Abstract
Pseudomonas aeruginosa is a common respiratory pathogen that causes injurious airway inflammation during acute pneumonia. Peroxisome proliferator-activated receptor (PPAR)-γ is involved in the regulation of metabolic and inflammatory responses in different cell types and synthetic agonists of PPAR-γ exert anti-inflammatory effects on myeloid cells in vitro and in models of inflammation in vivo. We sought to determine the effect of the PPAR-γ agonist pioglitazone on airway inflammation induced by acute P. aeruginosa pneumonia, focusing on bronchial epithelial cells. Mice pretreated with pioglitazone or vehicle (24 and 1 h) were infected with P. aeruginosa via the airways. Pioglitazone treatment was associated with increased expression of chemokine (Cxcl1, Cxcl2, and Ccl20) and cytokine genes (Tnfa, Il6, and Cfs3) in bronchial brushes obtained 6 h after infection. This pro-inflammatory effect was accompanied by increased expression of Hk2 and Pfkfb3 genes encoding rate-limiting enzymes of glycolysis; concurrently, the expression of Sdha, important for maintaining metabolite flux in the tricarboxylic acid cycle, was reduced in bronchial epithelial cells of pioglitazone treated-mice. Pioglitazone inhibited bronchoalveolar inflammatory responses measured in lavage fluid. These results suggest that pioglitazone exerts a selective proinflammatory effect on bronchial epithelial cells during acute P. aeruginosa pneumonia, possibly by enhancing intracellular glycolysis.
Keywords: pioglitazone, Pseudomonas aeruginosa, innate immunity, inflammation
Graphical Abstract
Graphical Abstract.
The peroxisome proliferator-activated receptor (PPAR)-? is involved in the regulation of metabolic and inflammatory responses and here we investigated the effect of the PPAR-? agonist pioglitazone on airway inflammation induced by Pseudomonas aeruginosa, focusing on bronchial epithelial cells. Mice pretreated with Pioglitazone presented with increased expression of chemokine and cytokine genes in bronchial brushes obtained 6 hours after infection, accompanied by increased expression of genes encoding rate limiting enzymes of glycolysis; suggesting that pioglitazone exerts proinflammatory effects on bronchial epithelial cells during acute P. aeruginosa pneumonia.
Introduction
Pseudomonas (P.) aeruginosa is a Gram-negative bacterium that causes severe infections of the lower respiratory tract in patients with a compromised immune system or impaired airway clearance mechanisms [1, 2]. These infections are associated with high mortality rates worldwide [1, 3]. Antibiotics, the first-line treatment for lower respiratory tract infections, exclusively target the bacterium, and the emergence of multi-drug resistant P. aeruginosa strains has become a major burden [1, 2, 4]. Hence, the development of new therapeutic strategies able to modulate the host immune response during P. aeruginosa pneumonia is of great interest. P. aeruginosa lung infections have been reported to induce a hyperinflammatory state that can injure the airways [5, 6]. Therefore, targeting exacerbated inflammatory responses during Pseudomonas pneumonia represents a potential novel therapeutic option that might improve the clinical outcome of patients suffering from this infection.
The peroxisome proliferator-activated receptor (PPAR)-γ is a major regulator of cell metabolism and inflammatory responses in different cell types [7, 8]. Thiazolidinediones are synthetic agonists of PPAR-γ that were originally designed as anti-diabetics [7]. However, due to their anti-inflammatory effects PPAR-γ agonists have received much attention as potential drugs capable of modulating airway inflammatory responses in the context of bacterial infections [8–11]. Treatment with the PPAR-γ agonist pioglitazone of mice with P. aeruginosa pneumonia reduced tumor necrosis factor (TNF)-α and interleukin (IL)-1β release in bronchoalveolar lavage fluid (BALF) [12]. Moreover, PPAR-γ activation augmented phagocytosis and killing of P. aeruginosa by macrophages, and pretreatment with the PPAR-γ agonist pioglitazone resulted in reduced bacterial burdens in the lungs of mice infected with this bacterium via the airways [13].
The respiratory epithelium plays a major role in innate host defense during P. aeruginosa pneumonia, at least in part through recognition of flagellin, the principal component of the flagellum of Pseudomonas, by epithelial toll-like receptor 5 [14–18]. Knowledge of the effect of PPAR-γ agonists on the respiratory epithelium during bacterial infection of the airways is limited. Therefore, we here sought to determine the in vivo effect of pioglitazone on airway epithelial cells during murine P. aeruginosa-induced lung infection and inflammation.
Materials and methods
Ethical statement
All experiments were reviewed and approved by the Central Authority for Scientific Procedures on Animals (CCD) and the Animal Welfare Body (IvD) of the Amsterdam-UMC, University of Amsterdam. The animal care and use protocol adhered to the Dutch Experiments on Animals Act (WOD) and European Directive of 22 September 2010 (Directive 2010/63/EU) in addition to the Directive of 6 May 2009 (Directive 2009/41/EC).
Animals
C57BL/6NCrl mice were purchased from Charles River (Maastricht, The Netherlands). Mice were kept in the Animal Research Institute Amsterdam facility in Amsterdam UMC under standard care. All experiments were carried out with 8–10-week-old female mice.
P. aeruginosa infection
P. aeruginosa (PAK) was kindly provided by Mustapha Si-Tahar (INSERM, Tours, France). P. aeruginosa was cultured in Luria-Bertani (LB) medium at 37ºC to log-phase; 5 × 106 colony-forming units (CFU) in 50 μl of normal saline was administered intranasally as described previously [18, 20, 22]. Twenty four and one hour prior to the infection, mice received 20 mg/kg body weight of pioglitazone (or vehicle; 10% DMSO in PBS) by intraperitoneal injection, a dose previously shown to exert an anti-inflammatory effect in a model of Klebsiella-induced pneumonia and sepsis [9]. Mice were terminated 6 h after induction of pneumonia.
BALF and epithelial brushes
BALF was collected by flushing the lungs via the trachea twice with 500 μl of 0.2 mM EDTA/PBS. Thereafter, epithelial brushes were performed as described by Chen K. et al. [34]. Briefly, PE50 tubing was sanded with sandpaper (P240) and treated with RNaseZap (Thermo Fisher Scientific, Waltham, MA, USA) before being inserted into the main bronchus via the trachea to collect epithelial tissue for RNA extraction. BALF was spun down at 1250 RPM for 10 min at 4ºC, supernatants were stored for cytokine determination and cells were used to determine total cell counts (Beckman Coulter, Fullerton, CA, USA) and FACs analysis as described below.
Chemokine and cytokine measurements
All chemokines and cytokines were measured by mouse-specific ELSA’s according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Flow cytometry
Cells were stained and analyzed on a FACS Canto II cytometer (BD Biosciences). The following antibodies were purchased from BD Biosciences unless otherwise listed: CD45 (30-F11), CD11c (HL3), CD11b (M1/70), Siglec-F (E50-2440), Ly-6C (AL-21), and Ly-6G (1A8; Biolegend, San Diego, CA, USA). Fixable Viability Dye kit (BD Biosciences) was used to exclude dead events. After gating for live CD45+ cells, alveolar macrophages were defined as CD11c+SiglecF+CD11b− cells; neutrophils as CD11c−CD11b+Ly6G+ cells; and monocytes as CD11c−CD11b+Ly6G- cells. Ly6C expression was used to differentiate patrolling (Ly6C−) from inflammatory (Ly6C+) monocytes. Data were analyzed using FlowJo software (Treestaar Inc, Ashland, OR, USA).
mRNA extraction and RT-PCR
Total RNA from epithelial brushes was isolated using a Nucleospin RNA isolation kit (Macherey-Nagel, Düren, Germany) following the manufacturer’s instructions. cDNA was synthetized using the M-MLV Reverse Transcriptase kit (Promega, Madison, WI, USA) in the presence of RNase inhibitor (Thermo Fisher Scientific) with 300 ng of DNase I (Roche) treated total RNA. qPCR was performed on LightCycler 480 (Roche) using the SensiFAST SYBR No-ROX Kit (Bioline, London, UK). Data were normalized to Actb as a housekeeping gene. Primers used in this study are listed in Table 1.
Table 1:
Primer sequences for the quantitative RT-PCR.
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| Actb | CTCTGGCTCCTAGCACCATGAAGA | GTAAAACGCAGCTCAGTAACAGTCCG |
| Pparg | CCTGCGGAAGCCCTTTGGTGACT | CCTCGATGGGCTTCACGTTCAGCA |
| Cxcl1 | CCACTGCACCCAAACCGAAG | TCCGTTACTTGGGGACACCT |
| Cxcl2 | CACTCTCAAGGGCGGTCAA | TCTTTGGTTCTTCCGTTGAGG |
| Ccl20 | AGACAGATGGCCGATGAAGC | CTGCTTTGGATCAGCGCACA |
| Tnfa | CGAGTGACAAGCCTGTAGCC | CCTTGAAGAGAACCTGGGAGT |
| Il6 | CTTCCTACCCCAATTTCCAATGCT | TCTTGGTCCTTAGCCACTCCTT |
| Il1b | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| Csf3 | CCTGGAGCAAGTGAGGAAGA | CAGCTTGTAGGTGGCACACA |
| Hk2 | GTGTGCTCCGAGTAAGGGTG | CAGGCATTCGGCAATGTGG |
| Pfkfb3 | CAACTCCCCAACCGTGATTGT | TGAGGTAGCGAGTCAGCTTCT |
| Sdha | GGAACACTCCAAAAACAGACCT | CCACCACTGGGTATTGAGTAGAA |
| Cd36 | ATGGGCTGTGATCGGAACTG | GTCTTCCCAATAAGCATGTCTCC |
| Acsl1 | TGCCAGAGCTGATTGACATTC | GGCATACCAGAAGGTGGTGAG |
| Cpt1 | CTCCGCCTGAGCCATGAAG | CACCAGTGATGATGCCATTCT |
Statistical analysis
All the analyses were done using GraphPad Prism 7.03. The number of replicates and the statistical tests used for each data is described in the figure legends. In most cases, Welch’s t-test was used. A P value < 0.05 was considered statistically significant.
Results
Infection with P. aeruginosa reduces Pparg expression in bronchial epithelial cells in vivo
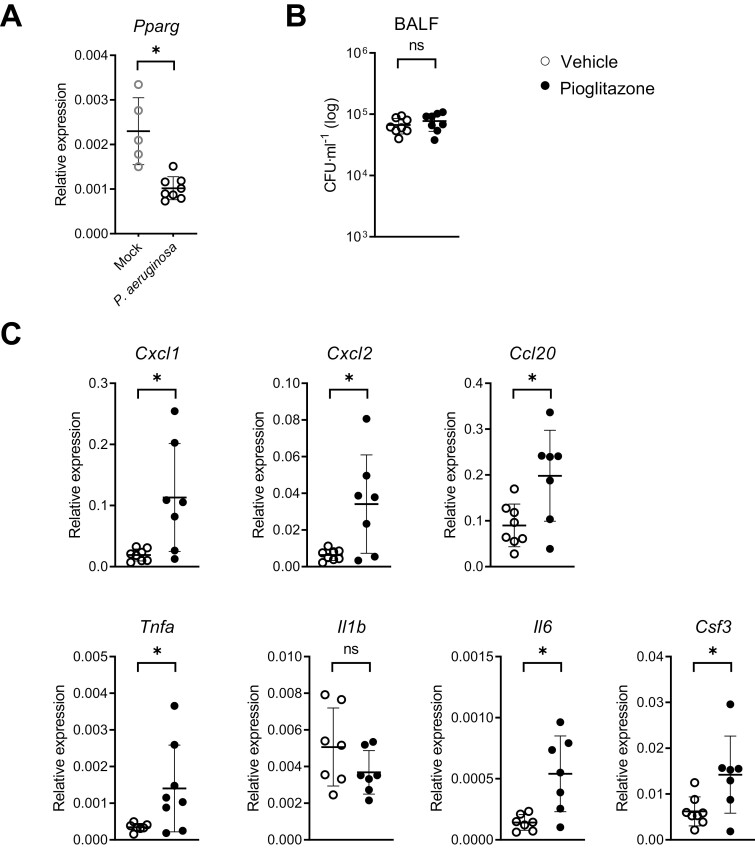
Previous studies reported decreased expression of PPARG in BALF cells from patients infected with P. aeruginosa [19] and in in vitro infected macrophages [13]. To determine the effect of P. aeruginosa infection on Pparg expression in airway epithelial cells in vivo, we inoculated mice intranasally with this bacterium and collected bronchial brushes 6 thereafter. Our group previously showed that bronchial brushes are highly enriched for bronchial epithelial cells as determined by the expression of the pan-epithelial cell marker Epcam and the club cell marker Scgb1a1 [20–22]. Bronchial brushes had reduced Pparg expression after infection with P. aeruginosa relative to uninfected mice (Fig. 1A).
Figure 1:
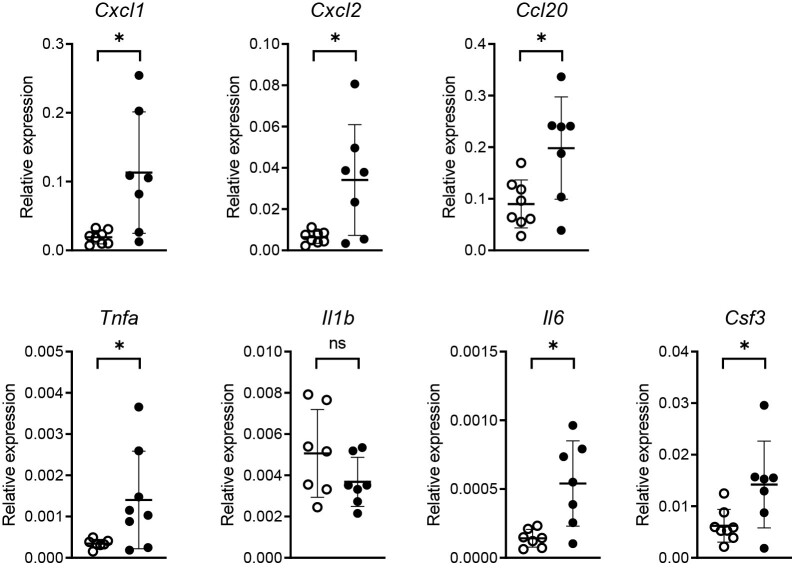
(A) Relative mRNA expression for Pparg analyzed by RT-PCR in lung epithelial brushes 6 h after P. aeruginosa infection or PBS administration (mock) via the airways. (B) Bacterial loads in BALF in vehicle and pioglitazone treated mice 6 h following induction of pneumonia. (C) Relative mRNA expression for the indicated immune-mediators analyzed by RT-PCR in lung epithelial brushes of mice 6 h after P. aeruginosa infection pre-treated with pioglitazone or vehicle. Graphs show mean and SD, and every dot represents one individual mouse. P values were calculated using Welch’s t-test. ∗P < 0.05, ns (not significant).
Pioglitazone enhances the expression of chemokine and cytokine genes in bronchial epithelial cells during acute P. aeruginosa pneumonia
To obtain insight into the effect of PPAR-γ stimulation in the respiratory epithelium during acute Pseudomonas pneumonia we treated mice with the PPAR-γ agonist pioglitazone or vehicle 24 and 1 h prior to P. aeruginosa infection via the airways and collected bronchial brushes and BALF 6 h thereafter. Pioglitazone did not affect bacterial loads in BALF (Fig. 1B), allowing an unbiased comparison of inflammatory responses between treatment groups (i.e. not confounded by different proinflammatory environments secondary to differences in bacterial numbers). Our group recently reported that acute Pseudomonas pneumonia is associated with a strong induction of genes encoding mediators of mucosal immunity in bronchial epithelial cells relative to bronchial brushes from uninfected control mice [20, 21]. Relative to vehicle control treatment, pioglitazone administration enhanced the expression of chemokine genes Cxcl1, Cxcl2, and Ccl20 and cytokine genes Tnfa, Il6, and Csf3 in bronchial epithelial cells, while not affecting Il1b expression (Fig. 1C).
Effect of pioglitazone on bronchoalveolar inflammatory responses during acute Pseudomonas pneumonia
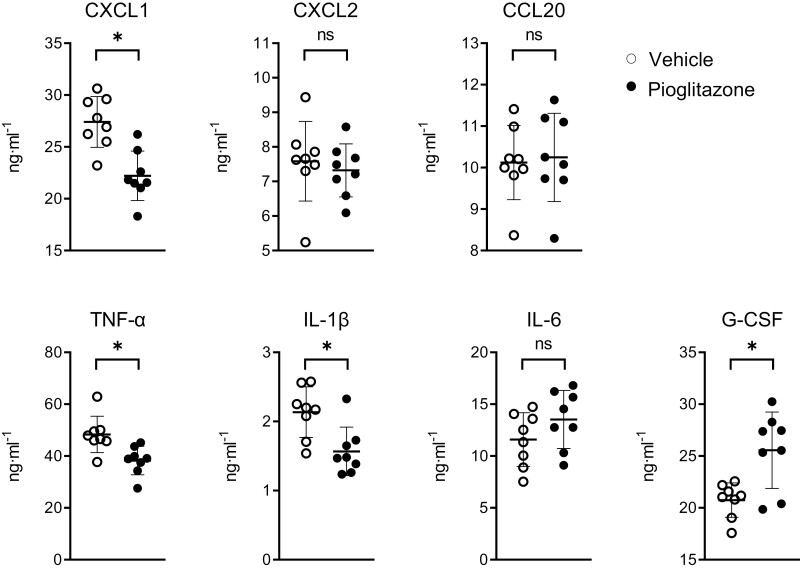
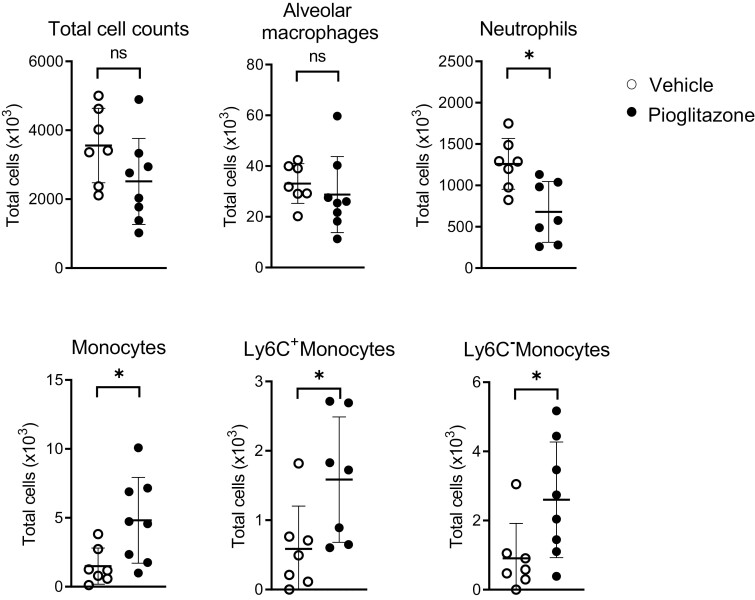
Our finding of an immune-enhancing effect of pioglitazone on bronchial epithelial cells was remarkable, considering the widely reported anti-inflammatory effects of PPAR-γ agonists in models of lung inflammation [8, 10, 11, 23], including murine Pseudomonas pneumonia [12, 13]. We, therefore, investigated the effect of pioglitazone regarding inflammation in the bronchoalveolar compartment in our model. To this end, we harvested BALF 6 h after infection with P. aeruginosa and analyzed chemokine/cytokine release (Fig. 2) and cell influx (Fig. 3). Pioglitazone reduced CXCL1, TNF-α, and IL-1β protein levels in BALF, while not affecting CXCL2, CCL20, and IL-6 concentrations. BALF levels of granulocyte colony-stimulating factor (G-CSF, encoded by Csf3) were increased in pioglitazone-treated mice when compared to mice that received vehicle control. Whilst pioglitazone did not impact total cell counts in BALF after P. aeruginosa infection, it reduced neutrophil numbers. Although total monocyte numbers in BALF were low in both treatment groups, pioglitazone treatment was associated with increased monocyte numbers, which was caused by the rises in both Ly6C− (patrolling) monocytes and Ly6C+ (inflammatory) monocytes. The number of alveolar macrophages was not altered by pioglitazone. These results are in agreement with previous studies [12, 13] and suggest a pro-inflammatory effect of pioglitazone specifically on respiratory epithelial cells.
Figure 2:
Chemokines and cytokines concentration in BALF after 6 h inoculation with P. aeruginosa to mice pre-treated with pioglitazone or vehicle. Graphs show mean and SD, and every dot represents one individual mouse. P values were calculated using Welch’s t-test. ∗P < 0.05, ns (not significant).
Figure 3:
Total cell count, alveolar macrophage, neutrophil and monocyte numbers, as well as Ly6C+ (inflammatory) and Ly6C− (patrolling) monocytes, were evaluated by flow cytometry in BALF in vehicle and pioglitazone treated mice 6 h following induction of pneumonia.Graphs show mean and SD, and every dot represents one individual mouse. P values were calculated using Welch’s t-test. ∗P < 0.05, ns (not significant).
Pioglitazone increases the expression of genes involved in glycolysis
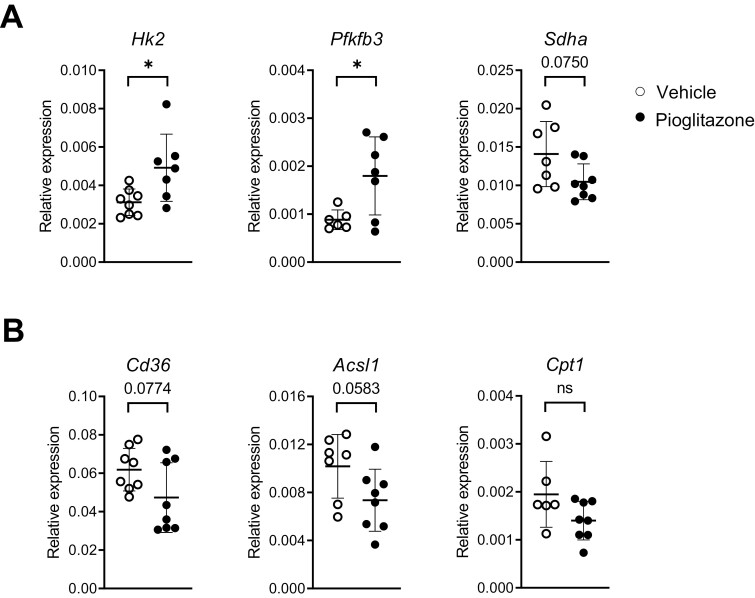
We recently reported an important role for glycolysis in the induction of inflammatory responses, including CXCL1, CCL20, and G-CSF release, by human bronchial epithelial cells stimulated with flagellin, an important immune-enhancing component of P. aeruginosa [24]. In accordance, inhibition of glycolysis by rapamycin abrogated Cxcl1, Cxl2, and Csf3 expression in bronchial brushes of mice administered with flagellin via the airways [24]. These data prompted us to study the impact of pioglitazone on the epithelial cell expression of genes involved in glycolysis. Of interest, pioglitazone treatment was associated with enhanced expression of Hk2 in bronchial brushes, the gene encoding hexokinase-2, the rate-limiting enzyme that mediates the first step of glycolysis by catalyzing the phosphorylation of D-glucose to D-glucose 6-phosphate (Fig. 4A). In accordance, pioglitazone also increased the expression of epithelial Pfkfb3, the gene encoding 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3, another rate-limiting enzyme in glycolysis (Fig. 4A). In contrast, the epithelial expression of Sdha, encoding succinate dehydrogenase complex – subunit A, tended to be decreased in pioglitazone treated mice (P = 0.075 versus vehicle, Fig. 4A); SDHA participates in the tricarboxylic acid cycle by converting succinate to fumarate. Together these data suggest that pioglitazone administration resulted in a metabolic shift to glycolysis in bronchial epithelial cells during acute P. aeruginosa pneumonia. Pioglitazone tended to reduce the expression of genes involved in fatty acid uptake (Cd36) and fatty acid metabolism (Acsl1); differences with vehicle-treated mice did not reach statistical significance (Fig. 4B).
Figure 4:
(A) Relative mRNA expression for the indicated glycolytic enzymes analyzed by RT-PCR in lung epithelial brushes from vehicle or pioglitazone treated mice 6 h after infection with P. aeruginosa. (B) Relative mRNA expression for the indicated fatty-acids metabolism enzymes analyzed by RT-PCR in lung epithelial brushes of mice from (A). Graphs show mean and SD, and every dot represents one individual mouse. P values were calculated using Welch’s t-test. ∗P < 0.05.
Discussion
PPAR-γ has been implicated as an important regulator of lung immunity by controlling the function of multiple cell types in the respiratory tract. The capacity of PPAR-γ to inhibit pro-inflammatory responses by monocytes and macrophages was reported more than 30 years ago [25, 26]. Since then many studies documented the anti-inflammatory effects of PPAR-γ agonists in models of lung inflammation [8–11, 23]. We here report remarkable pro-inflammatory effects of pioglitazone treatment in bronchial epithelial cells in mice infected with viable P. aeruginosa via the airways, as reflected by increased expression of chemokine and cytokine genes. Pioglitazone enhanced the epithelial expression of genes involved in glycolysis, providing a possible mechanistic link with its effect on proinflammatory gene expression.
In contrast with the abundant literature on the anti-inflammatory effects of PPAR-γ agonists on monocytes and macrophages, knowledge of the role of PPAR-γ in respiratory epithelial cells is more limited. PPAR-γ agonists inhibited IL-8 secretion by airway epithelial cell lines stimulated with a mixture of proinflammatory cytokines [12, 27], whilst the effect on Pseudomonas induced cytokine production was more variable [12]. Several studies reported on the function of endogenous PPAR-γ in the respiratory epithelium. Mice with targeted deletion of Pparg in bronchial epithelial cells presented exacerbated lung inflammation during allergen-induced airway disease, with increased cytokine concentration in BALF and increased cytokine expression in airway epithelial cells [28]. In intestinal epithelial cells, pioglitazone enhanced barrier function after infection with Pseudomonas [29]. In accordance with an anti-inflammatory role of endogenous PPAR-γ in colon epithelium, mice with an epithelial-specific deletion of Pparg showed an enhanced susceptibility to chemically induced colitis [30]. Moreover, PPAR-γ ligands reduced cytokine gene expression in colon cancer cell lines and attenuated colonic inflammation in a mouse colitis model [31]. As such, existing literature suggests an anti-inflammatory role for PPAR-γ activation in epithelial cells. We found enhanced gene expression of multiple chemokines and cytokines in bronchial epithelial cells in pioglitazone-treated mice with acute P. aeruginosa pneumonia. Although a definite explanation of these distinct findings is lacking, differences in the context of PPAR-γ stimulation (e.g. analyses of epithelial cell lines stimulated in vitro versus primary bronchial epithelial cells harvested from mice after airway infection in vivo) might play a role.
Pioglitazone enhanced the gene expression of rate-limiting enzymes of glycolysis hexokinase-2 and PFKFB3, whilst expression of the gene encoding SDHA (essential for an adequate metabolite flux in the tricarboxylic acid cycle) tended to be reduced. In agreement, Hk2 has been described as a transcriptional target for PPAR-γ and pioglitazone increased hepatic HK2 levels in mice with fatty liver disease [32]. We recently showed that the primary human bronchial epithelial cells require glycolysis to sustain the production of pro-inflammatory mediators upon stimulation with flagellin, including CXCL1, CCL20, and G-CSF [24], of which the expression was enhanced in bronchial epithelial cells of pioglitazone treated mice with P. aeruginosa pneumonia. Moreover, inhibition of the mTOR (mechanistic target of rapamycin) pathway prevented the induction of glycolysis and limited the secretory capacity of bronchial epithelial cells in response to flagellin in vitro and abolished expression of Hk2, Cxcl1, Cxcl2, and Csf3 in bronchial epithelial cells of mice challenged with flagellin in vivo [24]. Together these data suggest a functional link between pioglitazone-induced metabolic rewiring toward glycolysis and its stimulatory effect on chemokine/cytokine gene expression in bronchial epithelial cells. Further studies, in which glycolysis is inhibited specifically in epithelial cells, are needed to obtain further support for this potential mechanism of action underlying the effect of pioglitazone.
Pioglitazone exerted several anti-inflammatory effects in mice infected with P. aeruginosa via the airways, including inhibition of CXCL1, TNF-α, and IL-1β release and neutrophil recruitment in BALF. These data are consistent with earlier reports demonstrating the anti-inflammatory effects of PPAR-γ agonists in mouse models of P. aeruginosa pneumonia [12, 13] and other lung inflammation models [10, 11]. The discrepancy between pioglitazone effects expression of genes in bronchial epithelial cells and protein release in BALF likely can be explained by additional cellular sources for chemokines and cytokines besides the respiratory epithelium, particularly macrophages, which are expected to be inhibited in function by pioglitazone [10, 33]. These results further indicate that the selective pro-inflammatory effects of pioglitazone on the bronchial epithelium do not have a major impact on typical inflammatory responses measured in BALF, which result from an interplay of different activated – predominantly myeloid – cell types.
Concluding remarks
PPAR-γ agonists exert broad anti-inflammatory effects on myeloid cells in vitro and models of inflammation in vivo. While we here confirm anti-inflammatory effects of pioglitazone in the bronchoalveolar compartment of mice during acute P. aeruginosa pneumonia, we found a selective pro-inflammatory effect on bronchial epithelial cells, as indicated by enhanced gene expression of several chemokines and cytokines, which was associated with increased expression of genes encoding rate-limiting enzymes of glycolysis. These results may have relevance for the administration of PPAR-γ agonists to patients with inflammatory lung disorders that affect the respiratory epithelium.
Acknowledgements
The authors thank Marieke ten Brink and Joost Daalhuisen (Center for Experimental and Molecular Medicine, Amsterdam UMC) for their technical support carrying the animal experiments.
Glossary
Abbreviations
- BALF
bronchoalveolar lavage fluid
- CFU
colony-forming units
Contributor Information
Bianca L Ferreira, Center of Experimental and Molecular Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Infection & Immunity Institute, Amsterdam, The Netherlands; Division of Infectious Diseases, Department of Medicine, Escola Paulista de Medicina, Universidade Federal de Sao Paulo, Sao Paulo, Brazil.
Ivan Ramirez-Moral, Center of Experimental and Molecular Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Infection & Immunity Institute, Amsterdam, The Netherlands.
Natasja A Otto, Center of Experimental and Molecular Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Infection & Immunity Institute, Amsterdam, The Netherlands.
Reinaldo Salomão, Division of Infectious Diseases, Department of Medicine, Escola Paulista de Medicina, Universidade Federal de Sao Paulo, Sao Paulo, Brazil.
Alex F de Vos, Center of Experimental and Molecular Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Infection & Immunity Institute, Amsterdam, The Netherlands.
Tom van der Poll, Center of Experimental and Molecular Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Infection & Immunity Institute, Amsterdam, The Netherlands; Division of Infectious Diseases, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Author contributions
Conceptualization: I.R-M., B.L.F. and T.v.d.P. Methodology: I.R-M., B.L.F. and N.A.O. Validation: I.R-M. and B.L.F. Formal analysis: I.R-M. and B.L.F. Investigation: I.R-M, B.L.F. and N.A.O. Resources: T.v.d.P. Writing - Original draft: I.R-M., B.L.F. and T.v.d.P. Writing - review and editing: I.R-M, B.L.F., N.A.O., A.F.d.V., R.S. and T.v.d.P. Visualization: I.R-M. and B.L.F. Funding acquisition: T.v.d.P.
Funding statement
Bianca Lima Ferreira was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (grant 2019/02224-0); Ivan Ramirez-Moral was funded by the Era-Net Joint Programming Initiative on Antimicrobial Resistance/ZonMW (grant 50-52900-98-201); and Natasja Otto was funded by ZonMW (grant 40-00812-98-14016).
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Data availability statement
The data underlying this study are available in the article. Any further information is available from the corresponding author on request.
References
- 1. Ramírez-Estrada S, Borgatta B, Rello J. Pseudomonas aeruginosa ventilator-associated pneumonia management. Infect Drug Resist 2016, 9, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lynch JP 3rd, Zhanel GG, Clark NM. Emergence of antimicrobial resistance among Pseudomonas aeruginosa: implications for therapy. Semin Respir Crit Care Med 2017, 38, 326–45. [DOI] [PubMed] [Google Scholar]
- 3. Tumbarello M, De Pascale G, Trecarichi EM, Spanu T, Antonicelli F, Maviglia R, et al. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med 2013, 39, 682–92. [DOI] [PubMed] [Google Scholar]
- 4. Melsen WG, Rovers MM, Groenwold RH, Bergmans DC, Camus C, Bauer TT, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis 2013, 13, 665–71. [DOI] [PubMed] [Google Scholar]
- 5. Lambiase A, Raia V, Del Pezzo M, Sepe A, Carnovale V, Rossano F. Microbiology of airway disease in a cohort of patients with cystic fibrosis. BMC Infect Dis 2006, 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin CK, Kazmierczak BI. Inflammation: a double-edged sword in the response to Pseudomonas aeruginosa infection. J Innate Immun 2017, 9, 250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 2013, 19, 557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy AT, Lakshmi SP, Reddy RC. PPARγ in bacterial infections: a friend or foe? PPAR Res 2016, 2016, 7963540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramirez-Moral I, Ferreira BL, de Vos AF, van der Poll T. Post-treatment with the PPAR-γ agonist pioglitazone inhibits inflammation and bacterial growth during Klebsiella pneumonia. Respir Res 2021, 22, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stegenga ME, Florquin S, de Vos AF, van der Poll T. The thiazolidinedione ciglitazone reduces bacterial outgrowth and early inflammation during Streptococcus pneumoniae pneumonia in mice. Crit Care Med 2009, 37, 614–8. [DOI] [PubMed] [Google Scholar]
- 11. de Carvalho MV, Gonçalves-De-albuquerque CF, Silva AR. PPAR gamma: From definition to molecular targets and therapy of lung diseases. Int J Mol Sci 2021, 22(2), 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perez A, van Heeckeren AM, Nichols D, Gupta S, Eastman JF, Davis PB. Peroxisome proliferator-activated receptor-gamma in cystic fibrosis lung epithelium. Am J Physiol Lung Cell Mol Physiol 2008, 295, L303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bedi B, Yuan Z, Joo M, Zughaier SM, Goldberg JB, Arbiser JL, et al. Enhanced clearance of Pseudomonas aeruginosa by peroxisome proliferator-activated receptor gamma. Infect Immun 2016, 84, 1975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun 2005, 73, 7151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mijares LA, Wangdi T, Sokol C, Homer R, Medzhitov R, Kazmierczak BI. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J Immunol 2011, 186, 7080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hajjar AM, Harowicz H, Liggitt HD, Fink PJ, Wilson CB, Skerrett SJ. An essential role for non-bone marrow-derived cells in control of Pseudomonas aeruginosa pneumonia. Am J Respir Cell Mol Biol 2005, 33, 470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morris AE, Liggitt HD, Hawn TR, Skerrett SJ. Role of Toll-like receptor 5 in the innate immune response to acute P. aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol 2009, 297, L1112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anas AA, van Lieshout MH, Claushuis TA, de Vos AF, Florquin S, de Boer OJ, et al. Lung epithelial MyD88 drives early pulmonary clearance of Pseudomonas aeruginosa by a flagellin dependent mechanism. Am J Physiol Lung Cell Mol Physiol 2016, 311, L219–28. [DOI] [PubMed] [Google Scholar]
- 19. Griffin PE, Roddam LF, Belessis YC, Strachan R, Beggs S, Jaffe A, et al. Expression of PPARγ and paraoxonase 2 correlated with Pseudomonas aeruginosa infection in cystic fibrosis. PLoS One 2012, 7, e42241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qin W, Brands X, Van’t Veer C, de Vos AF, Scicluna BP, van der Poll T. Bronchial epithelial Tet2 maintains epithelial integrity during acute Pseudomonas aeruginosa Pneumonia. Infect Immun 2020, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin W, Brands X, van ‘t Veer C, de Vos AF, Scicluna BP, van der Poll T. Flagellin induces innate immune genes in bronchial epithelial cells in vivo: Role of TET2. Scand J Immunol 2021, 94, e13046. [DOI] [PubMed] [Google Scholar]
- 22. Qin W, Brands X, Van’t Veer C, F de Vos A, Sirard JC, J T H Roelofs J, et al. Bronchial epithelial DNA methyltransferase 3b dampens pulmonary immune responses during Pseudomonas aeruginosa infection. PLoS Pathog 2021, 17, e1009491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bassaganya-Riera J, Song R, Roberts PC, Hontecillas R. PPAR-gamma activation as an anti-inflammatory therapy for respiratory virus infections. Viral Immunol 2010, 23, 343–52. [DOI] [PubMed] [Google Scholar]
- 24. Ramirez-Moral I, Yu X, Butler JM, van Weeghel M, Otto NA, Ferreira BL, et al. mTOR-driven glycolysis governs induction of innate immune responses by bronchial epithelial cells exposed to the bacterial component flagellin. Mucosal Immunol 2021, 14, 594–604. [DOI] [PubMed] [Google Scholar]
- 25. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–6. [DOI] [PubMed] [Google Scholar]
- 26. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998, 391, 79–82. [DOI] [PubMed] [Google Scholar]
- 27. Wang AC, Dai X, Luu B, Conrad DJ. Peroxisome proliferator-activated receptor-gamma regulates airway epithelial cell activation. Am J Respir Cell Mol Biol 2001, 24, 688–93. [DOI] [PubMed] [Google Scholar]
- 28. Lakshmi SP, Reddy AT, Banno A, Reddy RC. Airway epithelial cell peroxisome proliferator-activated receptor γ regulates inflammation and mucin expression in allergic airway disease. J Immunol 2018, 201, 1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bedi B, Lin KC, Maurice NM, Yuan Z, Bijli K, Koval M, et al. UPR modulation of host immunity by Pseudomonas aeruginosa in cystic fibrosis. Clin Sci (Lond) 2020, 134, 1911–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adachi M, Kurotani R, Morimura K, Shah Y, Sanford M, Madison BB, et al. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 2006, 55, 1104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, et al. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest 1999, 104, 383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Panasyuk G, Espeillac C, Chauvin C, Pradelli LA, Horie Y, Suzuki A, et al. PPARγ contributes to PKM2 and HK2 expression in fatty liver. Nat Commun 2012, 3, 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reddy RC. Immunomodulatory role of PPAR-gamma in alveolar macrophages. J Investig Med 2008, 56, 522–7. [DOI] [PubMed] [Google Scholar]
- 34. Chen K, Eddens T, Trevejo-Nunez G, Way EE, Elsegeiny W, Ricks DM, et al. IL-17 Receptor signaling in the lung epithelium is required for mucosal chemokine gradients and pulmonary host defense against k. pneumoniae. Cell Host Microbe 2016, 20, 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study are available in the article. Any further information is available from the corresponding author on request.