Abstract
Introduction
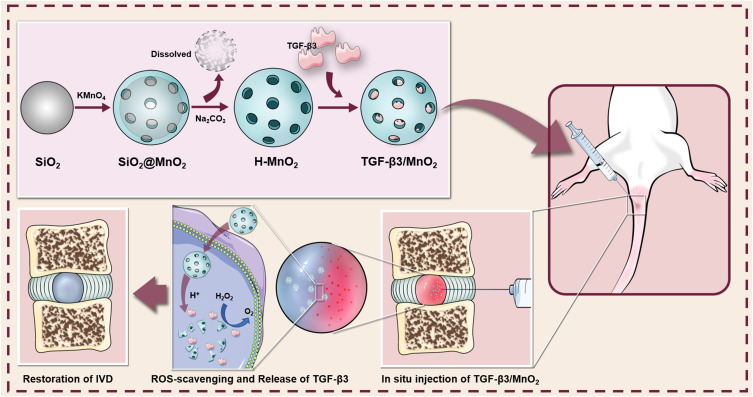
Intervertebral disc (IVD) degeneration (IDD) is one of the most widespread musculoskeletal diseases worldwide and remains an intractable clinical challenge. Currently, regenerative strategies based on biomaterials and biological factors to facilitate IVD repair have been widely explored. However, the harsh microenvironment, such as increased ROS and acidity, of the degenerative region impedes the efficiency of IVD repair. Here, an intelligent biodegradable nanoplatform using hollow manganese dioxide (H-MnO2) was developed to modulate the degenerative microenvironment and release transforming growth factor beta-3 (TGF-β3), which may achieve good long-term therapeutic effects on needle puncture-induced IDD.
Methods
Surface morphology and elemental analysis of the MnO2 nanoparticles (NPs) were performed by transmission electron microscopy and an energy-dispersive X-ray spectroscopy detector system, respectively. The biological effects of MnO2 loaded with TGF-β3 (TGF-β3/MnO2) on nucleus pulposus cells (NPCs) were assessed via cytoskeleton staining, EdU staining, qPCR and immunofluorescence. The efficacy of TGF-β3/MnO2 on needle puncture-induced IDD was further examined using MRI and histopathological and immunohistochemical staining.
Results
The MnO2 NPs had a spherical morphology and hollow structure that dissociated in the setting of a low pH and H2O2 to release loaded TGF-β3 molecules. In the oxidative stress environment, TGF-β3/MnO2 was superior to TGF-β3 and MnO2 NPs in the suppression of H2O2-induced matrix degradation, ROS, and apoptosis in NPCs. When injected into the IVDs of a rat IDD model, TGF-β3/MnO2 was able to prevent the degeneration and promote self-regeneration.
Conclusion
Use of an MnO2 nanoplatform for biological factors release to regulate the IDD microenvironment and promote endogenous repair may be an effective approach for treating IDD.
Keywords: intervertebral disc degeneration, hollow manganese dioxide, transforming growth factor beta-3, oxidative stress, endogenous repair
Graphical Abstract
Introduction
Low back pain, a leading cause of disability worldwide, is strongly associated with intervertebral disc degeneration (IDD), with IDD appearing in 40% of all cases of lower back pain.1–3 The loss of disc cell viability and functionality is thought to be critical to disrupting disc homeostasis.4 Excessive apoptosis and cellular senescence from IDD contribute to a reduced number of viable disc cells.5,6 Deficiencies in anabolic factors such as transforming growth factor beta (TGF-β) and insulin-like growth factor-1 may further reduce cellular viability and the production of extracellular matrix (ECM).7 In addition, the increased production of reactive oxygen species (ROS) and catabolic cytokines along with the accumulation of senescent disc cells may further deteriorate the microenvironment of degenerated intervertebral discs (IVDs).8 Based on these observations, the ideal biological therapeutics for IVD regeneration should provide the disc with viable and functional cells and improve the survival conditions within degenerating IVDs.9
TGF-β3, an important member of the TGF-β superfamily, is involved in the regulation of biological processes such as proliferation, survival, and differentiation.10,11 TGF-β3 upregulates the expression of genes involved in cartilage formation to promote cartilage repair and accelerate chondrogenic differentiation.12 TGF-β3 was shown to stimulate adipose-derived stem cell proliferation and chondrogenic differentiation in vitro.13 Importantly, TGF-β3 stimulation enhances cell survival and matrix deposition in the IVDs.14,15 Hence, targeting the supply of TGF-β3 could improve the metabolic imbalance of the ECM in IDD, and potentially slow its progression. However, supplying pure TGF-β3 to diseased tissues will cause local overdose, and the protein will be quickly washed away or degraded by body fluids.10 A way to deliver a sustained dose of TGF-β3 into target tissues could facilitate the IVD repair process.
Nanoparticles (NPs) have been developed as carriers for the delivery of a wide range of active pharmaceutical ingredients to overcome the limitations of free therapeutics and navigate biological barriers.16 Encapsulation in NPs can improve the stability and solubility of the cargo, promote transmembrane transport, and prolong drug circulation time, thereby increasing its safety and effectiveness.17 In recent years, manganese dioxide (MnO2)-based NPs have attracted interest in the field of medical science due to their high sensitivity to H2O2 and H+, which are abundant in the tumor, osteoarthritis, and IDD microenvironments.18–20 Yang et al developed a hollow MnO2 nanoplatform for the sustained release of anti-tumor drugs in a slightly acidic environment and in the presence of H2O2.21 Kumar et al reported that MnO2 NPs protect cartilage from inflammation induced by oxidative stress.22 However, little is known about the involvement of MnO2 NPs in the regulation of the IDD microenvironment. During IDD pathogenesis, the acidity and ROS levels within the degenerated IVDs significantly increase, which can inhibit normal cellular activity and accelerate IVD degeneration.9,23,24 Therefore, the application of MnO2 NPs might improve the harsh survival conditions in the disc microenvironment as a way to promote tissue regeneration.
In this study, we prepared an intelligent drug delivery system based on hollow MnO2 NPs encapsulating TGF-β3. The NPs exhibited satisfactory drug loading efficiency along with pH-responsive degradation. The hollow MnO2 NPs degraded to release the encapsulated TGF-β3 when exposed to excessive H2O2 and/or H+ in tissues and cells. The released TGF-β3 improved cell survival and ECM deposition by scavenging ROS, thereby alleviating the progression of IDD. Based on these results, the hollow MnO2-based responsive nanocarriers for drug delivery show promise for IVD repair.
Materials and Methods
Synthesis and Characterization of Hollow MnO2 Nanoparticles
Hollow MnO2 NPs were prepared as previously described.21,25 Briefly, solid SiO2 (sSiO2) NPs were first synthesized as follows: 14 mL of ethanol, 2 mL of deionized water, and 500 μL of NH3·H2O were mixed in a 50-mL round-bottom flask. The mixture was heated under magnetic stirring in an oil bath at 50 °C for 5 min. 500 μL of tetraethyl orthosilicate was then added dropwise and the mixture was stirred at 50 °C for 2 h to produce sSiO2 NPs. The resulting sSiO2 NPs were washed twice with ethanol and twice with water and then stored in water for further use. For the synthesis of sSiO2@MnO2 NPs, 600 mg of KMnO4 dispersed in 20 mL of water was added dropwise into the sSiO2 NPs during sonication. The mixture was ultrasonicated for 1 h, then stirred overnight at room temperature. The resulting sSiO2@MnO2 NPs were washed three times with deionized water and centrifuged at 14,800 rpm/min. Finally, the prepared sSiO2 coated with mesoporous MnO2 was dissolved in Na2CO3 at 60 °C for 12 h. The resulting hollow MnO2 NPs were then centrifuged and washed several times with water. The surface morphology and elemental analysis of the NPs were determined by transmission electron microscopy (TEM; FEI Tecnai F20, Hillsboro, OR, USA) and an energy-dispersive X-ray spectroscopy detector system (EDS, Oxford X-MAX, Oxford, UK), respectively.
Encapsulation of TGF-β3 into Hollow MnO2 Nanoparticles
For TGF-β3 loading, hollow MnO2 NPs (5 mg) were added into 1 mL of phosphate-buffered saline (PBS; pH 7.4) containing TGF-β3 (1 μg; Sigma-Aldrich, St. Louis, MO, USA) and stirred for 12 h at room temperature. Subsequently, the NPs were centrifuged, and the supernatant was removed.
Examination of in-vitro Release of TGF-β3 from MnO2 Nanoparticles
The release of TGF-β3 from the NPs in vitro was quantified by ELISA (Sangon Biotech, Shanghai, China). The NPs were incubated in 1 mL of PBS at different pH values (6.5 and 7.4) in the absence or presence of 100 μM H2O2. At predetermined time points, the samples were centrifuged, the supernatant was collected, and an equal amount of fresh PBS was added to continue the release process. All collected solutions were kept frozen at –20°C for subsequent analysis with TGF-β3-ELISA.
Isolation and Culture of Nucleus Pulposus Cells
To isolate nucleus pulposus cells (NPCs), six-week-old male Sprague-Dawley rats were sacrificed and their lumbar and caudal IVDs were harvested under aseptic conditions. We then separated gel-like nucleus pulposus tissues from the discs and treated them with 0.25% (w/v) type II collagenase (Yeasen, Shanghai, China) for 4 h at 37 °C. The obtained cell suspension was centrifuged at 1200 rpm for 3 min and then cultured with DMEM/F12 containing 10% fetal bovine serum (HyClone, Logan, UT, USA) and 1% penicillin/streptomycin (Gibco, Grand Island, NY, USA) in a humidified incubator at 37 °C with 5% CO2. NPCs at passage 2 were used for all of the experiments in this study.
Cell Culture
NPCs at passage 2 were plated on a cell culture dish at an initial density of 5000 cells/cm2. When the cells were attached, H2O2 solution was diluted with DMEM/F12 medium and then added into the dishes to a final concentration of 100 μM to induce oxidative stress in the NPCs. To further investigate whether NPs altered oxidative stress, after treatment with H2O2 for 12 h, the medium was replenished by fresh medium containing TGF-β3, MnO2, and TGF-β3/MnO2 for 24 h, respectively.
In vitro Cytotoxicity Assay
The cell toxicity of the NPs was determined by examining the viability of NPCs treated with NPs using a CCK-8 assay (NCM Biotech, Suzhou, China). Briefly, cells (5×103 per well) were seeded into 96-well plates and cultured for 12 h to allow cell attachment. The cells were then incubated with a series of increasing concentrations of hollow MnO2 NPs for 24 h. After incubation, the cell medium was removed and replaced with 100 μL of culture medium and 10 μL of CCK-8 reagent for another 2 h for color development. Optical density was measured using a microplate reader (BioTek, VT, USA) at 450 nm.
Cellular Uptake Experiments
To assess the cellular uptake efficiency of the NPs, bovine serum albumin (BSA), and fluorescein isothiocyanate-labeled BSA (FITC-BSA; Solarbio, Beijing, China) were encapsulated into hollow MnO2 NPs. After encapsulation, NPCs (5×103 per well) were cultured in 48-well plates at 37 °C for 12 h. After complete adhesion, the cells were washed twice with PBS followed by the addition of 100 μL of fresh medium containing NPs loaded with BSA or FITC-BSA. Incubation was then continued for 12 h. Next, the cells were washed three times with PBS to remove the residual NPs, fixed with 4% paraformaldehyde solution for 15 min, and stained with TRITC-phalloidin and DAPI. Finally, images were obtained using a fluorescence microscope.
Intracellular ROS Assay
An ROS assay kit (Beyotime, Shanghai, China) was used to measure the accumulation of intracellular ROS in the treated cells. The cells were first exposed to DCFH-DA solution (10 µM) and then incubated for 1 h at 37 °C. After a wash with PBS, the fluorescence of the cells was observed with a fluorescence microscope.
Cell Proliferation Assay
Cell proliferation was measured using a BeyoClick™ EdU Cell Proliferation Kit with Alexa Fluor 488 (Beyotime, Shanghai, China). Briefly, immediately before H2O2 treatment in all groups, EdU was added to label cells undergoing active DNA synthesis. After 6 h, the cells were fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.3% Triton X-100 in PBS for 10 min. Subsequently, the samples were incubated with click reaction solution at room temperature for 30 min, and the nuclei were counterstained with Hoechst 33342 dye. Images were acquired using a fluorescence microscope.
Quantitative Polymerase Chain Reaction Analysis
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract the total RNA from NPCs under different conditions. The RNA concentrations were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). RNA (1 μg) was reverse-transcribed into cDNA using a 5X All-In-One RT MasterMix (abm, Vancouver, BC, Canada) according to the manufacturer’s instructions. Subsequently, qPCR was performed using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). The primer sequences (Sangon Biotech, Shanghai, China) of the genes used in this study are listed in Table 1. The expression of each gene was normalized by the housekeeping gene GAPDH. Relative changes in mRNA level were analyzed using the 2−ΔΔCT method.
Table 1.
Primers for qPCR
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| SOD1 | CGGCTTCTGTCGTCTCCTTGC | AACTGGTTCACCGCTTGCCTTC |
| SOD2 | GCTGGAGGCTATCAAGCGTGAC | TTAGAGCAGGCGGCAATCTGTAAG |
| CAT | GGCCTGACTGACGCGATTGC | CTGCTCCTTCCACTGCTTCATCTG |
| Adamts5 | TCCTCTTGGTGGCTGACTCTTCC | TGGTTCTCGATGCTTGCATGACTG |
| MMP3 | CAGTCCTGCTGTGGCTGTGTAC | AACCTCCATGCCAGCATCTTCTTC |
| BAX | CACCAGCTCTGAACAGATCATGA | TCAGCCCATCTTCTTCCAGATGGT |
| Col-II | ACGCTCAAGTCGCTGAACAACC | ATCCAGTAGTCTCCGCTCTTCCAC |
| iNOS | GAGACGCACAGGCAGAGGTTG | CAGGAAGGCAGCAGGCACAC |
| COX-2 | TTCCAGTATCAGAACCGCATTGCC | CCGTGTTCAAGGAGGATGGAGTTG |
| ACAN | GGCGT CCAAA CCAAC CCGAG | GGCGT CCAAA CCAAC CCGAG |
| MCL1 | TCATCTCCCGCTACCTGC | ACTCCACAAACCCATCCC |
| BCL2 | CACCCCTGGCATCTTCTCCTT | AGCGTCTTCAGAGACAGCCAG |
| GAPDH | GACATGCCGCCTGGAGAAAC | AGCCCAGGATGCCCTTTAGT |
Immunofluorescence
NPCs were fixed in 4% paraformaldehyde for 15 min and then permeabilized with 0.3% Triton X-100 in PBS for 10 min. Non-specific binding was blocked by a commercial blocking reagent (Beyotime, Shanghai, China) followed by primary antibodies incubation at 4 °C overnight (rabbit anti-Col-II, rabbit anti-iNOS, Abcam, Cambridge, UK). After overnight incubation, samples were incubated with second antibodies (Beyotime, Shanghai, China) for 1 h. Nuclei were stained with DAPI and images were acquired using a fluorescence microscope. Semi-quantitative fluorescence analysis was performed using Image J software (NIH, Bethesda, MD, USA).
Examination of Caudal Full (Form of IDD) Using Rat Model
All procedures followed the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Soochow University. After complete anesthesia, the rat tails were disinfected, and Co7-8 IVDs were punctured sequentially with 21G needles to induce degeneration. To ensure that trauma was induced, a needle was used to puncture the center of the discs, rotated for 5 s then the position maintained for 30s. Each disc was then injected with 20 µL of material by a 33 G needle; in the negative control group, the discs were injected with PBS instead of NPs. After surgery, the rats were placed in a warm and ventilated location.
Magnetic Resonance Imaging
Four and eight weeks after surgery, the water contents of the IVDs were evaluated on magnetic resonance imaging (MRI, 3.0 T; Siemens, Germany) based on the signal intensity in sagittal T2-weighted images. The MRI images were evaluated by another blinded researcher using the classification for intervertebral disc degeneration (Table 2), as previously reported.26
Table 2.
Classification of Disc Degeneration
| Grade | Structure | Distinction of Nucleus and Annulus | Signal Intensity | Height of Intervertebral Disc |
|---|---|---|---|---|
| I | Homogeneous, bright white | Clear | Hyperintense, isointense to cerebrospinal fluid | Normal |
| II | Inhomogeneous with or without horizontal bands | Clear | Hyperintense, isointense to cerebrospinal fluid | Normal |
| III | Inhomogeneous, gray | Unclear | Intermediate | Normal to slightly decreased |
| IV | Inhomogeneous, gray to black | Lost | Intermediate to hypointense | Normal to moderately decreased |
| V | Inhomogeneous, black | Lost | Hypointense | Collapsed disc space |
Histopathological and Immunohistochemical Analysis of Intervertebral Disc Regeneration
After sacrifice, the IVDs were removed from each rat and placed in 10% formalin for 48 h, decalcified in 10% EDTA for 30 d, and then embedded in paraffin blocks. The specimens were cut into 5-µm-thick histopathological sections followed by staining with either H&E or Safranin-O/Fast Green. The histopathological grade was calculated as described in previous work27 and the specific definition of histopathological grade was shown in Table 3. To observe the expressions of type II collagen and iNOS in the harvested tissues, the sections were immunohistochemically stained for collagen type II and iNOS (Abcam, Cambridge, UK). Semi-quantitative immunohistochemical stain analysis was performed using Image J software.
Table 3.
Histological Grading Scale
| Category | Grade |
|---|---|
| I. Cellularity of the annulus fibrosus | 1. Fibroblasts comprise more than 75% of the cells 2. Neither fibroblasts nor chondrocytes comprise more than 75% of the cells 3. Chondrocytes comprise more than 75% of the cells |
| II. Morphology of the annulus fibrosus | 1. Well-organized collagen lamellae without ruptured or serpentine fibers 2. Inward bulging, ruptured or serpentine fibers in less than one third of the annulus 3. Inward bulging, ruptured or serpentine fibers in more than one third of the annulus |
| III. Border between the annulus fibrosus and nucleus pulposus | 1. Normal, without any interruption 2. Minimal interruption 3. Moderate or severe interruption |
| IV. Cellularity of the nucleus pulposus | 1. Normal cellularity with stellar shaped nuclear cells evenly distributed throughout the nucleus 2. Slight decrease in the no. of cells with some clustering 3. Moderate or severe decrease (>50%) in the number of cells with all the remaining cells clustered and separated by dense areas of proteoglycans |
| V. Morphology of the nucleus pulposus | 1. Round, comprising at least half of the disc area in midsagittal sections 2. Rounded or irregularly shaped, comprising one quarter to half of the disc area in midsagittal sections 3. Irregularly shaped, comprising less than one quarter of the disc area in midsagittal sections |
Statistical Analysis
All data are expressed as mean ± standard deviation. Statistical significance was evaluated using a one-way analysis of variance (GraphPad Software, USA) with Tukey’s multiple comparison test to further evaluate differences between groups. P values less than 0.05 were considered statistically significant.
Results
Synthesis and Characterization of MnO2 Nanoparticles
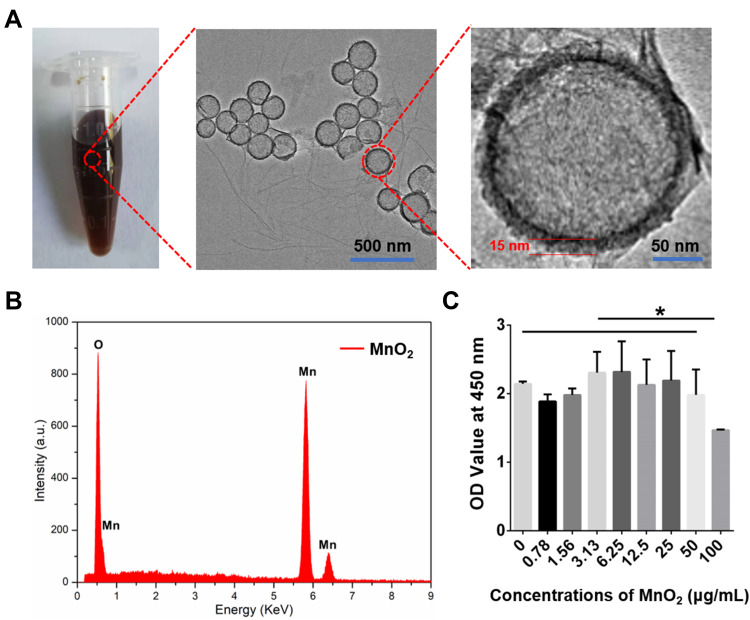
The MnO2 NPs were successfully synthesized and used as carriers for TGF-β3 delivery. The TEM images of the MnO2 NPs clearly show the spherical morphology and hollow structure of the product, and the thickness of the MnO2 shell was approximately 15 nm (Figure 1A). The corresponding EDS spectrum proved the presence of the Mn and O elements derived from MnO2 and also confirmed the hollow structure of MnO2 without element Si (Figure 1B). In addition, the cytotoxicity of the MnO2 NPs was tested by CCK-8 assay, which found that the MnO2 NPs did not exhibit obvious toxicity to NPCs, even at high concentrations up to 50 μg/mL (Figure 1C). Hence, the MnO2 NPs were used at a concentration of 50 μg/mL in further experiments.
Figure 1.
Synthesis and characterization of MnO2 NPs. (A) Digital picture and TEM images of MnO2 NPs. (B) EDS analysis of MnO2 NPs. (C) Relative viabilities of NPCs treated with various concentrations of MnO2 NPs for 24 h. *, p<0.05.
The Critical Examination of Controlled Drug Release
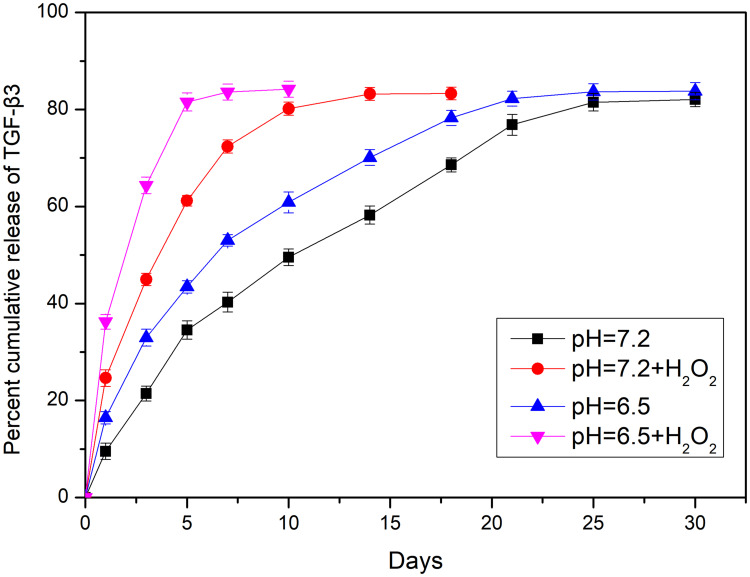
MnO2 is known to be stable under neutral and basic conditions; however, it decomposes into Mn2+ under low pH.28 A release test was performed to evaluate the degradation and loading of MnO2 at different pH values (7.4 and 6.5) in the absence or presence of 100 μM H2O2 for various treatment times lengths (Figure 2). The release curve shows that TGF-β3 was continuously released from MnO2 in a time-dependent manner. Compared with the slow drug release observed at pH 7.4, the rate of TGF-β3 release was higher at pH 6.5. Furthermore, incubation with H2O2 at pH 6.5 further accelerated drug release by triggering the decomposition of the MnO2 nanocarriers into Mn2+ ions.
Figure 2.
Nanoparticle decomposition and drug behaviors of TGF-β3/MnO2. In vitro release of TGF-β3 from MnO2 NPs at different pH values (7.4 and 6.5) in the absence or presence of 100 μM H2O2.
In vitro Cellular Uptake of MnO2 Nanoparticles
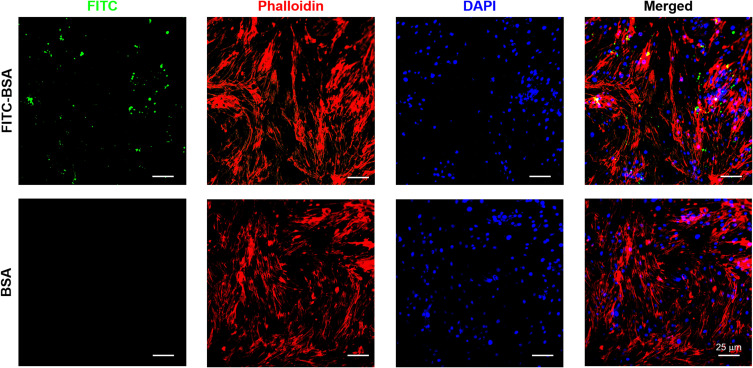
The endocytosis of NPs in NPCs was investigated by loading FITC-BSA into the MnO2 NPs. As shown in Figure 3, the fluorescence signal of the FITC-BSA-loaded MnO2 NPs was strong, indicating that the MnO2 NPs had excellent drug-loading ability. In particular, the fluorescence signal was primarily located in the cytoplasm with some signal found in the nucleus. This confirmed the high cellular uptake of MnO2 NPs.
Figure 3.
Representative images of NPC uptake of BSA/MnO2 and FITC-BSA/MnO2 for 12 h. Green, red and blue colors represent FITC-BSA/MnO2, F-actin, and DAPI fluorescence, respectively.
Antioxidant Activity of TGF-β3-Loaded MnO2 Nanoparticles (TGF-β3/MnO2) Against H2O2
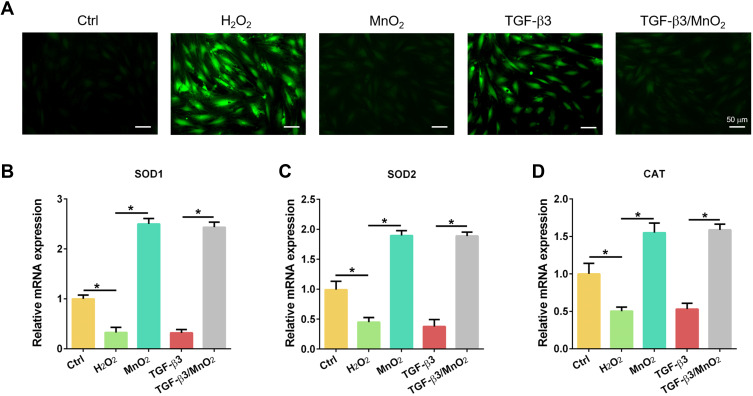
To investigate the ROS scavenging activity of TGF-β3/MnO2, the DCF fluorescence intensity was detected using an ROS assay kit. The ROS content increased after H2O2 treatment, but decreased following treatment with MnO2 and TGF-β3/MnO2. However, treatment with TGF-β3 did not significant reverse H2O2-induced ROS production (Figure 4A). In addition, the expressions of the antioxidative genes SOD1, SOD2, and CAT were significantly increased in cells treated with MnO2 and TGF-β3/MnO2 compared to cells treated with H2O2 and TGF-β3 (Figure 4B–D). Hence, the antioxidant activity of TGF-β3/MnO2 was mainly attributed to MnO2.
Figure 4.
Antioxidant activity of TGF-β3/MnO2 NPs. (A) Representative fluorescent images of NPCs stained with DCFH-DA in the Ctrl, H2O2, MnO2, TGF-β3, and TGF-β3/MnO2 groups, respectively. (B–D) qPCR analyses of the relative expression of the SOD1, SOD2, and CAT genes of the NPCs in Ctrl, H2O2, MnO2, TGF-β3, and TGF-β3/MnO2 groups, respectively. *, p<0.05.
Anti-Apoptotic and Proliferative Effects of TGF-β3/MnO2 Against H2O2
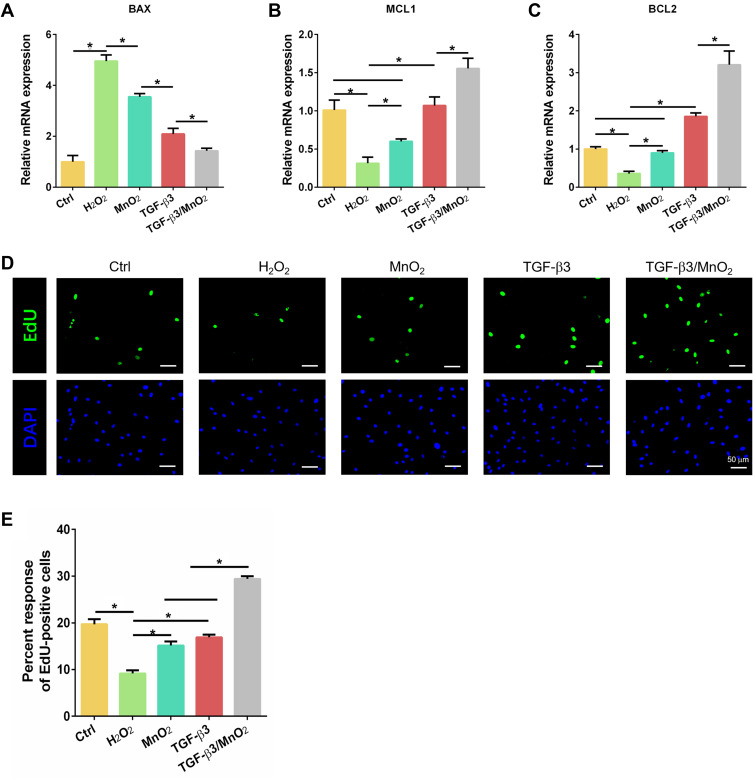
We evaluated the anti-apoptotic effects of TGF-β3/MnO2 against H2O2 using the expression of the apoptotic gene BAX and the anti-apoptotic genes BCL2 and MCL1 in cultured NPCs. The expression of BAX was enhanced in H2O2-treated cells compared to the control NPCs. In contrast, the enhanced expression of the BAX gene was significantly reduced by treatment with TGF-β3, MnO2, or TGF-β3/MnO2, with TGF-β3/MnO2 producing the greatest decrease in BAX expression (Figure 5A). Compared to the cells treated with H2O2, the expression of BCL2 and MCL1 was significantly increased by treatment with TGF-β3, MnO2, or TGF-β3/MnO2, with TGF-β3/MnO2 resulting in the highest expressions (Figure 5B and C). Further, EdU assay showed that cell proliferation rate, represented by the percentage of EdU-positive cells, was decreased to 9.6% in H2O2-treated cells. Interestingly, this number was significantly increased to 17.6% and 18.5% in following treatment with MnO2 and TGF-β3, respectively, and it was markedly enhanced to 28.4% in the setting of TGF-β3/MnO2 (Figure 5D and E).
Figure 5.
Anti-apoptotic and proliferative effects of TGF-β3/MnO2. qPCR analyses of the relative expression of apoptotic (BAX) (A) and anti-apoptotic (MCL1 and BCL2) (B and C) genes of NPCs in the Ctrl, H2O2, MnO2, TGF-β3, and TGF-β3/MnO2 groups, respectively. (D) Representative images of EdU staining of NPCs in the Ctrl, H2O2, MnO2, TGF-β3, and TGF-β3/MnO2 groups, respectively. Green represents EdU-labeled proliferating cells and blue represents nuclei. (E) Quantification of the percentage of EdU-positive cells in the Ctrl, H2O2, MnO2, TGF-β3, and TGF-β3/MnO2 groups, respectively. *, p<0.05.
Characterization of the Metabolic Effects of TGF-β3/MnO2
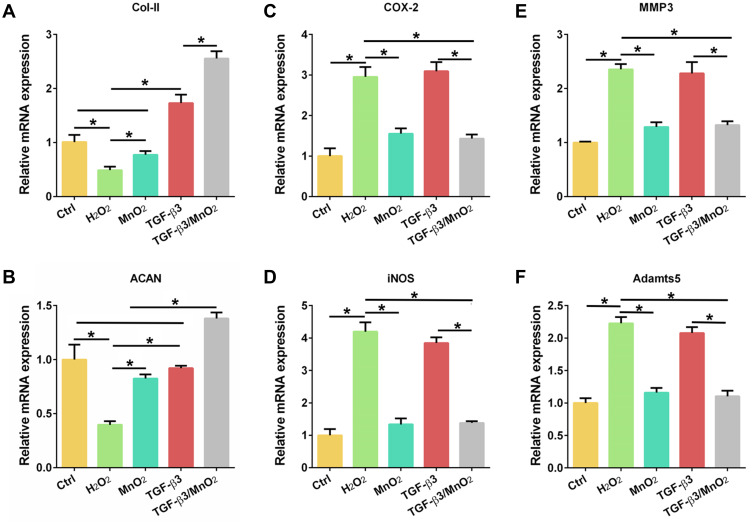
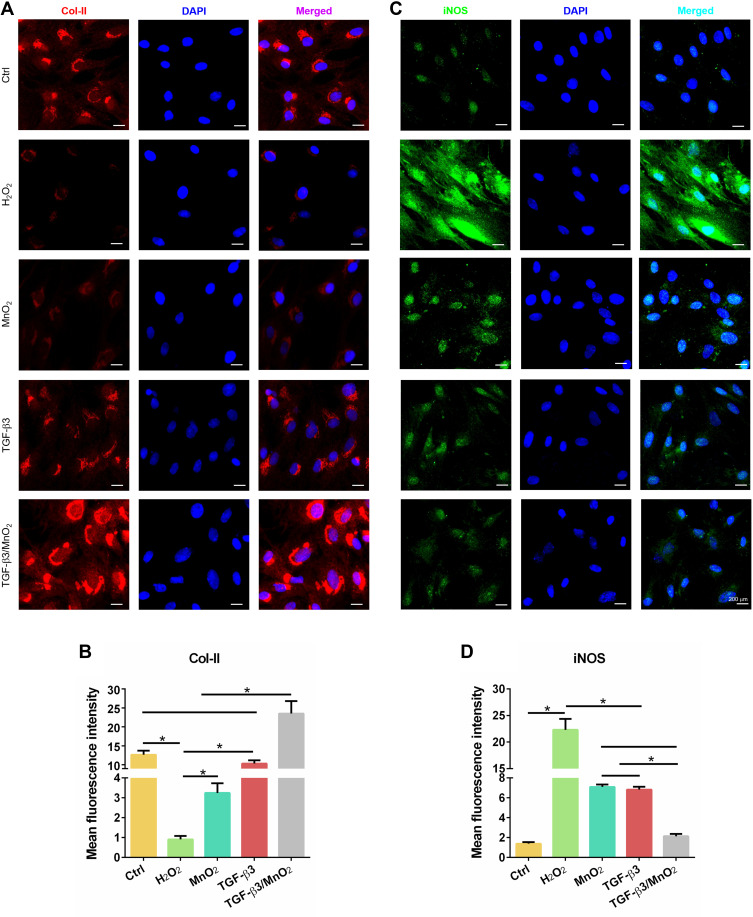
The above results suggest that TGF-β3/MnO2 exerted antioxidant, anti-apoptotic, and proliferative activities to protect cells from H2O2 damage. To further explore the effects of TGF-β3/MnO2 on ECM synthesis in NPCs treated with H2O2, we conducted qPCR measurements to investigate the expressions of genes related to anabolism, inflammation, and catabolism. Of the studied treatments, TGF-β3/MnO2 had the best ability to reverse the H2O2-induced expression of the ECM genes Col-II and ACAN (Figure 6A and B). Further, TGF-β3/MnO2 almost completely blocked the stimulatory effect of H2O2 on the expressions of the pro-inflammatory enzymes COX-2 and iNOS (Figure 6C and D) and the catabolic genes MMP3 and Adamts5 (Figure 6E and F). Immunofluorescent experiments also confirmed that Col-II associated fluorescence intensity in the TGF-β3/MnO2 group, was highest of the experimental groups (Figure 7A and B). Meanwhile, lower fluorescence intensity of iNOS was observed after TGF-β3/MnO2 treatment (Figure 7C and D).
Figure 6.
TGF-β3/MnO2 regulating the ECM metabolic balance of NPCs in vitro. qPCR analyses of the relative expression of anabolic (Col-II and ACAN) (A and B), pro-inflammatory (COX-2 and iNOS) (C and D), and catabolic (MMP3 and Adamts5) (E and F) genes in the NPCs of the Ctrl, H2O2, MnO2, TGF-β3, and TGF-β3/MnO2 groups, respectively. *, p<0.05.
Figure 7.
Immunofluorescent analysis of Col-II and iNOS expression in NPCs. Representative images of the immunofluorescence of Col-II (A) with semi-quantitative analysis (B) and iNOS (C) with semi-quantitative analysis (D) in the NPCs of the Ctrl, H2O2, MnO2, TGF-β3, and TGF-β3/MnO2 groups, respectively. Red, green and blue colors indicate Col-II protein, iNOS protein and DAPI fluorescence, respectively. *, p<0.05.
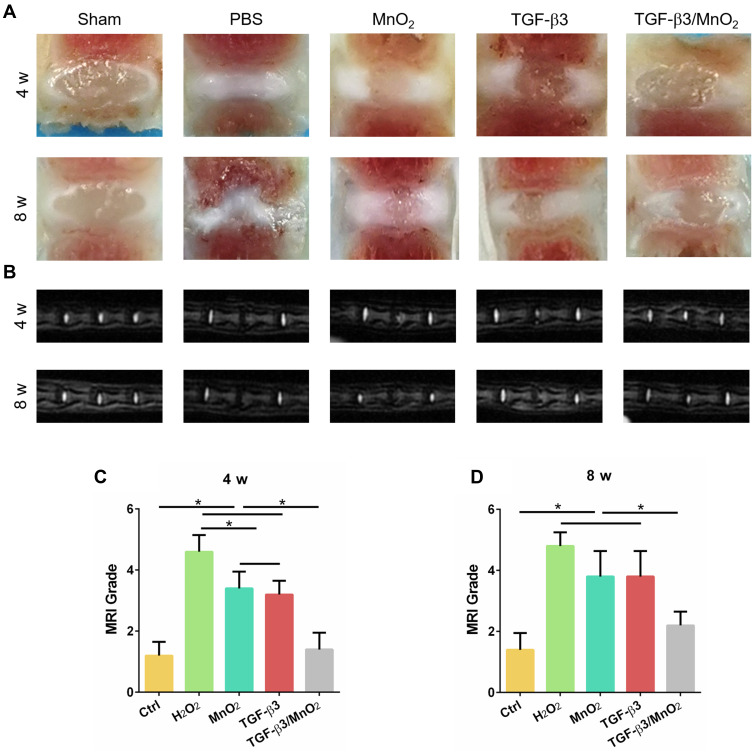
In vivo Evaluation of Intervertebral Disc Regeneration by TGF-β3/MnO2
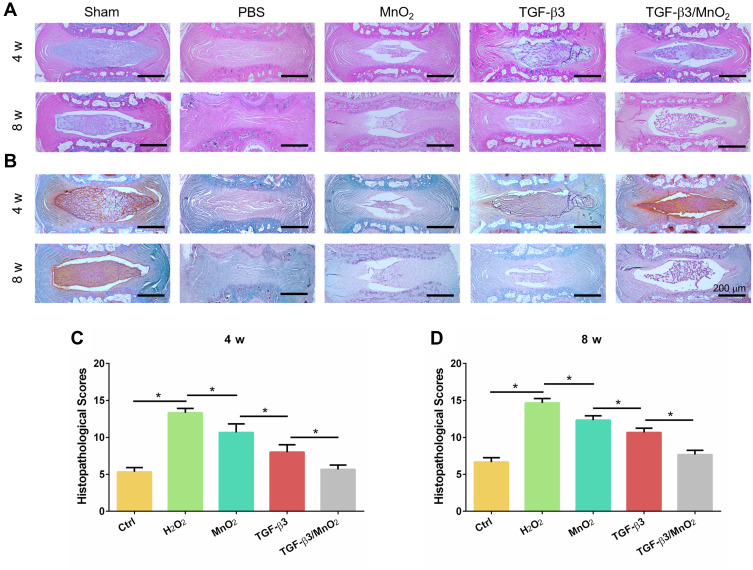
To assess the in vivo effect of TGF-β3/MnO2 treatment, a rat disc puncture model was established. PBS, MnO2, TGF-β3, or TGF-β3/MnO2 was injected into the rat IVDs after they were punctured with a 21G needle. The therapeutic efficacy was evaluated based on changes in the gross appearance (Figure 8A) and MRI images (Figure 8B). After puncture, the gross appearance and T2-weighted MRI signal intensity of the IVDs were similar in the sham group and the group treated with TGF-β3/MnO2. In contrast, discs treated with PBS, MnO2, and TGF-β3 lost their signal, and water was observed (Figure 8C and D). The morphologies of the discs in each group were also evaluated with H&E (Figure 9A) and Safranin O-Fast Green staining (Figure 9B). In the sham group, the discs contained elliptical nucleus pulposus (NP), well-organized collagen lamellae, and a clear boundary between the annulus fibrosus (AF) and NP. From four to eight weeks after puncture, the size of the NP region and proteoglycan content decreased in the TGF-β3/MnO2 group; however, these changes were relatively minor compared to the other experimental groups, and a clear tissue boundary between the AF and NP was still observed. In the PBS, MnO2, and TGF-β3 groups, the discs displayed degenerative characteristics such as decreased NP size and disorganized collagen fibers in the inner AF. The histopathological scores of the TGF-β3/MnO2 group were significantly lower after 4 weeks, which were closest to the Ctrl group. Conversely, the histopathological scores of the other experimental groups were significantly higher, a difference that were more obvious at week 8 (Figure 9C and D).
Figure 8.
Evaluation of disc degeneration with T2-weighted MRI and gross appearance. Representative images of disc gross appearance (A) and MRI signal intensity (B) at 4 and 8 weeks. Changes in MRI grade at 4 and 8 weeks after surgery (C and D). *, p<0.05.
Figure 9.
Histopathological evaluation in vivo. H&E (A) and Safranin O-Fast Green (B) images of IVDs in the rat caudal vertebrae at 4 and 8 weeks. The histopathological scores of different groups at 4 and 8 weeks after surgery (C and D). *, p<0.05.
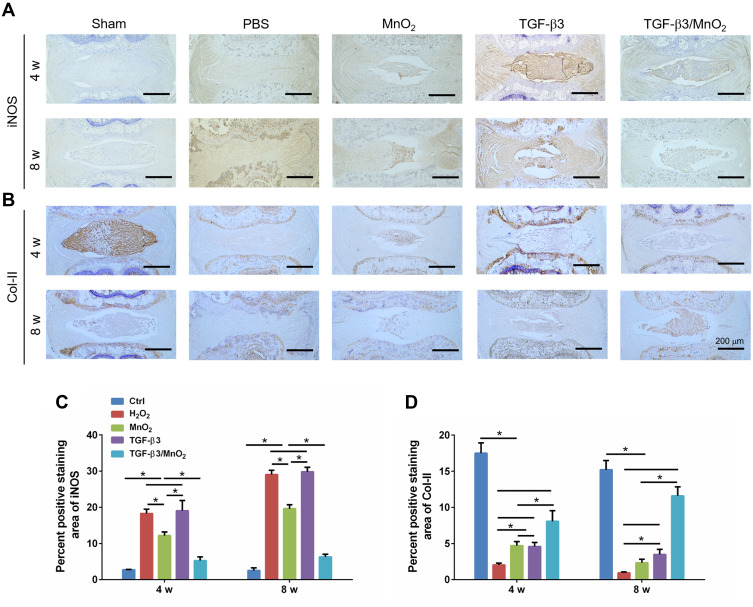
Finally, to verify the effectiveness of TGF-β3/MnO2, the expressions of iNOS and Col-II in the IVD tissue were further evaluated using immunohistochemistry. The expression of iNOS was significantly lower in the TGF-β3/MnO2 group than in the other experimental groups (Figure 10A). Conversely, the expression of Col-II, an important marker of ECM in the NP, was significantly higher in the TGF-β3/MnO2 group than in the other experimental groups (Figure 10B). Semi-quantitative analysis of the staining further illustrated these differences (Figure 10C and D). Therefore, from a macroscopic perspective, the in vivo experimental results indicate that the TGF-β3/MnO2 group had superior MRI signal and gross appearance compared with the other experimental groups. At the microscopic level, immunohistochemical staining revealed more residual NP, lower iNOS expression, and significantly higher Col-II expression in the TGF-β3/MnO2 group. This indicates that the imbalance in ECM synthesis in IDD, which is one of the main causes of the loss of IVD function, can be reversed, thereby delaying the progression of IVD degeneration. This effect was attributed to the ability of the MnO2 NPs to create an appropriate microenvironment/vehicle for TGF-β3 delivery to the IVDs. Moreover, TGF-β3 delivered via the MnO2 system effectively promoted cell proliferation and inhibited ECM degradation by reducing the acidity and scavenging ROS of the IVD microenvironment.
Figure 10.
Immunohistochemical analysis of iNOS and Col-II expression in each group. Representative images of the immunohistochemical staining of iNOS (A) and Col-II (B) at 4 and 8 weeks after surgery, and the semi-quantitative analysis of iNOS (C) and COL-II (D) at these timepoints. *, p<0.05.
Discussion
We have proposed a novel strategy for IDD treatment using a MnO2 nanoplatform that effectively releases TGF-β3 in response to the acidic microenvironment produced by IDD. This novel nanoplatform can also decrease ROS accumulation to further promote cell survival and endogenous repair. The mechanisms and effectiveness of the proposed therapeutic system were validated both in vitro using rat NPCs and in vivo using a rat IDD model. Growing evidence suggests that the progression of IDD is accompanied by a pronounced decline in cell density.29 The IVD is an avascular organ with a small cell population and a limited nutrient supply, resulting in poor endogenous repair capability.30,31 During aging and degeneration, excessive cellular senescence and death prevent the natural repair processes from occurring, exacerbating IDD.32,33 Anti-senescent therapy appears to have a curative effect on IDD.34,35
Regenerative medicine has emerged as a promising option for the prevention or even reversal of disc degeneration.36 Growth factors, which are an important component of regenerative medicine, act as mediators of the healing processes and represent potential molecular targets for stimulating and guiding regeneration.37 TGF-β3 plays an essential role in cell differentiation, cell adhesion, and ECM formation; TGF-β3 has been shown to promote the repair of cartilaginous organs including IVDs and upregulate the expressions of aggrecan and type II collagen.38 Peck et al reported that TGF-β3 enhanced cell survival and ECM synthesis of mesenchymal stem cells in low-oxygen and nutrient-limited microenvironments.39 Ashraf et al reported that TGF-β3 enhanced the ability of NPCs to form tissues, in part by decreasing cell death.40 Consistent with the results of the present study, TGF-β3 treatment blocked the inhibitory effect of H2O2 on NPC proliferation, likely by suppressing the apoptotic gene BAX and upregulating the proliferative genes MCL1 and BCL2. Further, TGF-β3 alleviated the H2O2-induced expression of ECM genes (ACAN and Col-II) and partly blocked the stimulatory effect of H2O2 on the expression of catabolic (MMP3 and Adamts5) and inflammatory genes (COX-2 and iNOS). Interestingly, the protective effect of TGF-β3 against H2O2 damage in NPCs was independent of the inhibition of oxidative stress, as evidenced by the lack of any effect of TGF-β3 on intracellular ROS and antioxidant genes. In a rat model of IDD induced by needle puncture, the reductions in water and Col-II contents were attenuated by TGF-β3 treatment, which is consistent with the recovery of the MRI signal at four weeks after injection. Regrettably, the effectiveness of TGF-β3 was diminished at 8 weeks after injection, as evidenced by decreased in NP area and ECM content. This is likely because growth factors are prone to degradation in the body.41 Furthermore, the iNOS expression was higher in the group treated with TGF-β3 alone compared to the sham group at both time points (4 w and 8 w). This may represent an increased inflammatory response and persistent oxidative stress within the IDD microenvironment, which may be another important reason why the positive effects of TGF-β3 attenuate over time.
Increasing evidence suggests that the harsh disc microenvironment inhibits the self-healing processes of degenerating IVDs and restricts the capacity for external intervention.33,42 Oxidative stress has been identified as a major risk component of pathological mechanism behind IDD.43,44 Oxidative stress increases the concentration of ROS, which is closely related to cell senescence and apoptosis.45 Further, oxidative stress enhances the degradation of the ECM and the inflammatory response in IVD cells and damages the mechanical function of the IVDs.8,46 These effects accelerate the progress of IDD and lead to low back pain. Disc degeneration has long been associated with increased acidity.47 The pH value of normal discs is 7.2, while the pH in severely degenerated discs is 6.2.48,49 A normal pH is necessary to maintain cellular functions, while an excessively acidic environment significantly inhibits cell viability and matrix synthesis.50 Gilbert et al reported that the increased pH was associated with decreased NPC proliferation and vitality, a shift towards matrix catabolism, and an increased expression of proinflammatory cytokines and pain-related factors.51 Therefore, targeting oxidative stress or acidity to improve the harsh microenvironment of disc degeneration may provide a new strategy for IDD treatment.
Based on the above considerations, MnO2 nanostructures that are decomposed in the presence of H+ or glutathione have attracted our attention.52 MnO2 nanostructures are also able to trigger the decomposition of H2O2 into water and oxygen.53 These characteristics enhance the therapeutic potential of the nanostructure, in particular as anti-tumor and anti-osteoarthritis vectors.54,55 However, to the best of our knowledge, MnO2 nanostructures have not yet been reported as a strategy for IDD therapy. In this study, we developed an intelligent nanoplatform based on MnO2 NPs for TGF-β3 delivery. The nanoplatform exhibited an ultrasensitive pH-triggered release and H2O2-responsive oxygen generation to relieve IVD hypoxia and reduce oxidative injury, resulting in long-term therapeutic effects. We successfully synthesized spherical MnO2 NPs with hollow structures. The NPs exhibited no obvious toxicity to the NPCs, even at high concentrations up to 50 μg/mL. The NPs were highly effective at drug loading. The encapsulated drug was precisely and controllably released upon exposure to H+ or H2O2. In vitro experiments demonstrated that TGF-β3/MnO2 had antioxidant and anti-apoptosis effects and promoted cell proliferation. Although TGF-β3 did not increase the antioxidant capacity of MnO2, the proliferative effect of TGF-β3 was enhanced by MnO2. There was increased production of ECM components (eg, ACAN and Col-II) in the presence of H2O2 in cells treated with TGF-β3/MnO2 compared to those treated with TGF-β3 alone. We hypothesized that the enhanced effects of TGF-β3 in the setting of MnO2 may be related to the elimination of H2O2 by MnO2, resulting in a better environment for cell survival. This effect may be derived from the reduction in inflammatory factors and catabolic makers.
To further investigate the role of TGF-β3/MnO2 in vivo, an IDD model was established in rats by needle puncture with half penetration. Needle puncture is the most common method used to establish injury models because of its repeatability and short-term degenerative changes.56 Our in vivo results showed that needle puncture induced rapid and severe disc degeneration, as indicated by the disappearance of the nucleus pulposus area and the imbalanced ECM. These altered morphological features were almost recovered at four weeks after treatment with TGF-β3/MnO2. Further, the MRI signal intensity and nucleus area were larger in the TGF-β3/MnO2 group compared to the TGF-β3 and MnO2 groups. Additionally, TGF-β3/MnO2 effectively slowed Col-II degradation and iNOS expression for up to eight weeks after treatment, which was not observed in the TGF-β3 and MnO2 groups. These findings reveal that MnO2 contributes to eliminating oxidative stress and controlling the release of TGF-β3, and the latter promoted cell survival, ECM deposition and enhanced endogenous repair.
The present study has some limitations. First, we only examined the effects of TGF-β3/MnO2 on the rat IDD model for eight weeks. A longer study period is needed to evaluate the long-term efficacy of the nanoplatform. Second, an upright model that is more similar to a human IDD should be used to further validate the results obtained in the rat IDD model. Third, the use of PBS solution as a vehicle increases the possibility of leakage. Thus, a better vehicle such as a hydrogel should be developed to improve drug bioavailability and avoid burst drug release.
Conclusion
In summary, we fabricated hollow mesoporous MnO2 NPs loaded with TGF-β3 as a multifunctional drug delivery system that is responsive to H+ and H2O2 in the IDD microenvironment. This nanoplatform can modulate the microenvironment to prolong the effects of TGF-β3, which achieved well persistent influence in IDD injury. Further studies will be needed to explore some suitable vehicles to enhance the curative effect and longer treatment periods to confirm the long-term efficacy of the nanoplatform. This injectable nanoplatform for controlled drug release provides a new strategy for tissue regeneration under local oxidative stress and acidic microenvironments.
Acknowledgments
This study was supported by the people’s livelihood Science and Technology Plan of Suzhou Municipal Government to Dr. Lifan Zhu (SS202090) and Ke Jiao Xing Wei Plan of Wujiang District (WWK202006) to Dr. Lifan Zhu.
Disclosure
No potential conflict of interest was reported by the authors.
References
- 1.Bonnevie E, Gullbrand S, Ashinsky B, et al. Aberrant mechanosensing in injured intervertebral discs as a result of boundary-constraint disruption and residual-strain loss. Nat Biomed Eng. 2019;3(12):998–1008. doi: 10.1038/s41551-019-0458-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji M, Jiang H, Zhang X, et al. Preclinical development of a microRNA-based therapy for intervertebral disc degeneration. Nat Commun. 2018;9(1):5051. doi: 10.1038/s41467-018-07360-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarzer A, Aprill C, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20(17):1878–1883. doi: 10.1097/00007632-199509000-00007 [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Rui Y, Lu J, Wang C. Cell and molecular biology of intervertebral disc degeneration: current understanding and implications for potential therapeutic strategies. Cell Prolif. 2014;47(5):381–390. doi: 10.1111/cpr.12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Maitre C, Freemont A, Hoyland J. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9(3):R45. doi: 10.1186/ar2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao C, Jiang L, Dai L. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11(12):2079–2088. doi: 10.1007/s10495-006-0290-7 [DOI] [PubMed] [Google Scholar]
- 7.Feng C, Liu H, Yang Y, Huang B, Zhou Y. Growth and differentiation factor-5 contributes to the structural and functional maintenance of the intervertebral disc. Cell Physiol Biochem. 2015;35(1):1–16. doi: 10.1159/000369670 [DOI] [PubMed] [Google Scholar]
- 8.Feng C, Yang M, Lan M, et al. ROS: crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxid Med Cell Longev. 2017;2017:5601593. doi: 10.1155/2017/5601593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, Shi R, Cai F, Wang Y, Wu X. Stem cell approaches to intervertebral disc regeneration: obstacles from the disc microenvironment. Stem Cells Dev. 2015;24(21):2479–2495. doi: 10.1089/scd.2015.0158 [DOI] [PubMed] [Google Scholar]
- 10.Roger Y, Sydow S, Burmeister L, Menzel H, Hoffmann A. Sustained release of TGF-β from polysaccharide nanoparticles induces chondrogenic differentiation of human mesenchymal stromal cells. Colloids Surf B Biointerfaces. 2020;189:110843. doi: 10.1016/j.colsurfb.2020.110843 [DOI] [PubMed] [Google Scholar]
- 11.Kimbrough-Allah M, Millena A, Khan S. Differential role of PTEN in transforming growth factor β (TGF-β) effects on proliferation and migration in prostate cancer cells. Prostate. 2018;78(5):377–389. doi: 10.1002/pros.23482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazemnejad S, Khanmohammadi M, Mobini S, et al. Comparative repair capacity of knee osteochondral defects using regenerated silk fiber scaffolds and fibrin glue with/without autologous chondrocytes during 36 weeks in rabbit model. Cell Tissue Res. 2016;364(3):559–572. doi: 10.1007/s00441-015-2355-9 [DOI] [PubMed] [Google Scholar]
- 13.Ma X, Ma X, Qian W, Zhao H, Ding J, Zhao T. Co-culture of adipose-derived stem cells and chondrocytes with transforming growth factor-beta 3 promotes chondrogenic differentiation. J Craniofac Surg. 2020;31(8):2355–2359. doi: 10.1097/SCS.0000000000006748 [DOI] [PubMed] [Google Scholar]
- 14.Haberstroh K, Enz A, Zenclussen M, et al. Human intervertebral disc-derived cells are recruited by human serum and form nucleus pulposus-like tissue upon stimulation with TGF-beta3 or hyaluronan in vitro. Tissue Cell. 2009;41(6):414–420. doi: 10.1016/j.tice.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 15.Risbud M, Di Martino A, Guttapalli A, et al. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine. 2006;31(8):884–890. doi: 10.1097/01.brs.0000209335.57767.b5 [DOI] [PubMed] [Google Scholar]
- 16.Mitchell M, Billingsley M, Haley R, Wechsler M, Peppas N, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang C, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi: 10.1016/j.cell.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansari M, Ahmad N, Haqqi T. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. 2020;129:110452. doi: 10.1016/j.biopha.2020.110452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang L, Yuan F, Yin X, Dong J. Responses and adaptations of intervertebral disc cells to microenvironmental stress: a possible central role of autophagy in the adaptive mechanism. Connect Tissue Res. 2014;55(5–6):311–321. doi: 10.3109/03008207.2014.942419 [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Xu L, Chao Y, et al. Hollow MnO as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun. 2017;8(1):902. doi: 10.1038/s41467-017-01050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Adjei IM, Brown SB, Liseth O, Sharma B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials. 2019;224:119467. doi: 10.1016/j.biomaterials.2019.119467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G, Deng Y, Xie Q, et al. Sirtuins and intervertebral disc degeneration: roles in inflammation, oxidative stress, and mitochondrial function. Clin Chim Acta. 2020;508:33–42. doi: 10.1016/j.cca.2020.04.016 [DOI] [PubMed] [Google Scholar]
- 24.Che H, Li J, Li Y, et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. eLife. 2020;9. DOI: 10.7554/eLife.52570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng W, Zhang H, Deng Y, Jiang A, Mei L. Dual-response oxygen-generating MnO2 nanoparticles with polydopamine modification for combined photothermal-photodynamic therapy. Chem Eng J. 2020;389:124494. doi: 10.1016/j.cej.2020.124494 [DOI] [Google Scholar]
- 26.Pfirrmann C, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011 [DOI] [PubMed] [Google Scholar]
- 27.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20 [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Niu D, Wu Q, et al. Iron oxide/manganese oxide co-loaded hybrid nanogels as pH-responsive magnetic resonance contrast agents. Biomaterials. 2015;53:349–357. doi: 10.1016/j.biomaterials.2015.02.101 [DOI] [PubMed] [Google Scholar]
- 29.Wang F, Cai F, Shi R, Wang X, Wu X. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage. 2016;24(3):398–408. doi: 10.1016/j.joca.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 30.Grunhagen T, Shirazi-Adl A, Fairbank J, Urban J. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42(4):465–477. doi: 10.1016/j.ocl.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Peroglio M, Alini M, Grad S. Potential and limitations of intervertebral disc endogenous repair. Curr Stem Cell Res Ther. 2015;10(4):329–338. doi: 10.2174/1574888X10666150305105114 [DOI] [PubMed] [Google Scholar]
- 32.Vergroesen P, Kingma I, Emanuel K, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057–1070. doi: 10.1016/j.joca.2015.03.028 [DOI] [PubMed] [Google Scholar]
- 33.Ma K, Chen S, Li Z, et al. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthritis Cartilage. 2019;27(1):41–48. doi: 10.1016/j.joca.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 34.Yi W, Lan H, Wen Y, et al. HO-1 overexpression alleviates senescence by inducing autophagy via the mitochondrial route in human nucleus pulposus cells. J Cell Physiol. 2020;235(11):8402–8415. doi: 10.1002/jcp.29684 [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Xie J, Jin M, et al. Sirt6 overexpression suppresses senescence and apoptosis of nucleus pulposus cells by inducing autophagy in a model of intervertebral disc degeneration. Cell Death Dis. 2018;9(2):56. doi: 10.1038/s41419-017-0085-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry N, Clouet J, Le Bideau J, Le Visage C, Guicheux J. Innovative strategies for intervertebral disc regenerative medicine: from cell therapies to multiscale delivery systems. Biotechnol Adv. 2018;36(1):281–294. doi: 10.1016/j.biotechadv.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 37.Frapin L, Clouet J, Chédeville C, et al. Controlled release of biological factors for endogenous progenitor cell migration and intervertebral disc extracellular matrix remodelling. Biomaterials. 2020;253:120107. doi: 10.1016/j.biomaterials.2020.120107 [DOI] [PubMed] [Google Scholar]
- 38.Tao Y, Zhou X, Liang C, et al. TGF-β3 and IGF-1 synergy ameliorates nucleus pulposus mesenchymal stem cell differentiation towards the nucleus pulposus cell type through MAPK/ERK signaling. Growth Factors. 2015;33:326–336. doi: 10.3109/08977194.2015.1088532 [DOI] [PubMed] [Google Scholar]
- 39.Peck S, Bendigo J, Tobias J, et al. Hypoxic preconditioning enhances bone marrow-derived mesenchymal stem cell survival in a low oxygen and nutrient-limited 3D microenvironment. Cartilage. 2019;12(4):512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashraf S, Chatoor K, Chong J, Pilliar R, Santerre P, Kandel R. Transforming growth factor β enhances tissue formation by passaged nucleus pulposus cells in vitro. J Orthop Res. 2020;38(2):438–449. doi: 10.1002/jor.24476 [DOI] [PubMed] [Google Scholar]
- 41.Blanquer S, Grijpma D, Poot A. Delivery systems for the treatment of degenerated intervertebral discs. Adv Drug Deliv Rev. 2015;84:172–187. doi: 10.1016/j.addr.2014.10.024 [DOI] [PubMed] [Google Scholar]
- 42.Sakai D, Andersson G. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11(4):243–256. doi: 10.1038/nrrheum.2015.13 [DOI] [PubMed] [Google Scholar]
- 43.Han Y, Li X, Yan M, et al. Oxidative damage induces apoptosis and promotes calcification in disc cartilage endplate cell through ROS/MAPK/NF-κB pathway: implications for disc degeneration. Biochem Biophys Res Commun. 2019;516(3):1026–1032. doi: 10.1016/j.bbrc.2017.03.111 [DOI] [PubMed] [Google Scholar]
- 44.Song D, Ge J, Wang Y, et al. Tea polyphenol attenuates oxidative stress-induced degeneration of intervertebral discs by regulating the Keap1/Nrf2/ARE pathway. Oxid Med Cell Longev. 2021;2021:6684147. doi: 10.1155/2021/6684147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B, Ke W, Wang K, et al. Mechanosensitive ion channel piezo1 activated by matrix stiffness regulates oxidative stress-induced senescence and apoptosis in human intervertebral disc degeneration. Oxid Med Cell Longev. 2021;2021:8884922. doi: 10.1155/2021/8884922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki S, Fujita N, Hosogane N, et al. Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res Ther. 2015;17(1):316. doi: 10.1186/s13075-015-0834-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Razaq S, Wilkins R, Urban J. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12(4):341–349. doi: 10.1007/s00586-003-0582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diamant B, Karlsson J, Nachemson A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia. 1968;24(12):1195–1196. doi: 10.1007/BF02146615 [DOI] [PubMed] [Google Scholar]
- 49.Nachemson A. Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop Scand. 1969;40(1):23–42. doi: 10.3109/17453676908989482 [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Tao H, Wang H, et al. Biological behavior of human nucleus pulposus mesenchymal stem cells in response to changes in the acidic environment during intervertebral disc degeneration. Stem Cells Dev. 2017;26(12):901–911. doi: 10.1089/scd.2016.0314 [DOI] [PubMed] [Google Scholar]
- 51.Gilbert H, Hodson N, Baird P, Richardson S, Hoyland J. Acidic pH promotes intervertebral disc degeneration: acid-sensing ion channel −3 as a potential therapeutic target. Sci Rep. 2016;6:37360. doi: 10.1038/srep37360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan W, Bu W, Shen B, et al. Intelligent MnO2 nanosheets anchored with upconversion nanoprobes for concurrent pH-/H2O2-Responsive UCL imaging and oxygen-elevated synergetic therapy. Adv Mater. 2015;27(28):4155–4161. doi: 10.1002/adma.201405141 [DOI] [PubMed] [Google Scholar]
- 53.Gordijo CR, Abbasi AZ, Amini MA, et al. Hybrid nanoparticles: design of hybrid MnO2-Polymer-Lipid nanoparticles with tunable oxygen generation rates and tumor accumulation for cancer treatment. Adv Funct Mater. 2015;25(12):1857–1587. [Google Scholar]
- 54.Chen L, Tiwari SR, Zhang Y, Zhang J, Sun Y. Facile synthesis of hollow MnO2 nanoparticles for reactive oxygen species scavenging in osteoarthritis. ACS Biomater Sci Eng. 2021;7(4):1686–1692. doi: 10.1021/acsbiomaterials.1c00005 [DOI] [PubMed] [Google Scholar]
- 55.Prasad P, Gordijo C, Abbasi A, et al. Multifunctional albumin-MnO2 nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS nano. 2014;8(4):3202–3212. doi: 10.1021/nn405773r [DOI] [PubMed] [Google Scholar]
- 56.Keorochana G, Johnson J, Taghavi C, et al. The effect of needle size inducing degeneration in the rat caudal disc: evaluation using radiograph, magnetic resonance imaging, histology, and immunohistochemistry. Spine J. 2010;10(11):1014–1023. doi: 10.1016/j.spinee.2010.08.013 [DOI] [PubMed] [Google Scholar]