomes contain vesicles produced by a broad variety of normal circulating or tissue cells and, in disease, by abnormal cells, such as cancer cells [10].
Melanoma is the most fatal among all skin cancers [13]. Melanoma cell-derived exosomes, named MTEX, may represent more than 50% of total plasma exosomes [14]. MTEX carry a variety of molecular and genetic cargos that modulate functions of recipient cells [9]. It has been reported that MTEX carry molecules that reprogram immune cells and hinder anti-tumor immune responses, changing patients’ responses to therapy and disease outcome [11]. Plasma-derived exosomes are heterogeneous mixtures of different EVs, and in melanoma, plasma contains numerous other exosomes in addition to MTEX that are of interest, because they carry information about cells of their origin [15]. In melanoma, immune cells are activated, and since activated T cells produce numerous exosomes, T cell-derived CD3+ exosomes represent a considerable fraction of total plasma exosomes [15]. They appear to reflect the immune competence of T cells in disease [15]. We have shown that these T cells are re-programmed by MTEX, and their functions are indicative of tumor-induced effects on the host immune system [14]. Therefore, both MTEX and T cell-derived (CD3+) exosomes in melanoma patients’ plasma are expected to serve as biomarkers of disease-induced alterations in the tumor or immune cells, respectively.
To achieve the isolation of vesicles produced by a specific cell type, such as MTEX or CD3+ exosomes, special separation approaches are required. As antibodies (Abs) are exquisitely specific for cognate antigens, there is a solid rationale for considering immune capture with Abs for the isolation of MTEX and NMTEX from total exosomes in melanoma patients’ plasma. To immunocapture MTEX, it is necessary to have an Ab that recognizes a protein uniquely expressed on the melanoma cell surface and is also present on MTEX. To isolate T cell-derived exosomes, an Ab is needed that recognizes the antigen such as CD3 that is expressed only on T cells and T cell-derived exosomes. The selection of Abs meeting the above criteria is the most critical part of the immunocapture-based methodology for exosome isolation from plasma or supernatants of cell lines. With the use of such Abs and immune capture methods successively applied to the isolation of MTEX and T cell-derived CD3+ exosomes, it is possible to isolate these vesicle subsets from the same specimen of a melanoma patient’s plasma.
We describe here an immunoaffinity-based capture method for MTEX and CD3+ T cell-derived exosomes, which has recently been developed in our laboratory [16,15]. Starting with the isolation of morphologically intact, biologically active bulk exosomes from plasma by mini size exclusion chromatography (mini-SEC) [12], we implemented immune-capture of MTEX using a monoclonal Ab developed by Dr. Soldano Ferrone [17] that recognizes an epitope of chondroitin sulfate peptidoglycan 4 (CSPG4), which is selectively expressed on melanoma cells of nearly all patients [18–20]. A commercially available anti-CD3 mAb was used to capture T cell-derived exosomes. The sequential immune capture of MTEX and then CD3+ exosomes on Ab-coated beads was applied to a single batch of total exosomes obtained by mini-SEC from a small volume (1mL) of melanoma patient’s plasma. The method has been routinely used by us to allow for reproducible recovery and subsequent molecular characterization of the two exosome fractions [14,15]. The overall objective of the procedure is to make MTEX and CD3+ exosomes available for molecular profiling and future biomarker analysis.
2. Materials
2.1. Isolation of exosomes from plasma using mini-SEC
Plasma of melanoma patients serves as a source of exosomes. Peripheral venous blood samples are collected from melanoma patients after obtaining a signed IRB approved consent from all blood donors. Blood samples are collected into heparinized (green top) tubes to obtain plasma.
1.5 mL Microcentrifuge Tubes (Thermo Fisher Scientific, catalog number: 05–408-129).
0.22μm syringe filter units (Millipore Sigma, catalog number: SLGP033RS).
3mL syringe without needle (BD Becton Dickinson, catalog number: 309656).
Sepharose CL-2B (GE Healthcare Life Sciences, catalog number: 17–0140-01) (See Note 1).
Econo-Pac® chromatography columns (1.5 × 12cm, 20mL bed volume, Bio-Rad, Catalog number: 7321010).
Eisco™ Premium Lab Metalware Set (Thermo Fisher Scientific, catalog number: 501042880).
Phosphate-buffered saline (Lonza, catalog number: BW17–516F).
Eppendorf 5415D Centrifuge.
Sorvall™ Legend RT Centrifuge (Thermo Fisher Scientific)
2.2. Characterization of exosomes recovered from plasma
Pierce™ BCA Protein Assay Kit (Pierce Biotechnology, catalog number: 23225)
Peirce™ Lane marker reducing sample buffer, 5X (Thermo Fisher Scientific, catalog number: 39000).
Immobilon-P PVDF Membranes (EMD Millipore, catalog number: IPVH00010)
Mini-PROTEAN® TGX™ Gels (Bio-Rad Laboratories, catalog number: 456–1084)
Anti-TSG101 antibody, 1:500 (Abcam, catalog number: ab30871)
Anti-CD63 antibody, 1:1000 (Thermo Fisher Scientific, catalog number: 10628D)
Anti-Calnexin antibody (Cell Signaling Technology, catalog number: 2679)
Anti-Grp94 antibody, 1:1000 (Cell Signaling Technology, catalog number: 2104)
Nanopore membrane (IZON, catalog number: NP150)
qNano instrument (IZON)
Uranyl acetate (Electron Microscopy Sciences, catalog number: 22400)
Transmission electron microscope, JEOL JEM1011 (JEOL)
Copper grids, 200 mesh, for TEM (Electron Microscopy Sciences, catalog number: G200TT-CU).
Eppendorf 5415D Centrifuge.
0.5mL 100 kDa Amicon Ultra Centrifugal Filter Units (EMD Millipore, catalog number: UFC510096)
2.3. Immunoaffinity-based MTEX or CD3+ exosome capture
ExoCap™ Streptavidin Kit (MBL International Corporation, catalog number: MEX-SA).
Biotin-labeled anti-human CD3 antibody (Hit3a clone, Biolegend, catalog number: 300303).
Biotin-labeled anti-human CD63 antibody (H5C6 clone, Biolegend, catalog number: 353018).
Monoclonal antibodies (mAbs) specific for CSPG4. We used two mAb clones: 763.74 for capture and 225.28 for detection. The clones recognize distinct and spatially-distant epitopes of CSPG4 and were generated, purified and characterized by Dr. Soldano Ferrone[17,21] (see Note 2).
Lightning-Link® Rapid Biotinylation Kit – Type B (Expedeon, Catalog number: 371–0010).
Eppendorf 5415D Centrifuge.
MagRack™ 6 (GE Healthcare Life Sciences, catalog number: 28948964)
2.4. Detection of MTEX or CD3+ exosomes
Monoclonal antibodies (mAbs) for CSPG4 clones 225.28 (see Note 2).
Expedeon Lightning-Link® Allophycocyanin antibody labeling kit (Expedeon, catalog number: 705–0010)
Mouse IgG2a isotype control (R&D Systems, catalog number: MAB0031) (see Note 2)
Mouse serum (Millipore Sigma, catalog number: M5905–5ml)
BD LSR Fortessa™ flow cytometer, BD Biosciences.
Eppendorf 5415D Centrifuge.
MagRack™ 6 (GE Healthcare Life Sciences, catalog number: 28948964).
3. Methods
3.1. Isolation of Exosome from plasma
3.1.1. Pre-clearing of blood samples
Centrifuge heparinized blood samples at 1000xg at room temperature (RT) for10min to separate cells from plasma.
Transfer the plasma layer into a new tube and centrifuge at 2000xg for 10min at RT to sediment cell debris and aggregates.
Carefully collect the upper layer in a fresh tube and divide into 1mL aliquots. The plasma can be used fresh or stored at −80°C for later use.
Thaw a stored plasma vial at RT or continue with freshly collected plasma. Centrifuge the plasma at 10,000×g for 30min at 4°C (see Note 3).
Collect centrifuged plasma and filter it using a 0.22µm syringe filter unit to remove the larger vesicles, bacteria, mitochondria, other organelles or aggregated materials.
3.1.2. Mini-size exclusion chromatography (Mini-SEC)
An illustration of the principle for size exclusion chromatography is presented in Fig. 1.
Fig. 1:
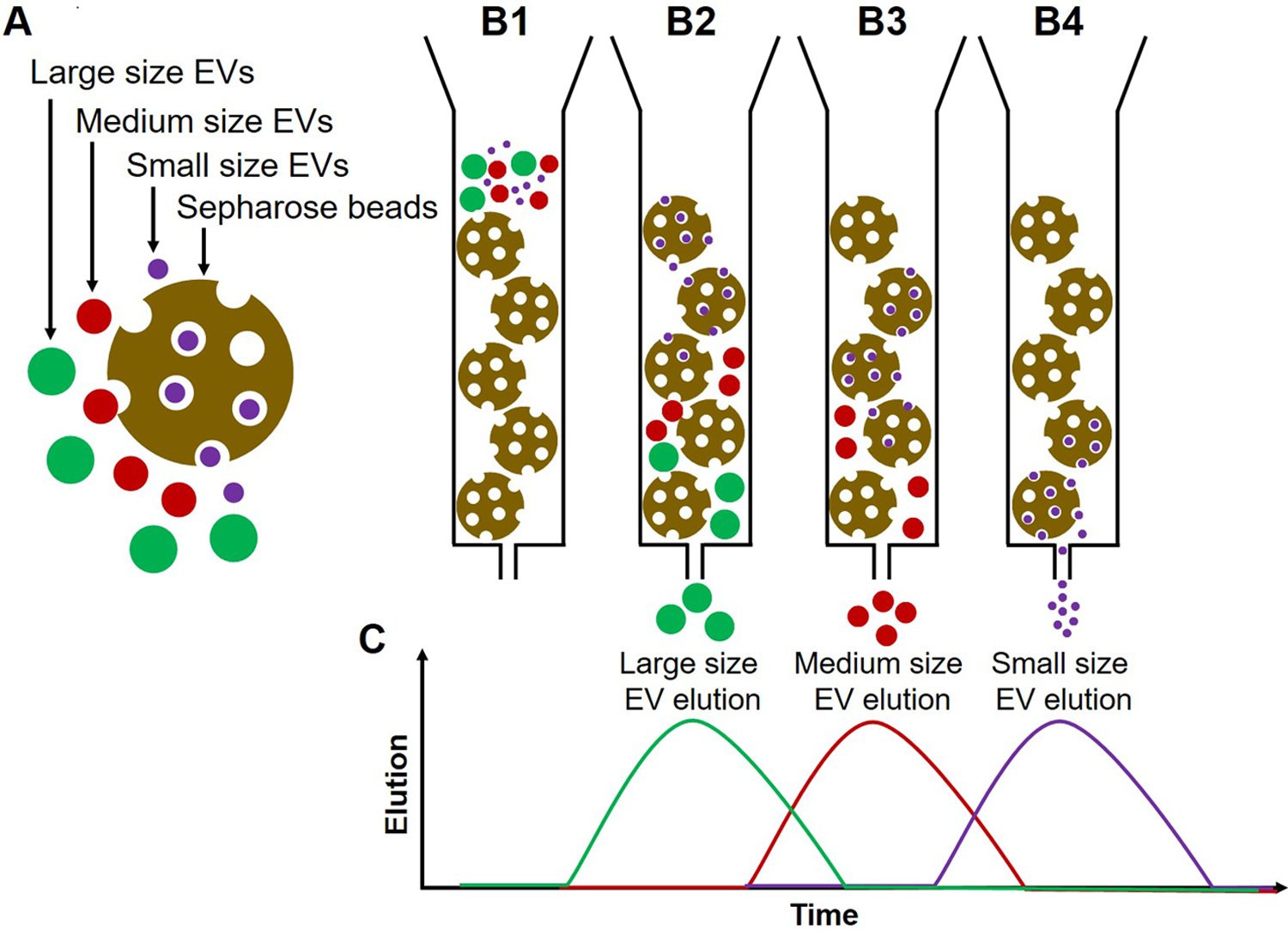
Illustration of the principle of size exclusion chromatography (SEC). (A) Sepharose beads (gold), large size EVs (green), medium-size EVs (red), and small size EVs (purple). (B1) The column is loaded with Sepharose beads and EVs are placed in the column. (B2) Larger EVs enter first into few pores of the Sepharose column, move down with the elution buffer and elute first. (B3) Medium EVs enter the pores later and thus elute after larger EVs (B4) Smaller EVs enter into many pores, move slowly through the column and elute later. (C) A histogram plot illustrating EV elution from the column; the elution time of EVs depends on their size. The vesicles elute in early fractions, while smaller protein aggregates or individual proteins are retained in the column and are eluted in later fractions.
Add Sepharose beads to the Econo-Pac® chromatography column until Sepharose bed volume reaches a 10mL mark on the column wall. Once the column is packed, place a porous frit at the top of the Sepharose bed using tweezers (see Note 4).
Wash the Sepharose column by passing 20mL PBS through the column.
Once PBS enters the Sepharose bed, place 1mL of clarified plasma onto the frit sitting on top of the column bed and wait until plasma enters the column (see Note 5).
Add 1mL PBS on the top of the column and collect the eluted fraction as fraction #1. Repeat this process two times and collect the eluent as fractions #2 and #3. Fractions #1 to #3 are waste fractions (see Note 6)
Add another 1mL of PBS and collect the corresponding eluent as the fraction # 4 in a 1.5 mL microcentrifuge tube. Fraction #4 contains the bulk of exosomes. It can be used immediately or stored at 4°C for no more than a week. Fraction #5 contains aggregated exosomes and is not collected.
3.2. Exosome characterization
It is necessary to check whether vesicles collected in the mini-SEC fraction #4 satisfy the criteria set for exosomes by the International Society for Extracellular Vesicles (ISEV) [22]. The following assays can be used for characterization of the isolated vesicles: protein levels, transmission electron microscopy (TEM), size distribution and particle concentration, and the presence of vesicle-specific protein makers (both positive and negative) by immunoblots and/or flow cytometry (Fig. 2). To avoid exosome aggregation, perform the TEM and qNano analysis immediately or within two days of exosome isolation.
Fig. 2:
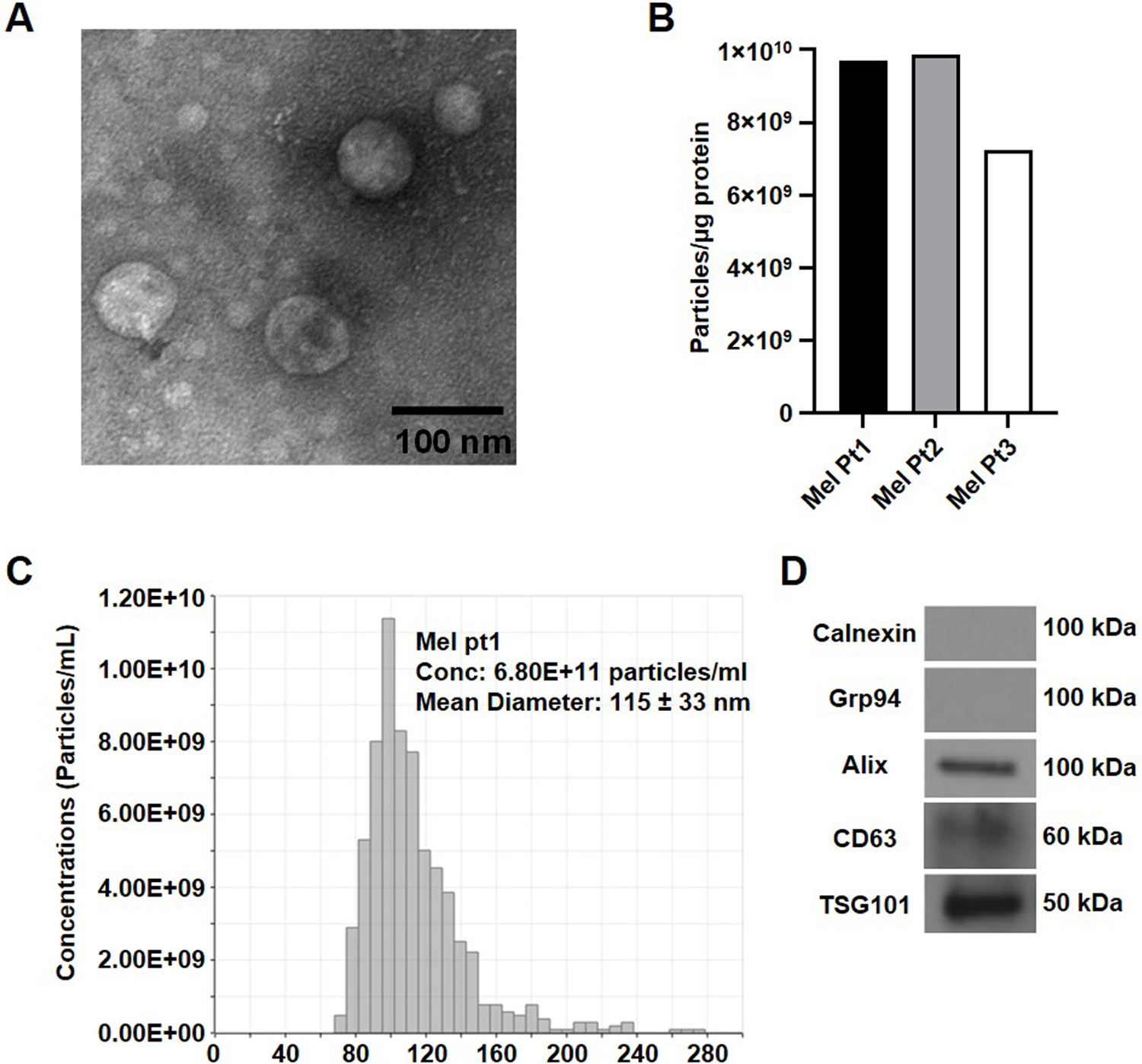
Characterization of exosomes isolated using mini-SEC from the plasma of melanoma cancer patients. (A) TEM images of exosomes recovered in the SEC fraction #4; (B) A bar graph presentation of the number of exosome particles per µg of protein in the SEC fraction #4 isolated from plasma of three different melanoma patients. (C) A representative size distribution profile for exosomes from plasma of a melanoma patient isolated by SEC and measured using qNano. (D) Western blot analysis of melanoma exosomes.
3.2.1. Protein measurements
Measure the protein concentration of exosomes using the BCA method following the manufacturer’s recommendation (see Note 7).
3.2.2. Transmission electron microscopy
Add a 3µL aliquot of exosomes on the grid and soak up the excess of exosomes using filter paper. Allow the grid to air dry for 3min (see Note 8).
Stain exosomes with 10µL of 1% (v/v) uranyl acetate solution in distilled water and remove excess uranyl acetate using filter paper. Allow the grid to air dry for 3min.
Observe exosome images from the TEM; note their morphology and diameter. Fig. 2A shows a TEM image of exosomes isolated by mini-SEC from melanoma patients’ plasma.
3.2.3. Measurements of exosome size and concentration by tunable resistive pulse sensing (TRPS)
Analyze the size and number of exosomes collected in fraction #4 using NP150 nanopore in a qNano instrument following the manufacturer’s instructions. (see Note 9).
3.2.4. Western blot analysis
Concentrate 0.5mL of fraction #4 to 250µg protein per mL using 100 kDa Amicon Ultra centrifugal filters and spinning the sample at 2000×g for 5min at RT.
Use 10µg exosomes for each western blot run. Add the Lane marker reducing buffer to each exosome sample. Denature proteins by heating the samples at 95°C for 5min.
Load 10µg protein equivalent of reduced exosomes in the gel sample wells and perform standard gel electrophoresis for western blot.
Transfer separated proteins into the PVDF membrane.
Examine the membrane for exosome protein markers such as CD9, TSG101, CD63 or other proteins of interest following the standard western blot protocols.
3.3. Separation of MTEX from -NMTEX
The exosomes isolated from patients’ plasma specimens by mini-SEC are a mixture of vesicles originating from many different cell types, including immune cells. EVs in melanoma patients’ plasma are enriched in exosomes derived from melanoma cells (i.e., MTEX). Immune capture of MTEX is performed first and it follows the established immune-affinity based method [16], which utilizes anti-CSPG4 mAb clone763.74 specific for a peptide epitope of the melanoma tumor antigen, chondroitin sulfate peptidoglycan 4 (CSPG4). This epitope is expressed on the surface of melanoma cells and on exosomes produced by these cells but is lacking from the surface of non-malignant cells or exosomes produced by these cells [20,19,18]. The success of immunocapture is strictly dependent on specificity of the capture Ab for the targeted antigen present on melanoma cells and on MTEX and on its absence from non-malignant cells and their exosomes (NMTEX). Thus, extensive testing for specificity and careful selection of mAbs to be used for immune capture is necessary. In addition, titrations of the selected mAb to determine the optimal ratios of mAb to MTEX and to beads used for their capture are critically important. Extensive preliminary titration steps are necessary to optimize the immune capture, and melanoma cell line-derived exosomes can be used in lieu of plasma-derived exosomes to establish and calibrate the conditions for immune capture. The MTEX capture procedure is illustrated in Fig. 3.
Fig. 3:
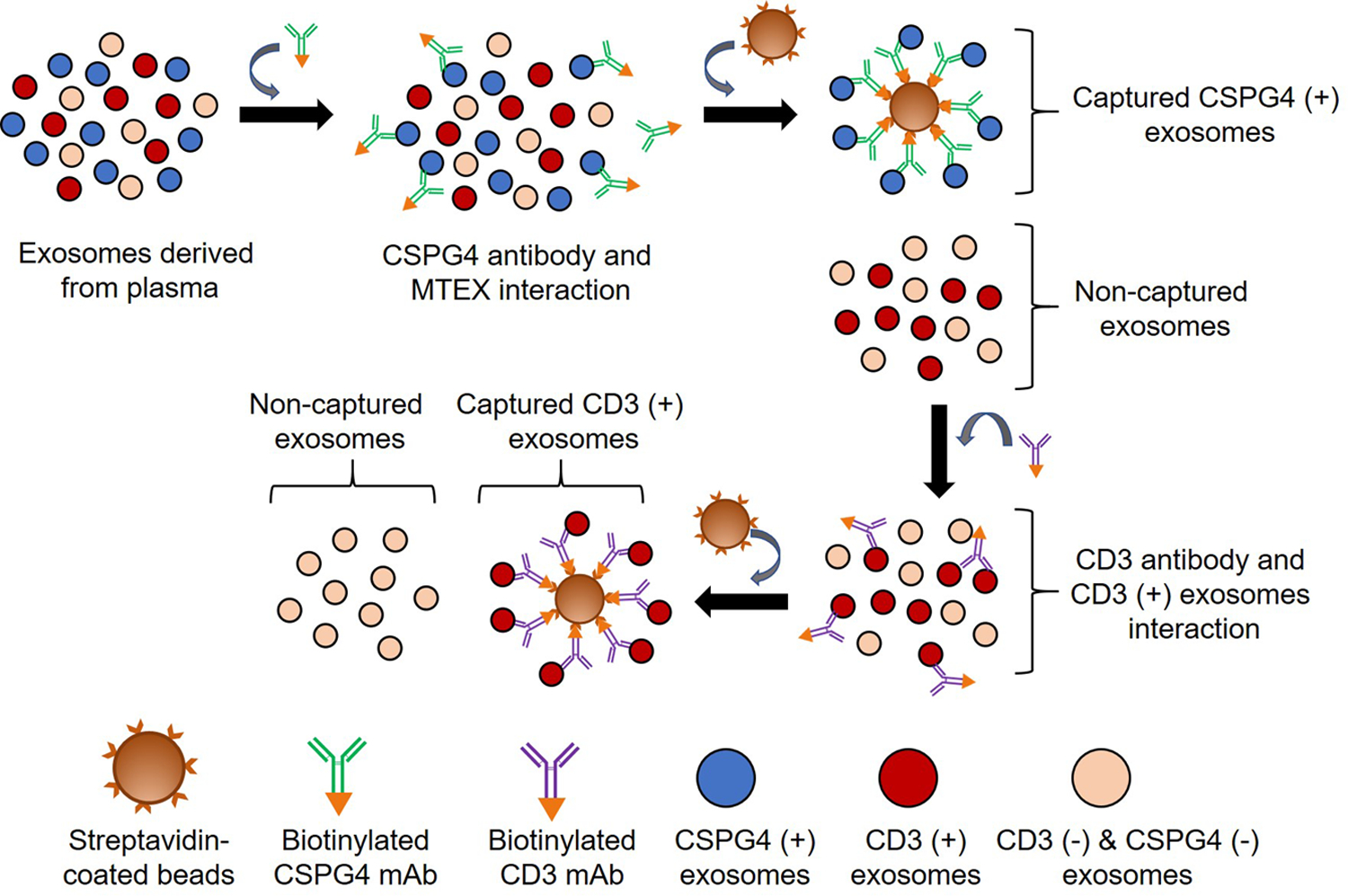
A schema of the immunoaffinity based capture method to sequentially capture MTEX and T cell-derived exosomes from total plasma exosomes. Exosomes were first co-incubated with biotin-labeled anti-CSPG4 mAb clone 763.74 and immune-captured on streptavidin-coated magnetic beads. The captured MTEX were recovered using a magnet. The non-captured exosomes (NMTEX) were then used to capture CD3+ exosomes using. biotin-labeled anti-CD3 mAb and streptavidin-coated magnetic beads. Finally, the exosome-antibody-bead complex was recovered using a magnet.
The non-captured exosomes, presumably the CSPG4(−) NMETX, are harvested and are used for the second immune capture to isolate T cell-derived CD3+ exosomes. A commercially available anti-CD3 mAb can be used. Thus, for each batch of total plasma exosomes isolated by SEC, two successive immune captures are performed, first to capture MTEX with biotinylated anti-CSPG4 mAbs and then to capture CD3+ T cell-derived exosomes with biotinylated anti- CD3 mAbs, In both successive captures, streptavidin-labeled beads are used for capture of biotin-labeled anti-CSPG4 or anti-CD3 mAbs complexed with the relevant exosomes (Fig. 3).
3.3.1. Immunoaffinity-based capture of MTEX on magnetic beads:
Measure the protein concentration of the fraction #4 collected from mini-SEC by BCA method following the manufacturer’s protocol (See Note 7).
Using 0.5mL of fraction #4, adjust protein concentration to 10µg/100µL either by using 100 kDa Amicon Ultra centrifugal filters at 2000×g or by diluting with PBS.
Place 10µg of exosome protein in a microcentrifuge tube and add 4µg of biotin-labeled anti-CSPG4 mAb clone 763.74. Incubate the mixture for 16–18h at 4°C with gentle agitation on a shaker (see Note 10 and 11).
Next day, combine the exosome/mAb mixture with 100µL of streptavidin-labeled beads and incubate for 2h at RT with gentle agitation (See Note 12).
Use a magnet to separate the magnetic beads from the supernatant. Beads contain the captured MTEX and the supernatant contains non-captured CSPG4 (−) NMTEX. Utilize the NMTEX fraction for the subsequent isolation of T cell-derived exosomes.
Captured MTEX are washed x3 with 100µL PBS using a magnet. Captured beads can be stored at 4°C for up to one week (See Note 11).
3.3.2. Immunoaffinity-based Capture of CD3+ exosomes from NMTEX
The NMTEX fraction contains non-captured exosomes in suspension, including CD3+ exosomes produced by T cells. Isolation of CD3+ exosomes from NMETX is illustrated in Fig. 3. To measure the protein concentration in the NMTEX fraction use the BCA method (See Note 7).
Concentrate the NMTEX fraction to 10µg/100µL, using 100kDa Amicon Ultra centrifugal filters at 2000×g.
Co-incubate 1µg of biotin-labeled anti-CD3 mAb and 10µg of concentrated NMTEX in 100µL PBS for 2h under gentle agitation at RT.
Add the above mixture into 50µL equivalent of streptavidin-labeled beads and incubate for 2h at RT with gentle agitation.
Remove the non-captured fraction using a magnet and store it in a fresh tube.
Wash the captured fraction x3 with 100µL PBS using a magnet.
3.3.3. Immunoaffinity-based capture of CD63+exosomes from the non-captured fractions
The success of immunocapture-based method relies on the complete capture of both CSPG4(+) and CD3+exosomes. Thus, the non-captured exosomes should be negative for CSPG4 and for CD3, respectively. Non-captured exosomes are incubated with biotinylated anti-CD63mAb specific for a tetraspanin carried by most exosomes. The immunocaptured CD63+ exosomes are tested by flow cytometry for the presence of CSPG4 or CD3 proteins.
Concentrate the non-captured fraction to 10µg/100µL using a 100kDa cutoff spin filter by centrifuging at 2000 × g at RT.
Add 1µg of biotin-labeled anti-CD63 mAb to 10µg of the concentrated non-captured fraction and incubate for 2h at RT with gentle agitation.
Combine the exosome/antibody mixture with a 50µL equivalent of streptavidin-coated beads and incubate for 2h at RT with gentle agitation.
Collect the captured fraction using a magnet and wash it x3 with 100µL of PBS.
3.4. Detection of antigens on the immunocaptured MTEX or CD3+ exosomes
By definition, the immunocaptured MTEX should be CSPG4+ and carry melanoma-associated antigens (MAA). Similarly, immunocaptured T cell-derived exosomes should be CD3+ and carry T cell-associated antigens such as PD-1 or CTLA4. The antigenic profiles of immunocaptured exosomes can be detected by on bead flow cytometry [15,16]. MTEX, CD3+ exosomes and CD63+ exosomes recovered from plasma exosomes using the isolation protocol described above are coupled to beads, as illustrated in Figs. 3 and 4. By using fluorochrome-labeled mAbs specific for antigens carried by these exosomes, it is possible to detect and quantitate relative levels of expression for various antigens on exosomes. The strategy of on-bead flow cytometry for exosome profiling is illustrated in Fig. 5 and is described elsewhere [15,16]. Relative mean fluorescence intensity values for each antigen present on MTEX, CD3+ or CD63+ exosomes can be determined as can ratios of costimulatory to immunosuppressive proteins on the exosome surface. The flow-cytometry based antigen detection on isolated MTEX and NMTEX is used for molecular profiling of the captured exosomes. An example of this detection strategy involves a search for the CSPG4 antigen on MTEX or NMTEX using fluorochrome-labeled anti-CSPG4 mAb clone 225.28.
Fig. 4:
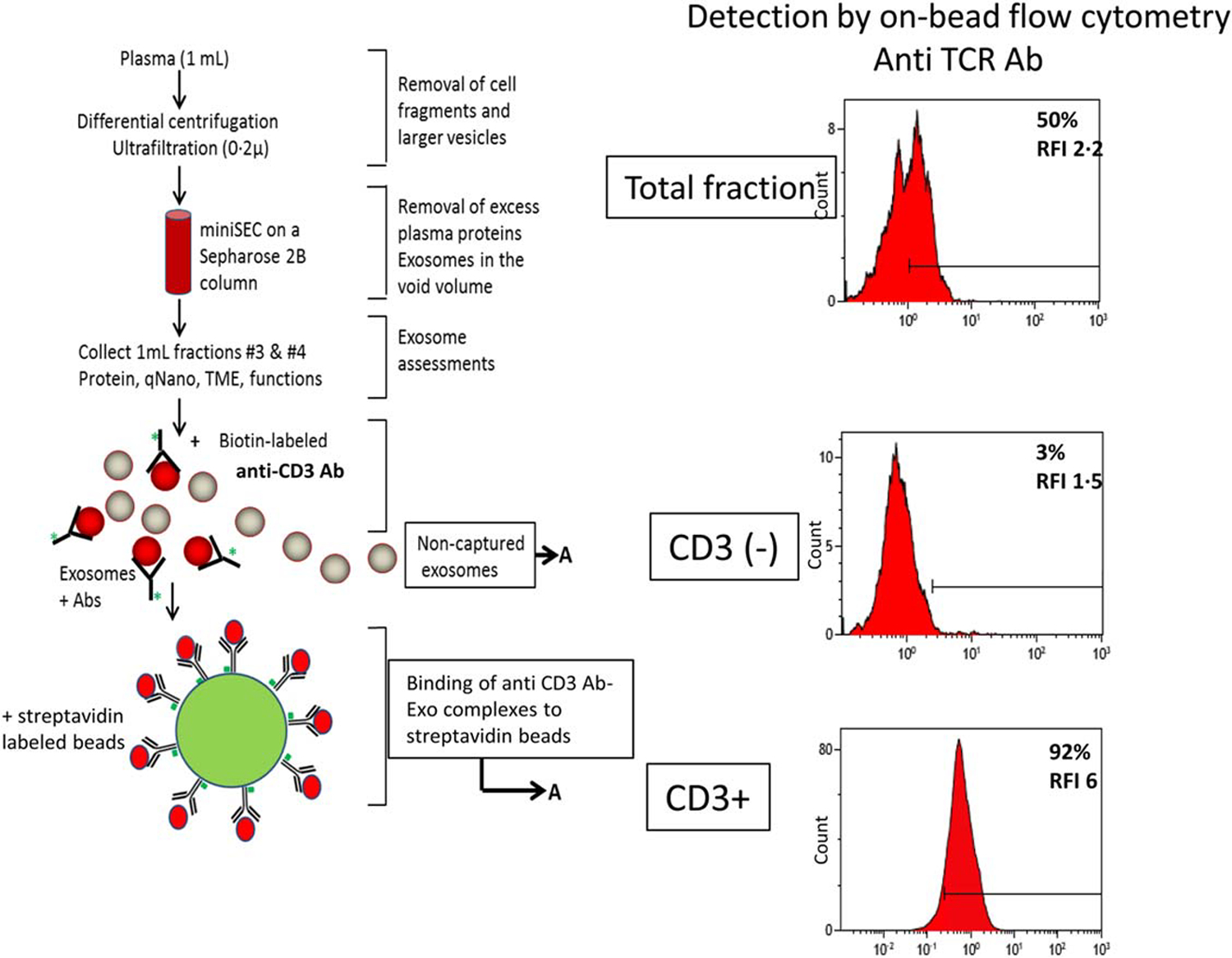
Immune capture of CD3+ exosomes and detection of the exosome cargo by on-bead flow cytometry. After exosome isolation from plasma by mini-SEC, immune capture with biotinylated anti-CD3 mAb antibody on beads separated CD3+ from uncaptured CD3neg exosomes. Proteins carried by CD3+ exosomes on beads are detected by using labeled mAbs specific for these proteins and on-bead flow cytometry. Non-captured CD3neg exosomes can be captured on beads using biotinylated ant-CD63 antibody for detection. The figure is adapted from Theodoraki et al. [15] and reproduced with permission of the Wiley Publishing Company.
Fig. 5:
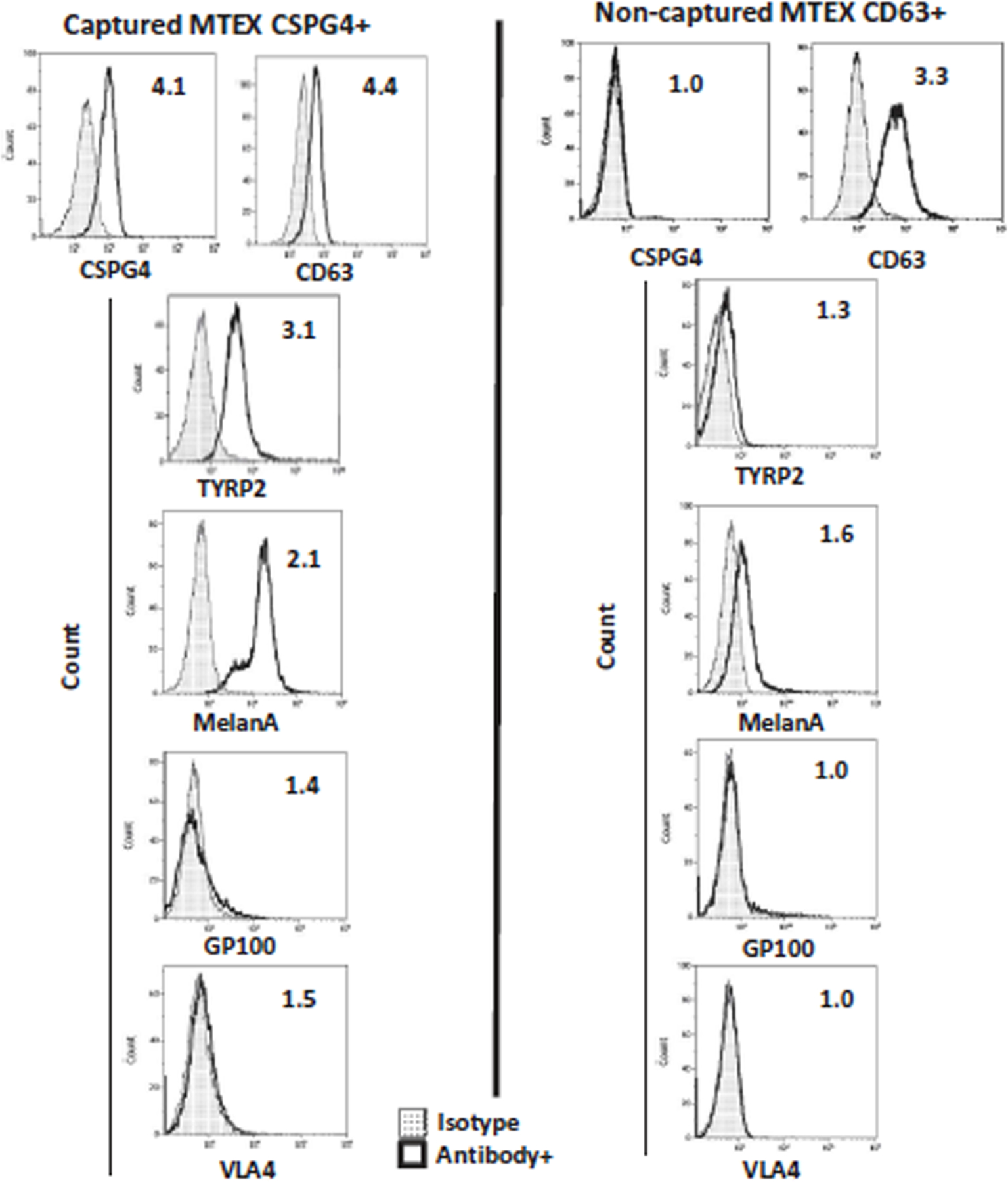
Representative on bead flow cytometry for detection of melanoma-associated antigens (MAAs) on the immunocaptured MTEX and NMTEX from plasma of a melanoma patient. NMTEX were re-captured on beads with biotinylated anti-CD63 mAb. The values within the histogram represent relative fluorescence values (RFI). RFI = MFI of detection Ab/MFI of isotype control Ab. Note that MAAs are carried exclusively by CSPG4(+) MTEX and are absent from non-captured exosomes (NMTEX). The figure is adapted from Sharma et al. [16] and reproduced with permission of the Taylor & Francis Group.
Place 4µL of MTEX captured on beads (see section 3.3.1) in a fresh microfuge tube. Adjust the volume to 98µL using PBS (see Note 13 and 14).
Add 2µL of mouse serum and incubate for 30min at RT.
Remove the supernatant using a magnet and disperse MTEX on beads in 100µL of 0.5% (v/v) mouse serum in PBS.
Add 4µg of APC-labeled anti-CSPG4 mAb clone 225.28, mix and incubate at RT for 1h with gentle agitation, protecting the microfuge tube from light. (for isotype control, use an equivalent level of APC-conjugated mouse IgG2a in place of anti-CSPG4 mAb in a separate microfuge tube)
Wash the beads x3 with 100µL of PBS using a magnet. Finally, disperse the beads in 300µL PBS for flow cytometry.
On-bead flow cytometry is performed with the gate set on MTEX/mAb/bead complexes. Relative fluorescence intensity (RFI) of the complexes is calculated by dividing the intensity value of mAb- stained MTEX by the intensity value of IgG isotype control.
Acknowledgements:
This work was in part supported by the NIH grant R01- CA 168628 to TLW.
4. Notes
GE Healthcare Bio-Sciences supplies Sepharose CL-2B gel in ethanol. To remove ethanol, wash Sepharose beads (GE Healthcare Bio-Sciences, cat. no. 17-0140-0) with 500mL of PBS x3. Allow Sepharose to settle for 4h between each washing step. Store washed Sepharose at 4°C in PBS.
We use mAbCSPG4 clone 763.74 to capture MTEX and clone 225.28 to detect CSPG4 on the captured MTEX. These Ab clones are not commercially available and can be obtained on request from Dr. Soldano Ferrone, Harvard U. Biotinylate CSPG4 mAb clone 763.74 using the Lightning-Link® Rapid Biotinylation Kit – Type B (Expedeon, Catalog number: 371-0010) and conjugate CSSPG4 mAb clone 225.28 with APC using Lightning-Link® Allophycocyanin antibody labeling kit (Expedeon, catalog number: 705-0010) following the manufacturer’s recommendations. For APC conjugation of mouse IgG2a isotype control, maintain the same weight ratio of IgG to APC as that used during labeling of CSPG4 with APC, following the manufacturer’s recommendations. Store conjugated antibodies and IgG at 4 °C.
Microvesicles (MVs) sediment at 10,000×g centrifugation, while small exosomes stay in solution. Pellet could be harvested for studies of MVs)
Caution needs to be taken to avoid air bubble formation inside the Sepharose bed while placing Sepharose beads into the column. If the air bubble is formed, add more PBS into the column and dislodge the Sepharose bed by pipetting, and then allow the Sepharose beads to settle down slowly. Carefully insert the frit in the column from top and orient frit horizontally using a tweezer, and then slowly lower the frit onto the Sepharose bed.
This method is standardized for 1mL plasma or other samples. To separate samples larger than 1mL in volume separation, a larger column can be used, but this will require prior standardization of the procedure to be able to recover “protein-contaminant free” exosomes. Alternatively, a series of several small column can be set up in parallel, each charged with 1mL of plasma as illustrated in ref [23].
The fractions #3 collected from mini-SEC contains microvesicles (MVs) and a few larger exosomes.
We use 25µL of fraction #4 for the protein measurement to make sure that it falls within the detection range of the BCA-based method. Most of the time, levels of protein recovered in fraction #4 are relatively low compared to cell lysates, for example. Thus, it is necessary to use exosome volumes large enough to allow for accurate protein measurements while using the BCA method (Pierce Biotechnology, catalog number: 23225).
Dilute exosomes prior to loading them onto the grid. Loading of highly concentrated exosomes will give crowded TEM images and prevent reliable estimations of exosome diameters and morphology.
Other methods, such as NanoSight or Zetasizer can be used to determine the size of exosomes but each method will give somewhat different values.
The immune capture of MTEX or CD3+ exosomes requires prior optimization, where various exosome protein concentrations ranging from 5 to 20µg are co-incubated with 0.5 to 4 µg of biotinylated anti-CSPG4 or anti-CD3 mAbs in the presence of 10 to 100µL of streptavidin-labeled beads per 100µL of reaction volume [15,16]. For MTEX capture,10µg exosome protein, 4µg CSPG4 antibody and 100µL MBL beads were found to be optimal [16], while 10µg exosome protein, 1µg anti-CD3 mAb and 50µL MBL streptavidin-labeled beads worked best for capture of T cell-derived exosomes [15]. Clearly, these parameters for immune capture have to be re-defined as new batches of mAbs are used for immune capture.
Make sure this is done with gentle agitation, just enough to make sure that beads are homogeneously distributed in the solution during the incubation time. High-speed vortex mixing or agitation of exosomes with magnetic beads may damage the exosomes. Thus, during washing steps, avoid vortex and instead, use the pipetting method to mix the exosome solution.
Wash streptavidin-coated beads once with PBS before use. Remove PBS completely from the beads and then add exosome/antibody mixture to the solid streptavidin-coated beads. To ensure that beads are homogeneously mixed, it is necessary to maintain the reaction volume at no less than 100µL in a 1.5mL microcentrifuge tube.
Flow cytometry-based detection can also be performed with CD63+ exosomes captured on beads (Fig. 4 and 5) or with CD3+ exosomes captured on beads (Fig. 4) using fluorochrome-labeled Abs specific for any protein of interest. The procedure can be used for ascertaining that immune capture was complete (i.e., MTEX are positive for CSPG4. while NMTEX are negative; or CD3+ exosomes are CD3+TCR+, while exosomes not captured by anti-CD3 mAb are negative). It is also used for detection of any antigen of interest on immunocaptured exosomes (i.e., for exosome profiling).
Background noise resulted from the autofluorescence of blank beads interfere with the flow cytometry-based detection. Also, often Abs has non-specific binding affinity for the beads. Thus, to determine the background caused by autofluorescence and non-specific binding, it is necessary to run blank beads and blank beads stained with Ab as controls along with the samples.
References
- 1.Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200 (4):373–383. doi: 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocucci E, Meldolesi J (2015) Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 25 (6):364–372. doi: 10.1016/j.tcb.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 3.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM (2015) Exosome mediated communication within the tumor microenvironment. J Control Release 219:278–294. doi: 10.1016/j.jconrel.2015.06.029 [DOI] [PubMed] [Google Scholar]
- 4.Xiao H, Lasser C, Shelke GV, Wang J, Radinger M, Lunavat TR, Malmhall C, Lin LH, Li J, Li L, Lotvall J (2014) Mast cell exosomes promote lung adenocarcinoma cell proliferation - role of KIT-stem cell factor signaling. Cell Commun Signal 12:64. doi: 10.1186/s12964-014-0064-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9 (6):654–659. doi: 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- 6.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M (2013) Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A 110 (18):7312–7317. doi: 10.1073/pnas.1220998110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu S, Yang Y, Allen CL, Maguire O, Minderman H, Sen A, Ciesielski MJ, Collins KA, Bush PJ, Singh P, Wang X, Morgan M, Qu J, Bankert RB, Whiteside TL, Wu Y, Ernstoff MS (2018) Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci Rep 8 (1):12905. doi: 10.1038/s41598-018-31323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560 (7718):382–386. doi: 10.1038/s41586-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiteside TL (2016) Exosomes and tumor-mediated immune suppression. J Clin Invest 126 (4):1216–1223. doi: 10.1172/JCI81136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinton LT, Sloane HS, Kester M, Kelly KA (2015) Formation and role of exosomes in cancer. Cell Mol Life Sci 72 (4):659–671. doi: 10.1007/s00018-014-1764-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18 (6):883–891. doi: 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL (2016) Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles 5:29289. doi: 10.3402/jev.v5.29289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domingues B, Lopes JM, Soares P, Populo H (2018) Melanoma treatment in review. Immunotargets Ther 7:35–49. doi: 10.2147/ITT.S134842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma P, Diergaarde B, Ferrone S, Kirkwood JM, Whiteside TL (2020) Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells. Sci Rep 10 (1):92. doi: 10.1038/s41598-019-56542-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theodoraki MN, Hoffmann TK, Whiteside TL (2018) Separation of plasma-derived exosomes into CD3((+)) and CD3((−)) fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clin Exp Immunol 192 (3):271–283. doi: 10.1111/cei.13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P, Ludwig S, Muller L, Hong CS, Kirkwood JM, Ferrone S, Whiteside TL (2018) Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles 7 (1):1435138. doi: 10.1080/20013078.2018.1435138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Sabbatino F, Wang X, Ferrone S (2014) Detection of chondroitin sulfate proteoglycan 4 (CSPG4) in melanoma. Methods Mol Biol 1102:523–535. doi: 10.1007/978-1-62703-727-3_28 [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Katayama A, Wang Y, Yu L, Favoino E, Sakakura K, Favole A, Tsuchikawa T, Silver S, Watkins SC, Kageshita T, Ferrone S (2011) Functional characterization of an scFv-Fc antibody that immunotherapeutically targets the common cancer cell surface proteoglycan CSPG4. Cancer Res 71 (24):7410–7422. doi: 10.1158/0008-5472.CAN-10-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S (2004) Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol 24 (4):267–296. doi: 10.1615/critrevimmunol.v24.i4.40 [DOI] [PubMed] [Google Scholar]
- 20.Schlingemann RO, Rietveld FJ, de Waal RM, Ferrone S, Ruiter DJ (1990) Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol 136 (6):1393–1405 [PMC free article] [PubMed] [Google Scholar]
- 21.Temponi M, Gold AM, Ferrone S (1992) Binding parameters and idiotypic profile of the whole immunoglobulin and Fab’ fragments of murine monoclonal antibody to distinct determinants of the human high molecular weight-melanoma associated antigen. Cancer Res 52 (9):2497–2503 [PubMed] [Google Scholar]
- 22.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr., Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr., Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7 (1):1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig N, Hong CS, Ludwig S, Azambuja JH, Sharma P, Theodoraki MN, Whiteside TL (2019) Isolation and Analysis of Tumor-Derived Exosomes. Curr Protoc Immunol 127 (1):e91. doi: 10.1002/cpim.91 [DOI] [PMC free article] [PubMed] [Google Scholar]


