Abstract
The gut–brain axis has presented a valuable new dynamic in the treatment of cancer and central nervous system (CNS) diseases. However, little is known about the potential role of this axis in neuro-oncology. The goal of this review is to highlight potential implications of the gut–brain axis in neuro-oncology, in particular gliomas, and future areas of research. The gut–brain axis is a well-established biochemical signaling axis that has been associated with various CNS diseases. In neuro-oncology, recent studies have described gut microbiome differences in tumor-bearing mice and glioma patients compared to controls. These differences in the composition of the microbiome are expected to impact the metabolic functionality of each microbiome. The effects of antibiotics on the microbiome may affect tumor growth and modulate the immune system in tumor-bearing mice. Preliminary studies have shown that the gut microbiome might influence PD-L1 response in glioma-bearing mice, as previously observed in other non-CNS cancers. Groundbreaking studies have identified intratumoral bacterial DNA in several cancers including high-grade glioma. The gut microbiome and its manipulation represent a new and relatively unexplored area that could be utilized to enhance the effectiveness of therapy in glioma. Further mechanistic studies of this therapeutic strategy are needed to assess its clinical relevance.
Keywords: fecal metabolites, glioblastoma, glioma, gut–brain axis, gut microbiome
Key Points.
There are gut microbiome differences among tumor-bearing mice/glioma patients.
The antibiotic effects on the microbiome may affect tumor growth and modulate the immune system.
Intratumoral bacterial DNA in several cancers including GBM has been identified.
Gliomas, a type of central nervous system (CNS) tumor, account for 80% of all malignant brain tumors and are the leading cause of death within the field of neuro-oncology.1 Despite the frequency of this diagnosis, glioma etiology is unclear. Although progress has been made in specific aspects of this disease, such as molecular markers that have prognostic significance, treatment strategies have been seemingly stagnant over the past decades.
Furthering research of the microbiome, which houses the commensal bacteria of the gut, has presented a valuable new dynamic in the treatment of cancer and CNS diseases.2–10 The microbiome can communicate with the brain via the gut–brain axis: a bidirectional feedback loop that utilizes fecal metabolites such as short-chain fatty acids (SCFAs) and neurotransmitters, among other pathways.11 Furthermore, recent research has illuminated the influence of the gut microbiome and bacteria-derived metabolites in the effectiveness and side effect protection of chemo-, radio-, and immunotherapies.6,12,13
In neuro-oncology, recent studies involving gliomas have shown that tumor growth affects the bacterial composition of the gut microbiome, fecal metabolites, and the innate immune system.14–16 This article reviews the literature on the gut microbiome, the gut–brain axis, and its relationship with CNS diseases and cancer, and explores the emerging evidence of the role that the gut microbiome plays in brain tumors, specifically glioma. We highlight the potential implications of the gut–brain axis in neuro-oncology and future areas of research.
Microbiome
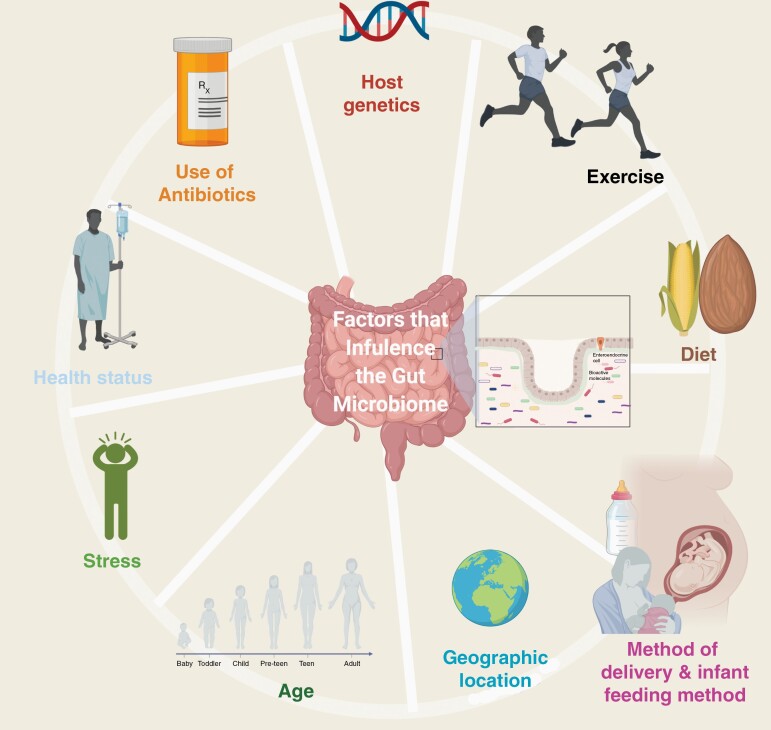
The microbiome has been a fascinating area of research since the ability to use next generation sequencing techniques to profile microbial communities within the body. The microbiome encompasses all the symbiotic microbes and their associated genes that have coevolved with humans. These microorganisms have created a homeostatically driven ecosystem within their host.17 The gut microbiome establishes early in life and varies within, and among populations, based on factors such as diet, ethnicity, and age.17–19 The role of diet on the gut microbiome is paramount, as diet directly influences the microbiome diversity and abundance in the gut, for example, higher fibers promote the growth of microbes specialized in the production of SCFAs like Bifidobacterium.20,21
Small, daily fluctuations in the gut microbiome composition occur, however, larger variations arise during a lifespan (Figure 1). The microbiome changes that occur with advanced age might be connected to the declining immune system and inflammatory responses.22 The gut microbiome functions include: modulation of immune activation and response, epithelial barrier integrity, nutrient absorption and storage, conversion of luminal compounds to metabolites, host–bacterial interactions at the mucosal surface, and long-term modulation of behaviors and brain processes.17,22–26
Figure 1.
Factors known to influence the gut microbiome composition.
Since the microbiome plays a critical role in normal physiological function, several studies have sought to establish the taxonomic composition and structure in healthy individuals. However, the definition of a “normal” gut microbiome remains inconclusive due to the high compositional variability in microbial taxa, even within healthy individuals and twins.18,27,28 Instead, a more practical approach is to look for a “functional core” in which specific properties, such as basic metabolic functions and regulatory pathways, are present and maintained.27,29 Large studies have shown that despite considerable interpersonal variation in microbial taxa composition, genes encoding specific metabolic functions are conserved.17,18 For example, the healthy gut microbiome can modulate their bacterial proportions via the host’s innate immunity, inducing the expression of specific genes that upregulate antimicrobial proteins.24
Dysbiosis, or a disruption in the normal functions or composition of the microbiome, is caused by a deviation from the functional core produced by factors such as antibiotics, lack of sufficient bacterial diversity, and microorganisms that react to therapies (including chemotherapy) causing toxicity or reducing pharmaceutical efficacy.17,30,31 Dysbiosis is an important aspect of the gut microbiome to consider, as it might potentially increase the risk of disease due to an inability to effectively respond to environmental changes.32 This lack of resilience leaves the host susceptible to disease. However, it is still unclear whether dysbiosis is a cause, or a response to, a particular disease state.17,32
The Gut–Brain Axis
The bidirectional communication between the CNS and the enteric nervous system forms a network called the gut–brain axis.11 Initially, it was believed that disruptions to the gut microbiome only created pathophysiological disorders locally in the gut, as seen in irritable bowel syndrome.26 However, more recent research has shown pathophysiological consequences of dysbiosis, not only in the gut, but also in the CNS.11,26,33 A large body of preclinical research, using germ-free (GF) and wild-type mice, has substantiated evidence that an alteration in brain signaling and behavior can be caused by manipulation of the gut microbiome.34,35 Specific alterations include memory dysfunction following bacterial infection, reduced neurotransmitter receptor expression in GF mice, and alterations in neuron excitability after probiotic administration, among many others.33,34,36 The translatability of these studies is limited because, although GF mice provide an organism with no microbial influence, the conditions in which they are reared have far-reaching physiological effects. These differences make extrapolating the data to human populations more difficult.
Human trials are more complex because of interindividual microbiome differences. To circumvent this, brain imaging has been used to link microbial ecology with various neural networks.22,26 Manipulation of the gut microbiome using antibiotics has shown increased subcortical and frontoparietal brain connectivity as well as improved cognitive function in a small cohort of minimal hepatic encephalopathic patients.37 Thus, both, preclinical and clinical evidence, show the cross-communication of the gut and the brain.
The gut affects neurological function via the neuroendocrine and immunological pathways, while the brain affects the composition of the microbiome via the autonomic nervous system (ANS).26 With neurons located centrally and peripherally, the ANS creates a complex feedback loop between the brain and the gut. Both branches of the ANS, in coordination with the enteric nervous system, via the hypothalamic–pituitary–adrenal axis, can induce CNS modulated changes to the microbiome including gut motility, rate of intestinal transit, and mucus secretion.22,26 Much of this feedback loop relies upon the vagus nerve. Afferent neurons of the vagus nerve carry signals from multiple innervated layers of the gut to the nucleus tractus solitarius (NTS) of the brain. The NTS, which has numerous different neurotransmitters and neuropeptide receptors can then act as a mediary to the brain. An integrated parasympathetic response is then conducted back through the vagus nerve, causing bodily and behavioral changes.22,38 Sympathetic innervation, through less direct pathways, mainly functions to maintain the integrity of the intestinal mucus layer.39
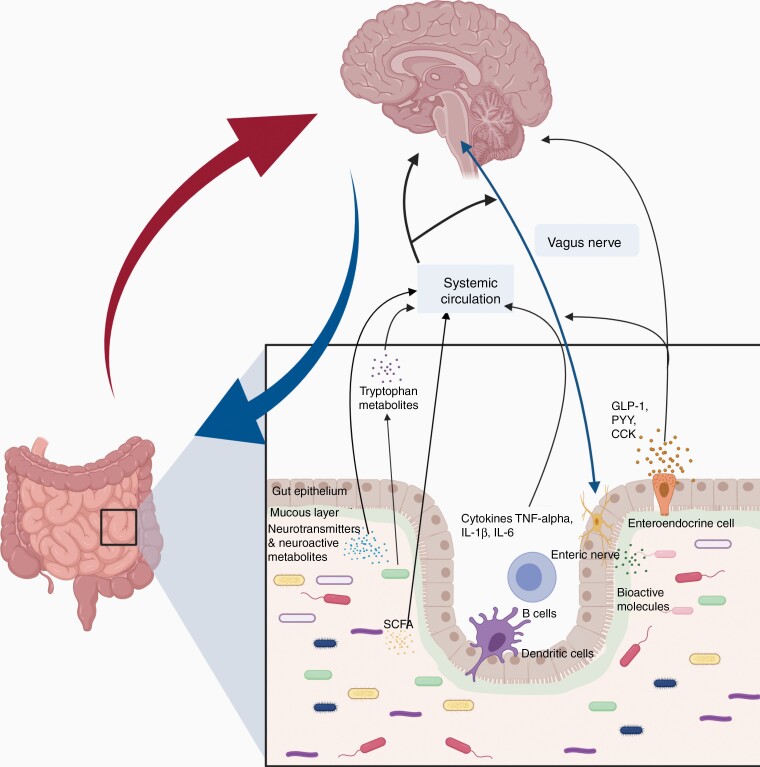
Another mechanism by which the axis operates is through metabolites. Blood and cerebral metabolites affect neuroendocrine responsiveness. The microbiome modulates metabolite levels by synthesizing metabolic reactions.25 Altered levels of SCFAs have been correlated with several CNS diseases and have been found in cerebrospinal fluid (CSF) and directly in the brain.15,22 Furthermore, SCFAs affect the immune response in many different ways including regulation of antigen-presenting cells and manipulation of T-cell production of IL-10, TH-1, and TH-17.40 Another family of molecules that play a role in the gut–brain axis is the neurotransmitters. The microbiome synthesizes and responds to several neurotransmitters including serotonin, norepinephrine, and other catecholamines. CNS diseases greatly skew the number of fecal neurotransmitters outside of the normal physiological range. This local change in the gut environment then creates systemic changes globally.15 A summary of the gut–brain axis communication is depicted in Figure 2.
Figure 2.
The gut–brain axis communication is bidirectional and mediated by several pathways. This communication is performed through hormonal (hypothalamic–pituitary–adrenal axis), metabolic (short-chain fatty acids and neurotransmitters), and immunologic pathways. Moreover, the gut microbiome interacts with the brain through the enteric nervous system and vagus nerve, which can be influenced either by the gut microbiota itself or indirectly through a local effect on the pathway by neuroactive compounds (eg, noradrenaline, serotonin, dopamine, tryptophan, and short-chain fatty acid).
Gut Microbiome and the Blood–Brain Barrier
The blood–brain barrier (BBB), an extension of the neural microvasculature composed of a network of endothelial cells sealed with tight junctions, contributes to maintaining CNS homeostasis.41 The BBB obstructs the diffusion of pathogens and hydrophilic molecules from the CSF and surrounding vasculature while permitting critical gases (O2, CO2) and lipid-soluble substances (glucose).41 However, the tight junctions of the BBB and their associated proteins are susceptible to degradation, which compromises its integrity.
The gut microbiome influences the development and maintenance of tight junctions of the BBB. GF mice, who lack a normal microbiome, have significantly higher levels of BBB permeability.42 It was observed that this is the result of reduced expression of occludin and claudin-5, key regulators of the BBB’s tight junctions.42 Interestingly, microbial colonization of GF mice gut has been shown to decrease permeability and increase the expression of tight junction proteins.42 Additionally, a study utilizing antibiotic-treated mice demonstrated that Lactobacillus and sodium butyrate administration reduces the permeability of the BBB in aged mice.43 Furthermore, gut microbial selective depletion of acetate and propionate-producing bacteria (through oral antibiotics) has been shown to increase BBB permeability in adult rhesus monkeys.44 This was exhibited by a reduced CSF/serum albumin ratio. Taken together these data demonstrate the important role of the gut microbiome in the BBB.
The BBB presents an obstacle to drug delivery in the brain. Although it is widely accepted that glioblastoma (GBM) patients have BBB disruption, it is now known, that all GBM patients have intact BBB tumor regions.45 Currently, efforts are being made to overcome the BBB to enhance drug delivery.46 Research is needed to further understand the relationship between the gut microbiome and the BBB, which might open novel methods to enhance drug delivery to treat CNS diseases, including gliomas.
Gut Microbiome and CNS Diseases
Accumulating evidence from both in vivo, and clinical studies, has implicated the gut microbiome in a variety of psychiatric, neurological, and neurodegenerative diseases.2,3,22,47–51 The relationship between multiple sclerosis (MS) and autism spectrum disorder (ASD) and the gut microbiome has been thoroughly investigated in both animals and humans.47,50,52,53 In both MS and ASD, SCFAs producing bacteria and SCFAs levels have been identified to be decreased.54,55 In stroke animal models, several studies have shown the role of the gut microbiome in outcome through the modulation of SCFAs.2,56 Meanwhile, in Alzheimer’s disease, the gut microbiome has been related to β-amyloid plaques and the pathophysiology of the disease.57 Other interesting findings had correlated amyotrophic lateral sclerosis, epilepsy, and Parkinson’s disease outcomes due to specific microbial-derived metabolites or drug metabolism from bacteria.3,58,59 Even though the findings of a strong relationship between the gut microbiome and CNS diseases are paving the path in neurosciences, there is still much that remains to discover.
Gut Microbiome and Cancer
It is estimated that microbes can be implicated in ~15%–20% of cancers.60 The recognition of the importance of the relationship between microbes in the gut and systemic tumors is increasing. The role of the gut microbiome in carcinogenesis,61 as well as, its influence in response to treatments like immunotherapy4,5 are interesting observations that could have a great impact in the field of oncology. Recent studies have revealed that human tumors harbor-specific bacteria that can be identified across tumor types,62,63 offering an interesting insight into the relationship between the microbiome and oncogenesis. However, the role of these tumor-associated bacteria is incompletely understood and more research is needed.
The Gut Microbiome and Chemotherapy
The microbiome plays an important role in the pathophysiology of cancer, not only in its formation, but also in the efficacy of treatments. The presence of commensal bacteria modifies anticancer drugs response by modulating the immune system and microenvironment.12,64
Chemotherapy utilizing CpG oligonucleotide, cyclophosphamide, and platinum-based agents cause translocation of gram-positive species into secondary lymphoid organs.12 Once there, naive CD4+ T cells are differentiated into TH-17 and TH-1 cells which produce interleukin-17 and interleukin-1, respectively, upon activation.12 Both proinflammatory cytokines contribute to tumor suppression and eradication.65 GF and antibiotic-treated mice show a marked reduction of TH-17 and TH-1 cells which correlates with an inability to effectively suppress tumorigenesis.12 Because chemotherapy’s tumoricidal properties might be affected by commensal bacteria, researchers have speculated about the possibility to enhance chemotherapy with “immune-stimulatory organisms.”66 However, more research is needed to push the boundaries of the vast array of possibilities offered by microbiome modulation with regard to the prevention and treatment of cancer.
The Gut Microbiome and Immunotherapy
The use of immunotherapy to promote antitumor immune response has revolutionized the treatment of multiple cancers, particularly melanoma, and epithelial tumors.67–69 Seminal studies on the gut microbiome have revealed the key role of specific gut bacteria (eg, Ruminococcaceae family, Akkermansia muciniphila, Bifidobacterium pseudolongum) in modulating the response to immune checkpoint inhibitors (ICIs) in both, melanoma and epithelial tumors.4–9 In addition, a recent study evaluating 52 patients with solid tumors treated with ICIs, identified high concentrations of some fecal (acetic acid, propionic acid, butyric acid, valeric acid) and plasma (isovaleric acid) SCFAs were associated with improved progression-free survival.70 The first human clinical trials exploring the safety of gut microbiome transplants in combination with ICIs for advanced melanoma have shown its safeness, long-term fecal microbiota transplant engraftment, and the response to anti-PD-1 therapy in a subset of previously PD-1-refractory cases.71,72 Despite these exciting advancements in melanoma and other cancer, how the microbiome might influence immunotherapy in glioma is an important but unanswered question that might identify patients that will respond to this therapy.
Gut Microbiome and Glioma
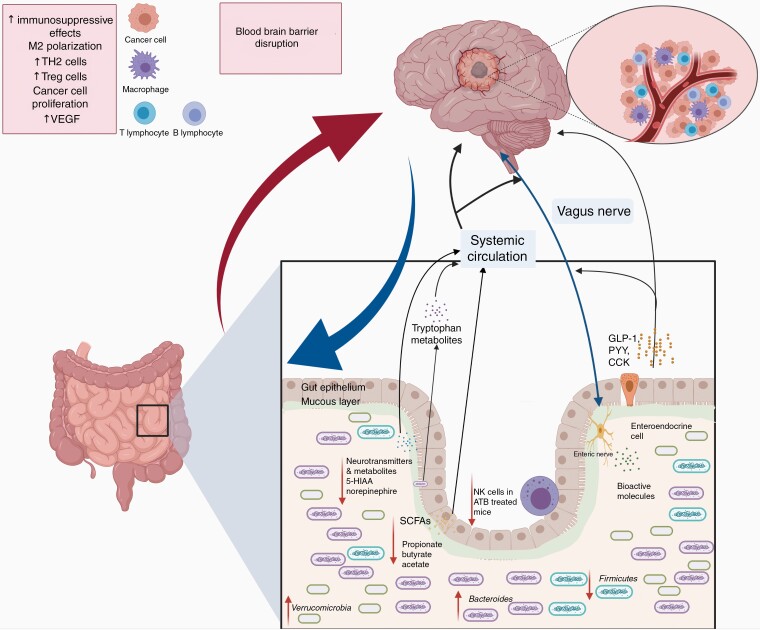
The relationship between the gut microbiome and glioma is a new and active field of investigation that might open therapeutic venues to a disease in which current treatment efficacy is limited. As observed in other CNS diseases and cancer, glioma mice models have shown that tumor development alters the gut microbiome prior to weight loss.14 Glioma-bearing mice present gut dysbiosis—measured by changes in the Firmicutes:Bacteroidetes ratio (F:B). In addition, to changes in Firmicutes and Bacteroidetes phyla, an increase in the relative abundance of the Verrucomicrobia phylum driven by Akkermansia, was observed. Interestingly, temozolomide (TMZ) administration hampered the changes observed in glioma-bearing mice.14 This study also demonstrated that the mice results translate to humans, as fecal samples derived from glioma patients compared to healthy controls showed similar taxa changes.14 A study evaluating fecal metabolite changes after tumor development demonstrated that the most important SCFAs (butyrate, acetate, propionate) and important neurotransmitters (norepinephrine, 5-hydroxyindoleacetic acid [5-HIAA]) were diminished after tumor development. The norepinephrine and 5-HIAA decreased levels were also identified in a small cohort of glioma patients compared to household member controls.15 In addition, a recent study by D’Alessandro et al.16 showed that glioma-bearing mice treated with antibiotics have increased tumor growth, changes in natural killer cells, and changes in microglia phenotype.16,73 Altogether, these studies demonstrate the interplay between the gut–brain axis in glioma mice models and humans (Figure 3). Moreover, these studies support the possible role of the gut microbiome in the modulation of brain tumor immunosuppression. However, studies evaluating the mechanisms in which the gut microbiome might provoke changes in the tumor microenvironment are needed (Table 1).
Figure 3.
Glioma alters the gut microbiome, fecal metabolites, and immune system. Illustrative figure derived from animal model data. Tumor growth with consequent blood–brain barrier disruption changes the relative taxa abundance (decrease in Firmicutes phylum and increase in Verrucomicrobiota and Bacteroidetes phyla) in mice. Similar results were observed in humans compared to the control in the same study. Glioma-bearing mice have been identified to present a decrease in short-chain fatty acids and important neurotransmitters (5-hydroxindoloacetic acid and norepinephrine). Moreover, it has been shown that antibiotic treatment induces NK cell impairment in glioma-bearing mice.
Table 1.
Summary of studies evaluating the role of the gut microbiome in glioma
| Study | Study subjects | Summary findings |
|---|---|---|
| D’Alessandro et al.16 | Glioma-bearing mice | Antibiotic treatment of glioma-bearing mice promoted tumor growth and changed the NK cell subsets and microglia phenotype. |
| Dono et al.15 | Glioma-bearing mice and glioma patients | Norepinephrine and 5-HIAA were decreased in mice and humans with glioma, compared to control. Additionally, glioma-bearing mice had decreased levels of SCFAs. |
| Patrizz et al.14 | Glioma-bearing mice and glioma patients | An increased in Verrucomicrobiota and Bacteroidetes and decrease in Firmicutes was observed in mice and humans. In mice, TMZ administration abrogated the microbial taxa changes. |
| Dees et al.76 | Humanized glioma-bearing mice | Humanized gut microbiome mouse models responded differently to PD-1 inhibitors. Taxa comparison between glioma-bearing mice that responded to anti-PD-1 revealed high abundance of Bacteroides cellulosilyticus. |
5-HIAA, 5-hydroxyindoleacetic acid; NK, natural killer cells; PD-1, programmed cell death protein 1; SCFA, short-chain fatty acids; TMZ, temozolomide.
The Gut Microbiome and TMZ
TMZ, part of the standard of care treatment for GBM, is a unique pharmacological oral agent. Studies have previously assessed the drug–microbiota interactions in chemotherapeutic agents.74 Cyclophosphamide (another alkylating agent) anticancer immune effects are modulated by the gut microbiome.12 A recent study evaluating the effects of TMZ on the gut microbiome identified that mice treated with serial oral TMZ gavage had significant changes in beta diversity (a measure of similarity or dissimilarity between 2 groups), Firmicutes:Bacteroidetes ratio (a ratio commonly utilized to describe dysbiosis, as it assesses the 2 most important microbiome phyla), Muribaculaceae and Ruminococcaceae families. However, these changes were not observed in a small cohort of glioma patients after TMZ and radiotherapy.14 Larger cohort studies are needed to evaluate if TMZ changes the gut microbiome of humans, as observed in mice.
The Gut Microbiome and Glioma Immunotherapy
Recent studies demonstrate how the composition of the gut microbiome in cancer patients influences the response to ICI.4,5 Importantly, the abundance of A. muciniphila has been correlated with clinical response to PD-1/PD-L1 therapy in patients with epithelial tumors.5 Furthermore, fecal transplantation from ICI responders into GF mice reversed the lack of response to PD-1 therapy associated with antibiotic-induced dysbiosis.5 To further validate this, mice receiving nonresponder microbiome were orally supplemented with A. muciniphila, acquiring sensitivity to PD-1 therapy.5 Recently, a study identified that bacterial-derived inosine promotes ICI effects in tumor models through TH-1 cell activation by a T-cell-specific A2AR in a context-dependent manner.6 Moreover, this study showed that A. muciniphila and B. pseudolongum promote ICIs effect utilizing the inosine-A2AR signaling pathway.6 This study provides a novel mechanistic by which the gut microbiome influence immunotherapy through a microbial–metabolite immune pathway.
Despite ICI’s success in many solid cancers and glioma preclinical models, little efficacy has been seen in GBM.75 Interestingly, the Akkermansia genus is increased in tumor-bearing mice and glioma patients compared to controls.14 Recent efforts have shown that individual-specific human gut microbiome influences immunotherapy response, in a humanized microbiome mouse model, utilizing healthy human donors.76 Further studies focusing on the influence of the gut microbiome in the treatment of glioma patients could potentially identify a subset of patients with a particular microbiome who may benefit from specific therapies, especially those involving the immune system (immunotherapy, viral therapy).
Intratumoral Microbiome in Gliomas
Intratumoral bacteria have been reported in several tumor types, including GBM.62 A recent study from 2 centers, evaluating intratumoral bacteria in 40 GBM tissue samples, detected 22 bacterial taxa, after stringent criteria to eliminate contaminating signals.77 Additionally, a tissue microarray of 32 GBM cases demonstrated intracellular tumor bacterial lipopolysaccharide and 16S rRNA staining in several cases.77 Although the significance of intratumoral bacteria is unknown, studies investigating its causality or leakage from ruptured vasculature and refugee due to gliomas’ immunosuppressive microenvironment are necessary. These efforts will pave our understanding of the role of the tumor microbiome in brain tumors.
Potential Opportunities and Future Perspectives
The gut microbiome offers interesting possibilities to enhance therapies that previously failed in gliomas (including GBM). Future studies should focus on identifying if gut microbiome signatures correlate with fecal metabolites and/or cytokines that might translate into survival differences in patients. Moreover, studies evaluating the potential of therapeutic modulation of the microbiome by probiotics/fecal transplant to enhance therapeutic options for gliomas (eg, immunotherapy, viral therapy) are needed. Lastly, recent studies showing the presence of bacteria in tumor tissue, oppose current views about tumors being sterile. Is the tumor microbiome related to glioma development? Or is this a result of leakage from the BBB disruption and refuge due to a glioma-induced immunosuppressive microenvironment? These and other questions that arose from the first’s studies evaluating glioma and the microbiome need to be addressed. Improving our understanding of the relationship between glioma and gut microbiome might help us in the treatment of this devastating disease.
Conclusions
In this review, we highlighted the potential implications of the gut–brain axis in neuro-oncology, in particular gliomas. Studies have demonstrated gut microbiome changes in tumor-bearing mice as well as glioma patients compared to controls. These microbiome differences might translate into fecal metabolite changes. The absence of a gut microbiome enhances tumor growth and decreases the innate immune system. Moreover, the gut microbiome might be related to the PD-L1 response in tumor-bearing mice. Understanding the synergy between the microbiome, antibiotics, intratumoral bacteria, the BBB, and the tumor microenvironment may pave the way for novel therapies. Studies evaluating the potential of therapeutic modulation of the microbiome by probiotics/fecal transplant to enhance therapeutic options for gliomas are needed.
Funding
Research reported in this publication was partly supported by the Center for Clinical and Translational Sciences, which is funded by the National Institutes of Health Clinical and Translational Award UL1 TR003167 from the National Center for Advancing Translational Sciences (Y.E.) and the NIH/NCI: K08CA241651 (L.Y.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest statement. None declared.
Authorship Statement
Study design: A.D., L.Y.B., and Y.E. Literature review: A.D., J.N., and A.G.R.-A. Manuscript writing: A.D., J.N., and A.G.R.-A. Manuscript revision and editing: A.D., B.C.M., N.J.A., L.Y.B., J.A.W., and Y.E. Study supervision: Y.E. Approved final manuscript: all authors.
References
- 1. Weller M, Wick W, Aldape K, et al. Glioma. Nat Rev Dis Prim. 2015;1:15017. doi: 10.1038/nrdp.2015.17 [DOI] [PubMed] [Google Scholar]
- 2. Spychala MS, Venna VR, Jandzinski M, et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018;84(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572(7770):474–480. [DOI] [PubMed] [Google Scholar]
- 4. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. [DOI] [PubMed] [Google Scholar]
- 6. Mager LF, Burkhard R, Pett N, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–1489. [DOI] [PubMed] [Google Scholar]
- 7. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377–388. [DOI] [PubMed] [Google Scholar]
- 11. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. [DOI] [PubMed] [Google Scholar]
- 12. Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo H, Chou WC, Lai Y, et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 2020;370(6516). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patrizz A, Dono A, Zorofchian S, et al. Glioma and temozolomide induced alterations in gut microbiome. Sci Rep. 2020;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dono A, Patrizz A, McCormack RM, et al. Glioma induced alterations in fecal short-chain fatty acids and neurotransmitters. CNS Oncol. 2020;9(2):CNS57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Alessandro G, Antonangeli F, Marrocco F, et al. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur J Immunol. 2020;50(5):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bäckhed F, Fraser CM, Ringel Y, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12(5):611–622. [DOI] [PubMed] [Google Scholar]
- 18. Huttenhower C, Gevers D, Knight R, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paoli A, Mancin L, Bianco A, et al. Ketogenic diet and microbiota: friends or enemies? Genes (Basel). 2019;10(7):534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh RK, Chang HW, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. [DOI] [PubMed] [Google Scholar]
- 23. Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73(6):1131S–1141S. [DOI] [PubMed] [Google Scholar]
- 24. Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK. Host-bacterial symbiosis in health and disease. Adv Immunol. 2010;107:243–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125(3):926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shafquat A, Joice R, Simmons SL, Huttenhower C. Functional and phylogenetic assembly of microbial communities in the human microbiome. Trends Microbiol. 2014;22(5):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turnbaugh PJ, Quince C, Faith JJ, et al. Organismal, genetic, and transcriptional variation in the deeply sequenced gut microbiomes of identical twins. Proc Natl Acad Sci U S A. 2010;107(16):7503–7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587(Pt 17):4153–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wallace BD, Wang H, Lane KT, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330(6005):831–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Das B, Nair GB. Homeostasis and dysbiosis of the gut microbiome in health and disease. J Biosci. 2019;44(5):1–8. [PubMed] [Google Scholar]
- 33. Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. [DOI] [PubMed] [Google Scholar]
- 35. Kennedy PJ, Clarke G, Quigley EM, et al. Gut memories: towards a cognitive neurobiology of irritable bowel syndrome. Neurosci Biobehav Rev. 2012;36(1):310–340. [DOI] [PubMed] [Google Scholar]
- 36. Xuelian M, Mao YK, Wang B, et al. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G868–G875. [DOI] [PubMed] [Google Scholar]
- 37. Ahluwalia V, Wade JB, Heuman DM, et al. Enhancement of functional connectivity, working memory and inhibitory control on multi-modal brain MR imaging with Rifaximin in Cirrhosis: implications for the gut-liver-brain axis. Metab Brain Dis. 2014;29(4):1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104(2):502–509. [DOI] [PubMed] [Google Scholar]
- 39. Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 40. Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014;14(6):277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009;9(suppl 1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wen J, Ding Y, Wang L, Xiao Y. Gut microbiome improves postoperative cognitive function by decreasing permeability of the blood-brain barrier in aged mice. Brain Res Bull. 2020;164(November):249–256. [DOI] [PubMed] [Google Scholar]
- 44. Wu Q, Zhang Y, Zhang Y, et al. Potential effects of antibiotic-induced gut microbiome alteration on blood-brain barrier permeability compromise in rhesus monkeys. Ann N Y Acad Sci. 2020;1470(1):14–24. [DOI] [PubMed] [Google Scholar]
- 45. Sarkaria JN, Hu LS, Parney IF, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20(2):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Idbaih A, Canney M, Belin L, et al. Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res. 2019;25(13):3793–3801. [DOI] [PubMed] [Google Scholar]
- 47. Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179–194. [DOI] [PubMed] [Google Scholar]
- 48. Li N, Wang X, Sun C, et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019;19(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Israelyan N, Margolis KG. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol Res. 2018;132 (June):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wallace CJK, Milev R. Erratum to: The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. 2017;16(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shahi SK, Freedman SN, Mangalam AK. Gut microbiome in multiple sclerosis: the players involved and the roles they play. Gut Microbes. 2017;8(6):607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sgritta M, Dooling SW, Buffington SA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101(2):246–259.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takewaki D, Suda W, Sato W, et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc Natl Acad Sci U S A. 2020;117(36):22402–22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu S, Li E, Sun Z, et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019;9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22(5):516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim MS, Kim Y, Choi H, et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut. 2020;69(2):283–294. [DOI] [PubMed] [Google Scholar]
- 58. Xie G, Zhou Q, Qiu CZ, et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J Gastroenterol. 2017;23(33):6164–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32(November):66–72. [DOI] [PubMed] [Google Scholar]
- 60. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615. [DOI] [PubMed] [Google Scholar]
- 61. Kadosh E, Snir-Alkalay I, Venkatachalam A, et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature. 2020;586(7827):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nejman D, Livyatan I, Fuks G, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Poore GD, Kopylova E, Zhu Q, et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579(7800):567–574. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64. Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183(7):4169–4175. [DOI] [PubMed] [Google Scholar]
- 66. Karin M, Jobin C, Balkwill F. Chemotherapy, immunity and microbiota—a new triumvirate? Nat Med. 2014;20(2):126–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Motzer RJ, Tannir NM, McDermott DF, et al. ; CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. [DOI] [PubMed] [Google Scholar]
- 69. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nomura M, Nagatomo R, Doi K, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020;3(4):e202895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Baruch EN, Youngster I, Ben-Betzalel G, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–609. [DOI] [PubMed] [Google Scholar]
- 73. D’alessandro G, Lauro C, Quaglio D, et al. Neuro-signals from gut microbiota: perspectives for brain glioma. Cancers (Basel). 2021;13(11):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Panebianco C, Andriulli A, Pazienza V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome. 2018;6(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Reardon DA, Brandes AA, Omuro A, et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dees KJ, Koo H, Humphreys JF, et al. Human gut microbial communities dictate efficacy of anti-PD-1 therapy in a humanized microbiome mouse model of glioma. Neurooncol Adv. 2021;3(1):vdab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yust-Katz S, Gigi E, Rosenber D, et al. TAMI-40. Tumor microbiome and glioblastoma (GBM). Neuro Oncol. 2020;22(suppl 2):ii221–ii222. [Google Scholar]