Abstract
We previously showed increased steroid-resistant CD28null CD8+ senescent lymphocyte subsets in the peripheral blood from patients with chronic obstructive pulmonary disease (COPD). These cells expressed decreased levels of the glucocorticoid receptor (GCR), suggesting their contribution to the steroid-resistant property of these cells. COPD is a disease of the small airways (SA). We, therefore, hypothesized that there would be a further increase in these steroid-resistant lymphocytes in the lung, particularly in the SA. We further hypothesized that the pro-inflammatory/cytotoxic potential of these cells could be negated using prednisolone with low-dose cyclosporin A. Blood, bronchoalveolar lavage, large proximal, and small distal airway brushings were collected from 11 patients with COPD and 10 healthy aged-matched controls. The cytotoxic mediator granzyme b, pro-inflammatory cytokines IFNγ/TNFα, and GCR were determined in lymphocytes subsets before and after their exposure to 1µM prednisolone and/or 2.5 ng/mL cyclosporin A. Particularly in the SA, COPD subjects showed an increased percentage of CD28null CD8 T-cells and NKT-like cells, with increased expression of granzyme b, IFNγ and TNFα and a loss of GCR, compared with controls. Significant negative correlations between SA GCR expression and IFNγ/TNFα production by T and NKT-like cells (eg, T-cell IFNγ R = −0.834, P = 0.031) and with FEV1 (R = −0.890) were shown. Cyclosporine A and prednisolone synergistically increased GCR expression and inhibited pro-inflammatory cytokine production by CD28null CD8− T and NKT-like cells. COPD is associated with increased pro-inflammatory CD28null CD8+ T and NKT-like cells in the SA. Treatments that increase GCR in these lymphocyte subsets may improve the efficacy of clinical treatment.
Keywords: COPD, pro-inflammatory CD28null CD8 T and NKT-like cells, small airways (SA), GCR
We previously showed increased steroid resistant CD28null CD8+ senescent lymphocyte subsets in peripheral blood from COPD patients and hypothesized that there would be a further increase in these steroid resistant lymphocytes in the lung. Particularly in the small airways, COPD subjects showed an increased percentage of CD28null CD8 T-cells and NKT-like cells, with increased expression of granzyme b, IFN? and TNFa and a loss of GCR, compared with controls. Treatments that increase GCR in these lymphocyte subsets may improve efficacy of clinical treatment in COPD.
Graphical Abstract
Graphical Abstract.
T cells are a major inflammatory cell type present in the lung in patients with chronic obstructive pulmonary disease (COPD) [1]. We previously investigated intracellular cytokine production in lymphocytes from the blood, bronchoalveolar lavage (BAL), and intraepithelial compartment obtained by bronchial brushings in patients with COPD and control groups [2]. Patients with COPD exhibited systemic inflammation as evidenced by increased IFNγ and TNFα in blood, BAL, and bronchial intraepithelial cell CD8+ T cells compared with the healthy controls. There was a negative correlation between forced expiratory volume in 1 sec (FEV1) and the percentage of BAL and bronchial intraepithelial CD8+ T cells producing TNFα [2]. Loss of CD28 co-stimulatory molecule expression is the most consistent biological indicator of premature senescent lymphocytes found in increased numbers in inflammatory syndromes due to persistent immune activation [3].
We have also shown an increase in CD28null CD8+ T and NKT-like senescent cells in the peripheral blood of patients with COPD [4–6]. These senescent cells expressed increased levels of cytotoxic mediators’ perforin and granzyme B, with increased production of pro-inflammatory cytokines IFNγ and TNFα, compared to their CD28+ counterparts [4, 5]. Reduced responsiveness to the anti-inflammatory effects of corticosteroids is a major barrier to the effective management of the majority of patients with COPD [7]. CD8+ T cells have been described as the central regulator of the inflammatory network in COPD [8]; thus targeting the inflammatory nature of these cells could be vital to reduce inflammation in patients with COPD. Our research to identify the mechanism/s of lack of steroid responsiveness in COPD recently showed that COPD was associated with loss of glucocorticoid receptor (GCR) from these pro-inflammatory senescent CD28null CD8+ T and NKT-like cells [5].
Small airway (SA) disease is a cardinal feature of COPD [9] and it has been suggested that SA disease must be targeted to attenuate the progression of COPD [9]. It is unknown whether these cytotoxic pro-inflammatory CD28null CD8+ T and NKT-like cells particularly target the SAs in COPD.
We, therefore, investigated intracellular granzyme b, IFNγ, and TNFα pro-inflammatory cytokine production by CD28± CD8+ and CD8− (CD4+) T and NKT-like lymphocyte subsets, and GCR expression in cultured peripheral blood, bronchoalveolar lavage (BAL), bronchial, and SA brushings from COPD patients and healthy controls using multiparameter flow cytometry. We hypothesized that CD28null CD8+ T and NKT-like cells, and their production of pro-inflammatory cytokines, would be increased, and GCR decreased, in intraepithelial lymphocytes from the small distal airways in patients with COPD.
Based on our previous findings of upregulation of heat shock protein 90 by blood CD28null T and NKT-like cells in the presence of 2.5 ng/mL cyclosporine A [10] we also investigated the effect of low dose cyclosporine A and standard-dose prednisolone on GCR expression and IFNγ and TNFα pro-inflammatory cytokine production by these cells.
Materials and methods
Patient and control groups
The subjects with COPD were specifically recruited for the study and informed written consent was obtained. All had no exacerbation of COPD disease for at least 6 weeks prior to this study. Subjects with other co-existing lung disease or malignancy or aged greater than 75 years were excluded. Ethics approval was obtained from the Royal Adelaide Hospital Human Ethics Committee. COPD was diagnosed using the GOLD criteria with clinical correlation [Stage I COPD: forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <70% but FEV1 ≥80% of predicted; Stage II COPD: FEV1 50–79% of predicted; Stage III COPD: FEV1 30–49% of predicted; Stage IV: <30% of predicted] [11]. The subjects with COPD were ex-smokers (>1 year) (n = 10) with an average of 39 pack years. No patients were receiving oral GCS. Healthy age-matched non-smoking volunteers (n = 11) with normal lung function and no history of lung disease were recruited as controls. All subjects underwent spirometry as a part of their routine clinical assessment. BAL, proximal, and distal brushings were collected as previously reported [12,13]. Briefly, using radiological guidance the brush tip was placed 2–3 cm from the pleural surface and SA brushings were obtained. Large airway (LA) brushings were obtained from segmented bronchi in the standard way [12].
Demographic details of the patient and control groups are shown in Table 1. Venous blood was collected into 10 U/mL preservative heparin (DBL, Sydney, Australia), and all samples were maintained at 4°C until processing. All subjects were submitted to the same protocol and analysis was performed retrospectively.
Table 1.
Demographic details of the study participants
| Subjects | Control | COPD GOLD 1 | COPD GOLD 2 | COPD GOLD 3 |
|---|---|---|---|---|
| Number | 11 | 1 | 7 | 2 |
| Age (years) | 56 (44–68)# | 51 | 54 (39-69) | 43 (39–69) |
| FEV1 % pred | 104.2 (95–103) | 82∗ | 60 (51–72)∗ | 45 (43–48)∗ |
| FEV1 % FVC | 68 (± 12) | 81∗ | 61 (36–61)∗ | 36 (35–37)∗ |
| Smoking pack years | 0 | 32 | 35 (25–44) | 34 (26–42) |
| Male/female | 5/6 | 1/0 | 3/4 | 1/1 |
Median (range)
∗Significantly decreased compared with control (P < 0.05 for all). (Wilcoxon, SPSS software).
Leucocyte counts
Full blood counts, including white cell differential counts, were determined on blood specimens using a CELL-DYN 4000 (Abbot Diagnostics, Sydney, Australia). Blood films and BAL cytospins were stained by the May-Grunwald-Giemsa method and leucocyte differential counts were checked by morphological assessment microscopically.
CD28± CD8± T, NKT-like cell subsets
Aliquots of blood were added to FACS tubes and red blood cells were lysed using FACSLyse (BD Biosciences, Sydney, Australia) as described previously [2,4,5].
BAL, and proximal and distal brushings samples, were centrifuged at 300g for 5 min. After decanting, cells were re-suspended in 2 mL of RPMI completed with 10%FCS (R10, Sigma, Sydney, Australia). Macrophages from BAL were adhered to plastic dishes incubated for 60 min in a humidified 5% CO2/95% air atmosphere at 37°C. Non-adherent cells were transferred to 10 mL sterile centrifuge tubes and cells, centrifuged at 300g for 5 min, and cells re-suspended in 2 mL of R10. All cells were permeabilized using FACSPerm (BD) as previously reported [2] then washed with wash buffer (0.5% BSA in Isoflow (Beckman Coulter, Sydney, Australia). Appropriately diluted monoclonal antibodies to CD3 perCP.CY5.5 (BD, Sydney, Australia), CD28 PECY7 (BD), CD56 APC (Beckman Coulter, Sydney, Australia), CD8 APC.CY7 (BD), and CD45 V500 (BD) were added for 15 min in the dark at RT. After further washing, cells were analysed within 1 h on a FACSCanto II flow cytometer using FACSDiva software (BD). Samples were analysed by gating lymphocytes using CD45 staining versus side scatter (SSC) as reported [2, 4,5]. A minimum of 350 000 low SSC events were acquired in list-mode format for analysis. T cells were identified as CD3+CD56−CD45+; CD8 and CD4 T-cell subsets were then identified by CD8+ and CD8− staining, NKT-like cells identified as CD3+CD56+CD45+ low FSC/SSC events [14].
Granzyme b expression in CD8+ and CD8− T, NKT-like cell subsets
Aliquots of blood, BAL, proximal and distal brushings were treated as above. Appropriately diluted monoclonal antibodies to CD3 perCP.CY5.5 (BD), CD28 PECY7 (BD), CD56 APC (Beckman Coulter), CD8 APC.CY7 (BD), granzyme B V450 (BD), and CD45 V500 (BD) were added for 15 min in the dark at RT. After further washing, cells were analysed within 1 h on a FACSCanto II flow cytometer using FACSDiva software (BD), and analysed as above.
GCR and intracellular cytokine expression in CD28± CD8± T and NKT-like cells
Blood, BAL, proximal and distal brushings were treated as above. To determine the expression of GCR and intracellular cytokine production in CD28± CD8+ and CD8− T and NKT-like cells, aliquots were stimulated as previously reported with phorbol myristate (25 ng/mL) (Sigma, Sydney, Australia) and ionomycin (1 μg/mL) (Sigma) in the presence of brefeldin A (1 μg/mL) (Sigma) and the tubes incubated in a humidified 5% CO2/95% air atmosphere at 37°C. At 16 h 100 μL of 20 mM EDTA/PBS was added to the culture tubes which were vortexed vigorously for 20 s to remove adherent cells. Red blood cells in blood were lysed and cells from all specimens permeabilized as described previously [2, 4–6]. Two millilitre of 0.5% bovine serum albumin (Sigma/Aldrich, Sydney, Australia)/Isoflow (Beckman Coulter, Sydney, Australia) was then added and the tubes were centrifuged at 300g for 5 min. After decanting the supernatant, Fc receptors were blocked with 10 μL of human immunoglobulin for 10 min in the dark at RT. After centrifuging and decanting the supernatant, 5 µL of appropriately diluted anti-GCR (clone 5E4, Serotec, Sydney, Australia; raised against a conserved sequence of the regulatory part of the receptor) as previously reported [5] was added for 15 min in the dark at RT. After a further wash, 5 μL of appropriately diluted secondary antibody rat-anti-mouse V450 (BD) was added for 15 min. After washing, appropriately diluted monoclonal antibodies to IFNγ FITC, TNFα PE, CD3 perCP.CY5.5, CD28 PE.CY7 (BD, Sydney, Australia), CD56 APC (Beckman Coulter, Sydney, Australia), CD8 APC.CY7 (BD) and CD45 V500 (BD) were added for 15 min in the dark at RT. After washing, cells were analysed within 1 h on a FACSCanto II flow cytometer using FACSDiva software (BD). Samples were analysed by gating lymphocytes using CD45 staining versus side scatter (SSC). A minimum of 350 000 low SSC events were acquired in list-mode format for analysis. T cell and NKT-like cell subsets were identified as reported above.
Effect of drugs on GCR and IFNγ/TNFα expression by in CD28± CD8± T and NKT-like cells
We have previously shown upregulation of heat shock protein 90 and a decrease in pro-inflammatory cytokine production by CD28null CD8+ T and NKT-like cells in the presence of 2.5 ng/mL cyclosporine A. We wanted to determine the effect of this very low dose cyclosporin A in combination with corticosteroid prednisolone on GCR expression by these steroid-resistant pro-inflammatory cells in the blood and the SAs. This was performed using SA lymphocytes from 3 COPD subjects only, due to restrictions of cell numbers in these samples. Aliquots of blood and distal brushings were incubated with ±1 µM prednisolone ± 2.5 ng/mL cyclosporin A in 10 mL sterile tubes in a humidified 5% CO2/95 % air atmosphere at 37oC for 24 h. These cultures were then stimulated for intracellular cytokine production then processed as for intracellular cytokines and GCR, IFNγ, and TNFα expression as described above.
Statistical analysis
Statistical analysis was performed using the non-parametric Kruskal–Wallis test. When P < 0.05, post hoc analysis was performed using the Mann–Whitney test. For post hoc analyses, all groups were compared with the healthy control subjects for all parameters. Pearson’s correlation tests were performed with SPSS software (SPSS Inc. IBM Chicago, USA) and differences between groups of P < 0.05 were considered significant.
Results
Increased CD8+, T, NKT-like, and NK cells in COPD
We noted a significant decrease in the percentage of peripheral blood CD3+ T cells and CD4+ T cells and an increase in CD8+ T cells and NKT-like cells in patients with COPD compared with the healthy control group (Table 2). There was no change in the percentage of peripheral blood NK cells between the COPD and the control group (Table 2). There was a significant decrease in the percentage of BAL CD3+ T cells and CD4+ T cells and an increase in the percentage of BAL CD8+ T, NKT-like, and NK cells in patients with COPD compared with control (Table 2). There was no change in any lymphocyte subsets in LAs between groups (Table 2). There was a significant decrease in the percentage of BAL CD3+ T cells and CD4+ T cells and an increase in the percentage of SA CD8+ T, NKT-like, and NK cells in patients with COPD compared with control (Table 2). These results are consistent with our previous reports [2,14,15].
Table 2.
Percentage of CD3+ T, CD4+ and CD8+ T, NKT-like and NK cells in blood, BAL, SA, and LA of patients with the COPD and control subjects
| COPD | CD3+ T cells | CD8+ T cells | CD4+ T cells | NKT-like cells | NK cells |
|---|---|---|---|---|---|
| Blood | ∗(31––86)# | ^75 (52–78) | ∗25 (14–32) | ^21 (6–39) | 16 (6–39) |
| BAL | ∗71 (53–90) | ^63 (58–71) | ∗34 (31–34) | ^12 (3–14) | ^15 (3–21) |
| LA | 74 (56–88) | 74 (56–88) | 22 (15–42) | 6 (1–8) | 18 (1–8) |
| SA | ∗41 (33–83) | ^75 (66–87) | ∗25 (16–31) | ^19 (5–23) | ^19 (1–23) |
| Control | CD3+ T cells | CD8+ T cells | CD4+ T cells | NKT-like cells | NK cells |
| Blood | 84 (71=89) | 48 (3–12) | 52 (28–65) | 6 (1–26) | 12 (1–26) |
| BAL | 78 (51–93) | 33 (13–68) | 66 (12–86) | 3 (1–4) | 1 (1–4) |
| LA | 77 (31–88) | 67 (31–88) | 31 (15–65) | 4 (1–7) | 17 (1–7) |
| SA | 79 (66–93) | 52 (41–69) | 48 (32–56) | 5 (2–19) | 3 (1–10) |
There was a significant decrease in the percentage of peripheral blood CD3+ T cells and CD4+ T cells and an increase in CD8+ T cells and NKT-like cells in patients with the COPD compared with the healthy control group. There was no change in the percentage of peripheral blood NK cells between COPD and control group. There was a significant decrease in the percentage of BAL, CD3+T cells, and CD4+ T cells and an increase in the percentage of BAL CD8+T, NKT-like, and NK cells in patients with COPD compared with control. There was no change in any lymphocyte subsets in LAs between groups. There was a significant decrease in the percentage of BAL CD3+T cells and CD4+ T cells and an increase in the percentage of SA CD8+T, NKT-like, and NK cells in patients with the COPD compared with control.
Median (range).
∗Significantly decreased compared with control (P < 0.05 for all).
Significantly increased compared with control (P < 0 .05 for all).
Increased CD28null CD8+ T and NKT-like cells in COPD
We noted a significant increase in the percentage of peripheral blood CD28null CD8+ T cells and CD28null CD8+ NKT-like cells in patients with COPD compared with the healthy control group (Table 3). There was no change in the percentage of peripheral blood CD28null CD8− T cells or NKT cells between the COPD and the control group (Table 3). Similarly these was a significant increase in the percentage of BAL, LA, and SA CD28null CD8+ T cells and CD28null CD8+ NKT-like cells in patients with COPD compared with the healthy control group (Table 2) but no change in CD28null CD8− (CD4+) T cells or NKT cells between COPD and control group (Table 3).
Table 3.
Percentage of CD28null CD8+ T and NKT-like cells in blood, BAL, SA, and LA from patients with COPD and control subjects
| COPD | T cells | NKT-like cells | ||
|---|---|---|---|---|
| CD28null CD8+ | CD28null CD4+ | CD28null CD8+ | CD28null CD4+ | |
| Blood | ^55 (38–63)# | 8 (3–12) | ^61 (36–71) | 6 (2–9) |
| BAL | ^62 (42–76) | 9 (4–14) | ^67 (48–91) | 7 (2–10) |
| LA | ^72 (54–92) | 7 (2–12) | ^74 (42–79) | 8 (1–14) |
| SA | ^86 (69–98)@ | 12 (3–18) | ^88 (71–99) | 11 (2–16) |
| Control | T cells | NKT-like cells | ||
| CD28null CD8+ | CD28null CD4+ | CD28null CD8+ | CD28null CD4+ | |
| Blood | 34 (18–42) | 6 (3–12) | 36 (23–44) | 5 (2–8) |
| BAL | 33 (13–46) | 6 (2–13) | 36 (18–53) | 6 (1–10) |
| LA | 31 (12–43) | 9 (3–16) | 33 (16–47) | 7 (2–14) |
| SA | 38 (22–53) | 11 (2–19) | 42 (25–62) | 10 (1–16) |
There was a significant increase in CD28null CD8+ T and NKT-like cells in blood, BAL, SA, and LA from patients with COPD compared with control subjects.
There was a significant increase in CD28null CD8+ T and NKT-like cells in SA compared with blood, BAL and LA from patients with the COPD
Median (range).
Significantly increased compared with control (P < 0.05 for all).
Significantly increased compared with blood, BAL and LA (P < 0.05 for all).
Percentages of granzyme b positive CD28± CD8± T and NKT-like subsets
There was a significant increase in the percentage of granzyme b positive blood CD28null CD8+ T and NKT-like cells in patients with COPD compared with control subjects (Table 4). Similarly, there was an increase in the percentage of granzyme b positive CD28null CD8+ T and NKT-like lymphocyte subsets from BAL, LAs, and SAs in patients with COPD compared with the control group (P < 0.05 for all) (Table 4).
Table 4.
Percentage of granzyme b positive CD28null CD8+ T and NKT-like cells in blood, BAL, SA, and LA from patients with the COPD and control subjects
| Granzyme b pos | CD28null CD8+ T cells | CD28null CD8+ NKT-like cells | ||
|---|---|---|---|---|
| Control | COPD | Control | COPD | |
| Blood | 25 (17–28) | ^70 (52–83) | 80 (38–88) | ^92 (66–98) |
| BAL | 20 (8–25) | ^50 (36–66) | 25 (12–32) | ^50 (32–68) |
| LA | 10 (2–15) | ^45 (36–61) | 15 (6–27) | ^75 (61–84) |
| SA | 8 (1–12) | ^75 (49–88) | 16 (4–23) | ^85 (68–96) |
There was a significant increase in granzyme b positive CD28null CD8+ T and NKT-like cells in blood, BAL, SA, and LA from patients with the COPD compared with control subjects.
Median (range).
Significantly increased compared with control (P < 0.05 for all).
Increased CD28± CD8± T and NKT-like lymphocyte subsets producing IFNγ and TNFα pro-inflammatory cytokines in COPD
There was an increase in the percentage of CD28null CD8+ T and NKT-like blood lymphocyte subsets producing IFNγ and TNFα pro-inflammatory cytokines in patients with COPD compared with the control group (P < 0.05 for all) (Table 5).
Table 5.
Percentage of CD28null CD8+ T and NKT-like cells producing interferon gamma (IFNγ) and tumour necrosis factor alpha (TNF α) in blood, BAL, SA, and LA from patients with the COPD and control subjects
| COPD | T cells | NKT-like cells | ||
|---|---|---|---|---|
| CD28null CD8+ | CD28null CD8+ | |||
| IFNγ | TNFα | IFNγ | TNFα | |
| Blood | ^55 (38–63)# | ^58 (42–66) | ^61 (36–71) | ^66 (38–73) |
| BAL | ^62 (42–76) | ^59 (39–73) | ^67 (48–91) | ^69 (41–78) |
| LA | ^72 (54–92) | ^67 (51–88) | ^74 (42–79) | ^78 (52–84) |
| SA | ^86 (69–98)@ | ^92 (72–97)@ | ^88 (71–99)@ | ^91 (69–96)@ |
| Control | T cells | NKT-like cells | ||
| CD28null CD8+ | CD28nullCD8+ | |||
| IFNγ | TNFα | IFNγ | TNFα | |
| Blood | 34 (18–42) | 36 (20–45) | 36 (23–44) | 35 (16–48) |
| BAL | 33 (13–46) | 33 (12–33) | 36 (18–53) | 36 (13–46) |
| LA | 31 (12–43) | 39 (14–41) | 33 (16–47) | 37 (13–51) |
| SA | 38 (22–53) | 31 (12–38) | 42 (25–62) | 30 (11–40) |
There was a significant increase in CD28null CD8+ T and NKT-like cells producing IFNγ and TNFα in blood, BAL, SA, and LA from patients with the COPD compared with control subjects. There was a significant increase in CD28null CD8+ T and NKT-like cells producing IFNγ and TNFα in SA compared with blood, BAL, and LA from patients with the COPD.
Median (range).
Significantly increased compared with control (P < 0.05 for all).
Significantly increased compared with blood, BAL and LA (P < 0.05 for all).
Similarly there was an increase in the percentage of CD28null CD8+T and NKT-like lymphocyte subsets from BAL, LA, and SA producing IFNγ and TNFα in patients with COPD compared with control group (P < 0.05 for all). (Table 5). There was a significant increase in the percentage of CD28null CD8+ T and NKT-like cells producing IFNγ/TNFα in SAs compared with LAs, BAL and blood in patients with COPD (Table 5) (P < 0.05 for all).
Percentages of CD28± CD8± T and NKT-like blood lymphocyte subsets expressing GCR
There was no difference in the percentage of CD28null CD8+ T and NKT-like lymphocyte subsets expressing GCR in patients with COPD compared with the control group (P < 0.05 for all) in either blood, BAL, LA or SAs ie, all CD28null CD8+T and NKT-like lymphocytes express reduced GCR regardless of the subject.
Correlation between SA CD28null CD8+GCR+T cells producing IFNγ/TNFα and patient FEV1
There was a negative correlation between the percentage of blood and SA CD28null CD8+ intraepithelial T-cells expressing GCR and producing IFNγ and TNFα in patients with COPD. Data for small airway GCR and IFNγ and TNFα are shown in Fig. 1a and b respectively.
Figure 1.
Negative correlation between the percentage of SA CD28null CD8+ intraepithelial T cells CD28null CD8+ intraepithelial T cells expressing GCR and producing IFNγ (a) and TNFα (b) in patients with COPD.
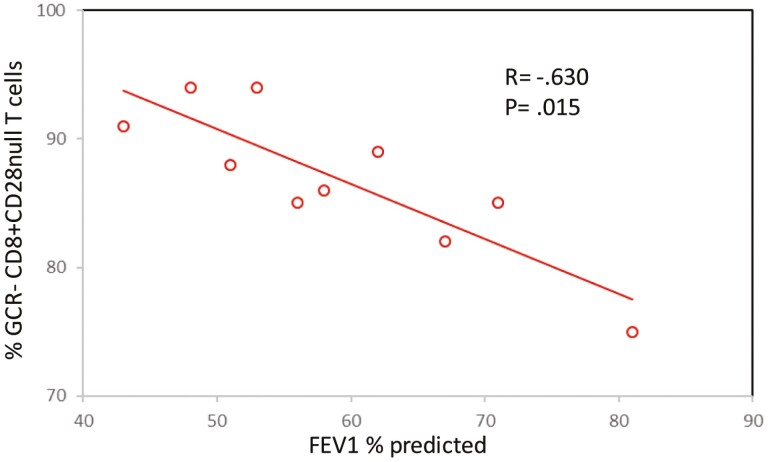
A negative correlation was identified between FEV1 and the percentage of blood and SA CD28null CD8+ T cells expressing GCR in patients with COPD. Data for SA FEV1 and GCR are shown in Fig. 2. There was no correlation between any other lymphocyte subset and FEV1 (P > 0.05 for all). There was no correlation between age or smoking history and blood or SA CD28null CD8+ intraepithelial T-cells expressing GCR and producing IFNγ and TNFα in patients with COPD.
Figure 2.
Negative correlation between FEV1 and the percentage of SA CD28null CD8+ T cells expressing GCR in patients with COPD.
Effect of 1 µM prednisolone ±2.5 ng/mL cyclosporin A on IFNγ/TNFα production by CD28null CD8+ T cells
Based on our previous findings of upregulation of heat shock protein by blood CD28null T and NKT-like cells in the presence of 2.5 ng/mL cyclosporine A [10], we also investigated the effect of low dose cyclosporine A and standard-dose prednisolone (1 µM) on GCR expression and IFNγ and TNFα pro-inflammatory cytokine production by these cells in vitro. In SA brushings, there were insufficient lymphocytes to investigate the effect of these two immunosuppressants individually. We, therefore, investigated the combined effects of 1 µM prednisolone + 2.5 ng/mL cyclosporine A in SA-derived lymphocytes, and in whole blood. The addition of 1 µM prednisolone and/or 2.5 ng/mL cyclosporine A resulted in the upregulation of GCR and decreased production of IFNγ/TNFα production by blood CD28null CD8+ T and NKT-like cells. The effect was synergistic when both immunosuppressants were used. The addition of 1 µM prednisolone and 2.5 ng/mL cyclosporine A resulted in the upregulation of GCR and decreased production of IFNγ/TNFα by SA intraepithelial CD28null T and NKT-like cells. Representative plots from a patient with COPD showing the combined effect of 1 µM prednisolone and 2.5 ng/mL cyclosporine A on the upregulation of GCR and decreased production of IFNγ by SA CD28null CD8+ T and NKT-like cells are shown in Fig. 3.
Figure 3.
Representative plots from patients with COPD showing the combined effect of 1 µM prednisolone and 2.5 ng/mL cyclosporine A on the upregulation of GCR and decreased production of IFNγ/TNFα by SA CD28null CD8+ T cells.
There was no difference in the combined effect of 1 µM prednisolone and 2.5 ng/mL cyclosporine A on the upregulation of GCR and decreased production of IFNγ/TNFα by CD28null T and NKT-like cells from blood or SA lymphocytes (P > 0.05 for all). The effect of 1 µM prednisolone, 2.5 ng/mL cyclosporine A, and 1 µM prednisolone + 2.5 ng/mL cyclosporine A compared with no drugs, on the percentage decrease in IFNγ production (Fig 4a), decrease in TNFα production (Fig 4b) and increase in GCR expression (Fig 4b) in blood CD28null T cells from all patients with COPD.
Figure 4.
Effect of 1 µM prednisolone, 2.5 ng/mL cyclosporine A, and 1 µM prednisolone + 2.5 ng/mL cyclosporine A compared with no drugs, on the percentage decrease in IFNγ production (a), decrease in TNFα production (b), and increase in GCR expression (b) in blood CD28null T cells from all patients with COPD.
Discussion
This is the first study to show an increase in intra-epithelial cytotoxic/pro-inflammatory lymphocyte subsets in the SAs compared with blood, BAL and LAs in patients with COPD. A previous study showed that CD8+ T cells are required for inflammation and lung destruction in cigarette smoke-induced emphysema in mice [8], indicating CD8+ T cells are a central regulator of the inflammatory network in COPD. Senescent CD28null T and NKT-like cells have been shown to be more pro-inflammatory and cytotoxic than their CD28 positive counterparts, show cytotoxicity to lung epithelial cells [15], and exhibit relative resistance to corticosteroids [5, 10, 14, 16–19]. We had previously shown that the CD8+ T cells from patients with COPD produce increased IFNγ and TNFα from blood, BAL, and bronchial brushings compared with aged-matched controls [2] and our current study further shows that CD28null CD8+ T and NKT-like cells are the most pro-inflammatory lymphocyte subsets in COPD. Their increase in the COPD lung supports the emerging concept of accelerated ageing and accumulation of aged cells in the lung of the patients with COPD [20]. Furthermore, in line with the notion of COPD as a disease of SAs, these senescent cells are in the greatest numbers in the small distal airways compared with blood and other areas of the lungs.
We showed that the percentage of T cells expressing GCR in the SAs is reduced, likely resulting in a reduced capacity to respond to corticosteroids, the mainstay of anti-inflammatory medication in COPD. Importantly, the loss of GCR by the increased numbers of senescent CD28null CD8+ T and NKT-like cells was shown to correlate with COPD disease severity. These findings go a long way in explaining why current anti-inflammatory treatments with glucocorticoids are ineffective in the treatment of patients with COPD [7] particularly in the SAs. There have been several theories suggested for the poor response of patients with COPD to the anti-inflammatory effects of glucocorticoids [7]. For glucocorticoids to be therapeutically effective they must first enter the cell and bind to the GCR. This complex is taken into the nucleus by various chaperones such as HSP90 and sirtuin-1 where HDAC2 is engaged to switch off pro-inflammatory cytokine production. We have shown that many steps are involved in the reduced responsiveness of senescent CD28null CD8+ T and NKT-like cells to steroids. The drug efflux pump Pgp1 is increased in peripheral blood T, NKT-like, and NK-cells from subjects with COPD [16]. However, there was no difference in Pgp-1 levels between CD28+ and CD28null T and NKT-like cells [16] suggesting this is not involved in GC resistance by these cells in COPD. We, however, did show that there are reduced levels of Hsp90 [10], sirtuin-1 [18], and HDAC2 [19] in CD28null CD8+ T and NKT-like cells compared with their CD28+ counterparts. Interestingly there were no differences between levels of these proteins in CD28null and CD28+ lymphocytes between patients with COPD and healthy control subjects although the percentages of CD28null lymphocytes were significantly greater in subjects with COPD. Unfortunately, lymphocyte cell numbers from the SA brushings were insufficient to investigate all patients with COPD and further mediators of steroid resistance including HSP90, sirtuin-1, and HDAC2. However, given the similar results in the blood of patients with COPD from our previous studies, it is highly likely that these other mechanisms of steroid-resistant are also present in these lymphocyte subsets in the SAs. Our finding that the addition of very low dose cyclosporine A resulted in increased expression of GCR in senescent CD28null CD8+ T and NKT-like cells is very significant given our previous findings of decreased PGP-1 [16] expression and increased HSP90 [10], sirtuin-1[18], and HDAC2 [19] also in the presence of this drug. The very low dose of cyclosporine A (2.5 ng/mL) is unlikely to cause any significant side effects known to be associated with the much higher levels of this drug used to prevent lung transplant rejection (80–250 ng/mL). Our findings argue for a larger clinical randomized control trial using low dose cyclosporine ± standard glucocorticoid dose examining pro-inflammatory cytokine production and levels of GCR, PGP-1, HSP-90, sirtuin-1, and HDAC2 in these cells. One could also speculate that these CD28null lymphocytes may be the precursor to other systemic GC-resistant diseases such as CVD, autoimmune disease, arthritis, IBD, ageing, and ageing associated with COPD [3,20–24]. Others have shown increased CD8+ T cells in the SAs of patients with severe COPD compared with mild COPD consistent with our findings [25].
We have previously used the technique of comparing pro-inflammatory intra-epithelial T cells between trachea and bronchi to identify COPD severity consistent with our current study [26]. An important addition to our current study would be the addition of a non-COPD smoker group and a COPD-smoker group to identify changes that may be attributed to smoking alone. A COPD group receiving inhaled steroids would also allow the determination of any effects of steroids on pro-inflammatory cytokine production by the various lymphocyte subsets.
In this regard, we have previously shown an increase in cytotoxic/pro-inflammatory CD8+ T cells in the blood of patients diagnosed with another SAs disease, bronchiolitis obliterans syndrome (BOS) following lung transplantation [27]. Importantly, these changes were noted up to 18 months preceding a fall in lung function of these patients suggesting phenotypic analysis of these cells in the blood may be predictive of pending BOS [27, 28]. A similar study determining granzyme b, GCR expression, and IFNγ/TNFα production in CD28null CD8+T and NKT-like cells in the SAs and blood in smokers who have not progressed to COPD would be important. There are currently no tests available that we are aware of to determine if smokers will develop COPD before a change in airway resistance or fall in lung function [29]. There is a need to specifically target SA disease to attenuate the progression of COPD [9, 29].
In conclusion, COPD is associated with increased intra-epithelial cytotoxic/pro-inflammatory CD28null CD8+ T and NKT-like in the blood, BAL, LA, and SAs, the latter being the largest site of these cell accumulation. Treatments that increase GCR in these lymphocyte subsets in the blood and airways in patients with COPD may improve (morbidity in COPD patients-omit) efficacy of clinical treatment.
Acknowledgements
The authors acknowledge the bronchoscopy suite team at the Royal Adelaide Hospital for helping them by supplying the patient samples.
Glossary
Abbreviations
- COPD
chronic obstructive pulmonary disease
- GCR
glucocorticoid receptor
- BAL
bronchoalveolar lavage
- IFNγ
interferon gamma
- TNFα
tumour necrosis factor alpha
- NKT-like
natural killer T-like
Contributor Information
Greg Hodge, Department of Thoracic Medicine, Royal Adelaide Hospital, Adelaide, South, Australia; Department of Medicine, University of Adelaide, Adelaide, South, Australia.
Hubertus Jersmann, Department of Thoracic Medicine, Royal Adelaide Hospital, Adelaide, South, Australia; Department of Medicine, University of Adelaide, Adelaide, South, Australia.
Hai B Tran, Department of Thoracic Medicine, Royal Adelaide Hospital, Adelaide, South, Australia.
Patrick F Asare, Department of Medicine, University of Adelaide, Adelaide, South, Australia.
Minnu Jayapal, Department of Medicine, University of Adelaide, Adelaide, South, Australia.
Paul N Reynolds, Department of Thoracic Medicine, Royal Adelaide Hospital, Adelaide, South, Australia; Department of Medicine, University of Adelaide, Adelaide, South, Australia.
Mark Holmes, Department of Thoracic Medicine, Royal Adelaide Hospital, Adelaide, South, Australia; Department of Medicine, University of Adelaide, Adelaide, South, Australia.
Sandra Hodge, Department of Thoracic Medicine, Royal Adelaide Hospital, Adelaide, South, Australia; Department of Medicine, University of Adelaide, Adelaide, South, Australia.
Funding
S.H. was funded by a University of Adelaide fellowship; H.T. by a Royal Adelaide Hospital Brine bequest; P.A. by a University of Adelaide postgraduate scholarship.
Competing interests
The authors declare they have no competing interests.
Author contributions
G.H. performed the concept and design of experiments, analysis, and interpretation of data and manuscript preparation; H.J. supplied and characterized patient specimens and helped draft the manuscript; H.T. helped with experiments and helped draft the manuscript; P.F.A. helped with experiments and helped draft the manuscript; M.J. helped with experiments and helped draft the manuscript; P.N.R. supplied and characterized patient specimens and helped draft the manuscript; M.H. supplied and characterized patient specimens and helped draft the manuscript. S.H. helped with study design, statistical analyses and helped draft the manuscript. All authors read and approved the final manuscript.
Declarations
Ethics approval and consent to participate. The subjects with COPD were specifically recruited for the study and informed written consent was obtained. Ethics approval was obtained from the Royal Adelaide
References
- 1. Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003, 22, 672–88. [DOI] [PubMed] [Google Scholar]
- 2. Hodge G, Nairn J, Holmes M, et al. Increased intracellular Th1 pro-inflammatory cytokine production in peripheral blood, bronchoalveolar lavage and intraepithelieal T cells of COPD subjects. Clin and Exp Immunol 2007, 150, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev 2005, 205, 158–69. [DOI] [PubMed] [Google Scholar]
- 4. Hodge G, Mukaro V, Reynolds PN, Hodge S. Role of increased CD8/CD28(null) T cells and alternative co-stimulatory molecules in chronic obstructive pulmonary disease. Clin Exp Immunol 2011, 166, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodge G, Jersmann H, Tran HB, Holmes M, Reynolds PN, Hodge S. Lymphocyte senescence in COPD is associated with loss of glucocorticoid receptor expression by pro-inflammatory/cytotoxic lymphocytes. Respir Res 2015, 16, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodge S, Hodge G, Nairn J, Holmes M, Reynolds PN. Increased airway granzyme b and perforin in current and ex-smoking COPD subjects. COPD 2006, 3, 179–87. [DOI] [PubMed] [Google Scholar]
- 7. Barnes PJ. Glucocorticoids: current and future directions. Brit J Pharmacol 2011, 163, 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol 2007, 178, 8090–6. [DOI] [PubMed] [Google Scholar]
- 9. Stewart JI, Criner GJ. The small airways in chronic obstructive pulmonary disease: pathology and effects on disease progression and survival. Curr Opin Pulm Med 2013, 19, 109–15. [DOI] [PubMed] [Google Scholar]
- 10. Hodge G, Roscioli E, Jersmann H, Tran HB, Holmes M, Reynolds PN, et al. Steroid resistance in COPD is associated with impaired molecular chaperone Hsp90 expression by pro-inflammatory lymphocytes. Respir Res 2016, 17, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. ; ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011, 365, 1184–92. [DOI] [PubMed] [Google Scholar]
- 12. Banerjee B, Kicic A, Musk M, Sutanto EN, Stick SM, Chambers DC. Successful establishment of primary small airway cell cultures in human lung transplantation. Respir Res 2009, 10, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hodge G, Hodge S, Liu H, Nguyen P, Holmes-Liew CL, Holmes M. Bronchiolitis obliterans syndrome is associated with increased senescent lymphocytes in the small airways. J Heart Lung Transplant 2021, 40, 108–19. [DOI] [PubMed] [Google Scholar]
- 14. Hodge G, Hodge S. Steroid resistant CD8+CD28null NKT-like pro-inflammatory cytotoxic cells in chronic obstructive pulmonary disease. Frontiers in Immunol 2016, 7, 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hodge G, Mukaro V, Holmes M, Reynolds PN, Hodge S. Enhanced cytotoxic function of natural killer and natural killer T-like cells associated with decreased CD94 (Kp43) in the chronic obstructive pulmonary disease airway. Respirology 2013, 18, 369–76. [DOI] [PubMed] [Google Scholar]
- 16. Hodge G, Holmes M, Jersmann H, Reynolds P, Hodge S. The drug efflux pump in pro-inflammatory lymphocytes is a target for novel treatment strategies in COPD. Resp Res 2013, 14, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodge G, Hodge S. Therapeutic targeting steroid resistant pro-inflammatory NK and NKT-like cells in chronic obstructive pulmonary disease. Int J Mol Sci 2019, 20, 1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hodge G, Tran HB, Reynolds PN, Jersmann H, Hodge S. Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes. Ther Adv Respir Dis 2020, 14, 1753466620905280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hodge G, Jersmann H, Tran HB, et al. Lymphocyte senescence in COPD is associated with decreased histone deacetylase 2 expression by pro-inflammatory lymphocytes. Resp Res 2015, 16, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barnes PJ. Targeting cellular senescence as a new approach to chronic obstructive pulmonary disease therapy. Curr Opin Pharmacol 2021, 56, 68–73. [DOI] [PubMed] [Google Scholar]
- 21. Téo FH, de Oliveira RT, Mamoni RL, Ferreira MC, Nadruz W Jr, Coelho OR, et al. Characterization of CD4+ CD28null T cells in patients with coronary artery disease and individuals with risk factors for atherosclerosis. Cell Immunol 2013, 281, 11–9. [DOI] [PubMed] [Google Scholar]
- 22. Thewissen M, Somers V, Hellings N, Fraussen J, Damoiseaux J, Stinissen P. CD4+ CD28null T cells in autoimmune disease: pathogenic features and decreased susceptibility to immunoregulation. J Immunol 2007, 179, 6514–23. [DOI] [PubMed] [Google Scholar]
- 23. Fasth AE, Snir O, Johansson AA, Nordmark B, Rahbar A, Af Klint E, et al. Skewed distribution of proinflammatory CD4+ CD28 null T cells in rheumatoid arthritis. Arthritis Res Ther 2007, 9, R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yokoyama Y, Fukunaga K, Ikeuchi H, Kamikozuru K, Hida N, Ohda Y, et al. The CD4+ CD28 null and the regulatory CD4+ CD25 high T-cell phenotypes in patients with ulcerative colitis during active and quiescent disease, and following colectomy. Cytokine 2011, 56, 466–70. [DOI] [PubMed] [Google Scholar]
- 25. Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, et al. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998, 157, 822–6. [DOI] [PubMed] [Google Scholar]
- 26. Hodge G, Reynolds PN, Holmes M, Hodge S. Differential expression of pro-inflammatory cytokines in intra-epithelial T cells between trachea and bronchi distinguishes severity of COPD. Cytokine 2012, 60, 843–8. [DOI] [PubMed] [Google Scholar]
- 27. Hodge G, Hodge S, Chambers D, Reynolds PN, Holmes M. Bronchiolitis obliterans syndrome is associated with absence of suppression of peripheral blood Th1 proinflammatory cytokines. Transplantation 2009, 88, 211–8. [DOI] [PubMed] [Google Scholar]
- 28. Hodge S, Hodge G, Ahern J, Liew CL, Hopkins P, Chambers DC, et al. Increased levels of T cell granzyme b in bronchiolitis obliterans syndrome are not suppressed adequately by current immunosuppressive regimens. Clin Exp Immunol 2009, 158, 230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higham A, Quinn AM, Cancado JED, Singh D. The pathology of small airways disease in COPD: Historical aspects and future direction. Resp Res 2019, 20, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]